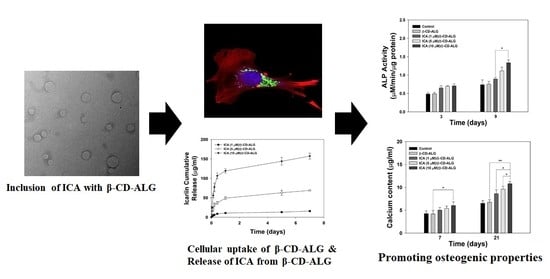
Investigating the In Vitro Osteogenic Properties of the Inclusion Nanocarrier of Icariin with Beta-Cyclodextrin-Alginate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fabrication of β-CD-Alginate (β-CD-ALG) Nanocarrier
2.2. Preparation of ICA-Loaded β-CD-ALG (ICA/β-CD-ALG)
2.3. Characterizations
2.4. In Vitro ICA Release
2.5. Cytotoxicity
2.6. Cell uptake of β-CD-ALG
2.7. ALP Activity
2.8. Calcium Deposition
2.9. Osteogenic Gene Markers
2.10. Statistical Analysis
3. Results
3.1. Physicochemical Characterization of β-CD-ALG with or without ICA
3.2. In Vitro ICA Release
3.3. Cytotoxic Test and Intracellular Uptake
3.4. ALP Activity
3.5. Calcium Content
3.6. Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ashammakhi, N.; Ahadian, S.; Darabi, M.A.; El Tahchi, M.; Lee, J.; Suthiwanich, K.; Sheikhi, A.; Dokmeci, M.R.; Oklu, R.; Khademhosseini, A. Minimally Invasive and Regenerative Therapeutics. Adv. Mater. 2019, 31, e1804041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Y.C.; Zhang, P.L.; Sun, Y.C.; Kang, Q.L.; Xu, J.; Zhang, C.F.; Chai, Y.M. Regeneration of large bone defects using mesoporous silica coated magnetic nanoparticles during distraction osteogenesis. Nanomedicine 2019, 21. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, C.E.; Borner, B.I.; Hasse, A.; Sieg, P. Donor site morbidity after microvascular fibula transfer. Clin. Oral. Investig. 2001, 5, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Silber, J.S.; Anderson, D.G.; Daffner, S.D.; Brislin, B.T.; Leland, J.M.; Hilibrand, A.S.; Vaccaro, A.R.; Albert, T.J. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine 2003, 28, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Lewandrowski, K.U.; Rebmann, V.; Passler, M.; Schollmeier, G.; Ekkernkamp, A.; Grosse-Wilde, H.; Tomford, W.W. Immune response to perforated and partially demineralized bone allografts. J. Orthop. Sci. 2001, 6, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Jaitak, V.; Kaul, V.K.; Kumar, N.; Singh, B.; Savergave, L.S.; Jogdand, V.V.; Nene, S. Simple and efficient enzymatic transglycosylation of stevioside by beta-cyclodextrin glucanotransferase from Bacillus firmus. Biotechnol. Lett. 2009, 31, 1415–1420. [Google Scholar] [CrossRef]
- Kurkov, S.V.; Loftsson, T. Cyclodextrins. Int. J. Pharm. 2013, 453, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Jansook, P.; Ogawa, N.; Loftsson, T. Cyclodextrins: Structure, physicochemical properties and pharmaceutical applications. Int. J. Pharm. 2018, 535, 272–284. [Google Scholar] [CrossRef]
- Loftsson, T.; Duchene, D. Cyclodextrins and their pharmaceutical applications. Int. J. Pharmaceut. 2007, 329, 1–11. [Google Scholar] [CrossRef]
- Devasari, N.; Dora, C.P.; Singh, C.; Paidi, S.R.; Kumar, V.; Sobhia, M.E.; Suresh, S. Inclusion complex of erlotinib with sulfobutyl ether-beta-cyclodextrin: Preparation, characterization, in silico, in vitro and in vivo evaluation. Carbohydr. Polym. 2015, 134, 547–556. [Google Scholar] [CrossRef]
- Bragagni, M.; Bozdag, M.; Carta, F.; Scozzafava, A.; Lanzi, C.; Masini, E.; Mura, P.; Supuran, C.T. Cyclodextrin complexation highly enhances efficacy of arylsulfonylureido benzenesulfonamide carbonic anhydrase inhibitors as a topical antiglaucoma agents. Bioorgan. Med. Chem. 2015, 23, 6223–6227. [Google Scholar] [CrossRef] [PubMed]
- Rekharsky, M.V.; Inoue, Y. Complexation Thermodynamics of Cyclodextrins. Chem. Rev. 1998, 98, 1875–1918. [Google Scholar] [CrossRef] [PubMed]
- Irie, T.; Uekama, K. Cyclodextrins in peptide and protein delivery. Adv. Drug Deliv. Rev. 1999, 36, 101–123. [Google Scholar] [CrossRef]
- Lysik, M.A.; Wu-Pong, S. Innovations in oligonucleotide drug delivery. J. Pharm. Sci. 2003, 92, 1559–1573. [Google Scholar] [CrossRef]
- Khan, J.; Alexander, A.; Saraf, S.; Saraf, S. Recent advances and future prospects of phyto-phospholipid complexation technique for improving pharmacokinetic profile of plant actives. J. Control Release 2013, 168, 50–60. [Google Scholar] [CrossRef]
- Ye, Y.; Jing, X.; Li, N.; Wu, Y.; Li, B.; Xu, T. Icariin promotes proliferation and osteogenic differentiation of rat adipose-derived stem cells by activating the RhoA-TAZ signaling pathway. Biomed. Pharmacother. 2017, 88, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, W.; Luo, B.; Chen, X.; Wen, W.; Zhou, C. Icariin immobilized electrospinning poly(l-lactide) fibrous membranes via polydopamine adhesive coating with enhanced cytocompatibility and osteogenic activity. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 399–409. [Google Scholar] [CrossRef]
- Qin, S.; Zhou, W.; Liu, S.; Chen, P.; Wu, H. Icariin stimulates the proliferation of rat bone mesenchymal stem cells via ERK and p38 MAPK signaling. Int. J. Clin. Exp. Med. 2015, 8, 7125–7133. [Google Scholar]
- Zhai, Y.K.; Guo, X.Y.; Ge, B.F.; Zhen, P.; Ma, X.N.; Zhou, J.; Ma, H.P.; Xian, C.J.; Chen, K.M. Icariin stimulates the osteogenic differentiation of rat bone marrow stromal cells via activating the PI3K-AKT-eNOS-NO-cGMP-PKG. Bone 2014, 66, 189–198. [Google Scholar] [CrossRef]
- Li, M.; Zhang, C.; Zhong, Y.; Zhao, J.Y. A Novel Approach to Utilize Icariin as Icariin-Derived ECM on Small Intestinal Submucosa Scaffold for Bone Repair. Ann. Biomed. Eng. 2017, 45, 2673–2682. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [Green Version]
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Yun, Y.P.; Shim, K.S.; Kim, H.J.; Kim, S.E.; Park, K.; Song, H.R. In Vitro Anti-Inflammation and Chondrogenic Differentiation Effects of Inclusion Nanocomplexes of Hyaluronic Acid-Beta Cyclodextrin and Simvastatin. Tissue Eng. Regen. Med. 2018, 15, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.M.; Chen, Y.; Hou, X.F.; Wu, X.; Gu, B.H.; Liu, Y. Sulfonato-beta-Cyclodextrin Mediated Supramolecular Nanoparticle for Controlled Release of Berberine. ACS Appl. Mater. Interfaces 2018, 10, 24987–24992. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ohba, S.; Shinkai, M.; Chung, U.I.; Nagamune, T. Icariin induces osteogenic differentiation in vitro in a BMP- and Runx2-dependent manner. Biochem. Biophys. Res. Commun. 2008, 369, 444–448. [Google Scholar] [CrossRef]
- Xia, L.; Li, Y.; Zhou, Z.; Dai, Y.; Liu, H.; Liu, H. Icariin delivery porous PHBV scaffolds for promoting osteoblast expansion in vitro. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 3545–3552. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Guo, Y.; Li, D.X.; Wang, R.; Fan, H.S.; Xiao, Y.M.; Zhang, L.; Zhang, X.D. The effect of loading icariin on biocompatibility and bioactivity of porous beta-TCP ceramic. J. Mater. Sci. Mater. Med. 2011, 22, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Ohba, S.; Komiyama, Y.; Shinkai, M.; Chung, U.I.; Nagamune, T. Icariin: A potential osteoinductive compound for bone tissue engineering. Tissue Eng. Part A 2010, 16, 233–243. [Google Scholar] [CrossRef]
- Zhu, L.; Ye, X.; Tang, G.; Zhao, N.; Gong, Y.; Zhao, Y.; Zhao, J.; Zhang, X. Biomimetic coating of compound titania and hydroxyapatite on titanium. J. Biomed. Mater. Res. A 2007, 83, 1165–1175. [Google Scholar] [CrossRef] [PubMed]
- Serigano, K.; Sakai, D.; Hiyama, A.; Tamura, F.; Tanaka, M.; Mochida, J. Effect of cell number on mesenchymal stem cell transplantation in a canine disc degeneration model. J. Orthop. Res. 2010, 28, 1267–1275. [Google Scholar] [CrossRef] [PubMed]
- Luu, H.H.; Song, W.X.; Luo, X.; Manning, D.; Luo, J.; Deng, Z.L.; Sharff, K.A.; Montag, A.G.; Haydon, R.C.; He, T.C. Distinct roles of bone morphogenetic proteins in osteogenic differentiation of mesenchymal stem cells. J. Orthop. Res. 2007, 25, 665–677. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.W.; Yun, Y.P.; Park, K.; Kim, S.E. Gentamicin and bone morphogenic protein-2 (BMP-2)-delivering heparinized-titanium implant with enhanced antibacterial activity and osteointegration. Bone 2012, 50, 974–982. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Yun, Y.P.; Shim, K.S.; Park, K.; Choi, S.W.; Shin, D.H.; Suh, D.H. Fabrication of a BMP-2-immobilized porous microsphere modified by heparin for bone tissue engineering. Colloids Surf. B Biointerfaces 2015, 134, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Ke, Y.; Zhang, Y.; Zhang, C.J.; Qian, W.; Zhang, G.L. Icariin stimulates MC3T3-E1 cell proliferation and differentiation through up-regulation of bone morphogenetic protein-2. Int. J. Mol. Med. 2012, 29, 435–439. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.F.; Cheng, C.; Wang, J.; Liu, X.Z.; Wei, H.; Han, Y.; Yang, S.H.; Wang, X. Icariin regulates the osteoblast differentiation and cell proliferation of MC3T3-E1 cells through microRNA-153 by targeting Runt-related transcription factor 2. Exp. Ther. Med. 2018, 15, 5159–5166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blair, H.C.; Larrouture, Q.C.; Li, Y.; Lin, H.; Beer-Stoltz, D.; Liu, L.; Tuan, R.S.; Robinson, L.J.; Schlesinger, P.H.; Nelson, D.J. Osteoblast Differentiation and Bone Matrix Formation In Vivo and In Vitro. Tissue Eng. Part B Rev. 2017, 23, 268–280. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.E.; Yun, Y.P.; Lee, J.Y.; Shim, J.S.; Park, K.; Huh, J.B. Co-delivery of platelet-derived growth factor (PDGF-BB) and bone morphogenic protein (BMP-2) coated onto heparinized titanium for improving osteoblast function and osteointegration. J. Tissue Eng. Regen. Med. 2015, 9, E219–E228. [Google Scholar] [CrossRef]
- Chen, M.; Cui, Y.; Li, H.; Luan, J.; Zhou, X.; Han, J. Icariin Promotes the Osteogenic Action of BMP2 by Activating the cAMP Signaling Pathway. Molecules 2019, 24, 3875. [Google Scholar] [CrossRef] [Green Version]








© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, S.; Lee, Y.S.; Jo, H.-S.; Jeong, W.K.; Kim, H.-J.; Song, M.H.; Park, K.; Kim, S.E. Investigating the In Vitro Osteogenic Properties of the Inclusion Nanocarrier of Icariin with Beta-Cyclodextrin-Alginate. Appl. Sci. 2020, 10, 4137. https://doi.org/10.3390/app10124137
Choi S, Lee YS, Jo H-S, Jeong WK, Kim H-J, Song MH, Park K, Kim SE. Investigating the In Vitro Osteogenic Properties of the Inclusion Nanocarrier of Icariin with Beta-Cyclodextrin-Alginate. Applied Sciences. 2020; 10(12):4137. https://doi.org/10.3390/app10124137
Chicago/Turabian StyleChoi, Somang, Yeong Seok Lee, Han-Saem Jo, Woong Kyo Jeong, Hak-Jun Kim, Mi Hyun Song, Kyeongsoon Park, and Sung Eun Kim. 2020. "Investigating the In Vitro Osteogenic Properties of the Inclusion Nanocarrier of Icariin with Beta-Cyclodextrin-Alginate" Applied Sciences 10, no. 12: 4137. https://doi.org/10.3390/app10124137
APA StyleChoi, S., Lee, Y. S., Jo, H. -S., Jeong, W. K., Kim, H. -J., Song, M. H., Park, K., & Kim, S. E. (2020). Investigating the In Vitro Osteogenic Properties of the Inclusion Nanocarrier of Icariin with Beta-Cyclodextrin-Alginate. Applied Sciences, 10(12), 4137. https://doi.org/10.3390/app10124137