Template-Assisted Electrochemical Synthesis of CdSe Quantum Dots—Polypyrrole Composite Nanorods
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
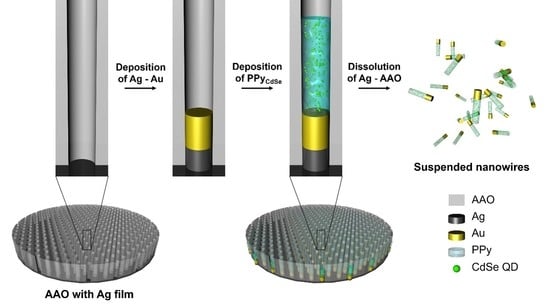
2.2. Synthesis of CdSe-Embedded PPy Nanorods
2.3. Characterization
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Little, W.A. Possibility of Synthesizing an Organic Superconductor. Phys. Rev. 1964, A134, 1415–1424. [Google Scholar] [CrossRef]
- Huang, D.; Yao, H.; Cui, Y.; Zou, Y.; Zhang, F.; Wang, C.; Shen, H.; Jin, W.; Zhu, J.; Xu, W.; et al. Conjugated-Backbone Effect of Organic Small Molecules for n-Type Thermoelectric Materials with ZT over 0.2. J. Am. Chem. Soc. 2017, 139, 13013–13023. [Google Scholar] [CrossRef] [PubMed]
- Fahlman, M.; Fabiano, S.; Gueskine, V.; Simon, D.; Berggren, M.; Crispin, X. Interfaces in organic electronics. Nat. Rev. Mater. 2019, 4, 627–650. [Google Scholar] [CrossRef]
- Yoon, C.O.; Reghu, M.; Moses, D.; Heeger, A.J.; Cao, Y.; Chen, T.-A.; Wu, X.; Rieke, R.D. Hopping transport in doped conducting polymers in the insulating regime near the metal-insulator boundary: Polypyrrole, polyaniline and polyalkylthiophenes. Synth. Met. 1995, 75, 229–239. [Google Scholar] [CrossRef]
- Yao, C.-J.; Zhang, H.-L.; Zhang, Q. Recent Progress in Thermoelectric Materials Based on Conjugated Polymers. Polymers 2019, 11, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alivisatos, A.P. Semiconductor Clusters, Nanocrystals, and Quantum Dots. Science 2006, 271, 933–937. [Google Scholar] [CrossRef] [Green Version]
- Liang, J.; Chen, D.; Yao, X.; Zhang, K.; Qu, F.; Qin, L.; Huang, Y.; Li, J. Recent Progress and Development in Inorganic Halide Perovskite Quantum Dots for Photoelectrochemical Applications. Small 2019, 16, 1903398. [Google Scholar] [CrossRef]
- Brenner, T.M.; Egger, D.A.; Kronik, L.; Hodes, G.; Cahen, D. Hybrid organic—inorganic perovskites: Low-cost semiconductors with intriguing charge-transport properties. Nat. Rev. Mater. 2016, 1, 15007. [Google Scholar] [CrossRef]
- Chen, Y.-C.; Chou, H.-L.; Lin, J.-C.; Lee, Y.-C.; Pao, C.-W.; Chen, J.-L.; Chang, C.-C.; Chi, R.-Y.; Kuo, T.-R.; Lu, C.-W.; et al. Enhanced Luminescence and Stability of Cesium Lead Halide Perovskite CsPbX3 Nanocrystals by Cu2+-Assisted Anion Exchange Reactions. J. Phys. Chem. C 2019, 123, 2353–2360. [Google Scholar] [CrossRef]
- Konstantatos, G.; Sargent, E.H. Nanostructured materials for photon detection. Nat. Nanotechnol. 2010, 5, 391–400. [Google Scholar] [CrossRef]
- Kind, H.; Yan, H.; Messer, B.; Law, M.; Yang, P. Nanowire Ultraviolet Photodetectors and Optical Switches. Adv. Mater. 2002, 14, 158–160. [Google Scholar] [CrossRef]
- Wang, X.; Liow, C.; Qi, D.; Zhu, B.; Leow, W.R.; Wang, H.; Xue, C.; Chen, X.; Li, S. Programmable photo-electrochemical hydrogen evolution based on multi-segmented CdS-Au nanorod arrays. Adv. Mater. 2014, 26, 3506–3512. [Google Scholar] [CrossRef] [PubMed]
- Tian, B.; Zheng, X.; Kempa, T.J.; Fang, Y.; Yu, N.; Yu, G.; Huang, J.; Lieber, C.M. Coaxial silicon nanowires as solar cells and nanoelectronic power sources. Nature 2007, 449, 885–889. [Google Scholar] [CrossRef]
- Lu, W.; Lieber, C.M. Nanoelectronics from the bottom up. Nat. Mater. 2007, 6, 841–850. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.L.M.; Gowda, S.R.; Shaijumon, M.M.; Ajayan, P.M. Hybrid Nanostructures for Energy Storage Applications. Adv. Mater. 2012, 24, 5045–5064. [Google Scholar] [CrossRef] [PubMed]
- Konstantatos, G.; Clifford, J.; Levina, L.; Sargent, E.H. Sensitive solution-processed visible-wavelength photodetectors. Nat. Photonics 2007, 1, 531–534. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Xu, J.; Zhang, Q.; Bando, Y.; Golberg, D.; Ma, Y.; Zhai, T. One-dimensional CdS nanostructures: A promising candidate for optoelectronics. Adv. Mater. 2013, 25, 3017–3037. [Google Scholar] [CrossRef]
- Bognitzki, M.; Czado, W.; Frese, T.; Schaper, A.; Hellwig, M.; Steinhart, M.; Greiner, A.; Wendorff, J.H. Nanostructured Fibers via Electrospinning. Adv. Mater. 2001, 13, 70–72. [Google Scholar] [CrossRef]
- Robel, I.; Bunker, B.A.; Kamat, P.V. Single-Walled Carbon Nanotube–CdS Nanocomposites as Light-Harvesting Assemblies: Photoinduced Charge-Transfer Interactions. Adv. Mater. 2005, 17, 2458–2463. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Liu, H.; Lai, S.-W.; Li, Y.; Li, Y.; Hu, W.; Wang, S.; Che, C.-M.; Zhu, D. Assembled Organic/Inorganic p−n Junction Interface and Photovoltaic Cell on a Single Nanowire. J. Phys. Chem. Lett. 2010, 1, 327–330. [Google Scholar] [CrossRef]
- Lauhon, L.J.; Gudiksen, M.S.; Wang, D.; Lieber, C.M. Epitaxial core–shell and core–multishell nanowire heterostructures. Nature 2002, 420, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Wang, X.; Geng, D.; Ozgit-Akgun, C.; Schneider, N.; Elam, J.W. Atomic layer deposition for nanomaterial synthesis and functionalization in energy technology. Mater. Horiz. 2017, 4, 133–154. [Google Scholar] [CrossRef]
- Ten Eyck, G.A.; Pimanpang, S.; Juneja, J.S.; Bakhru, H.; Lu, T.-M.; Wang, G.-C. Plasma-Enhanced Atomic Layer Deposition of Palladium on a Polymer Substrate. Chem. Vap. Depos. 2007, 13, 307–311. [Google Scholar] [CrossRef]
- Song, B.; Schneider, G.F.; Xu, Q.; Pandraud, G.; Dekker, C.; Zandbergen, H. Atomic-Scale Electron-Beam Sculpting of Near-Defect-Free Graphene Nanostructures. Nano Lett. 2011, 11, 2247–2250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.-H.; Jeong, Y.K.; Peters, J.A.; Nam, G.-H.; Jin, S.; Kim, J.-H. In Situ Fabrication of Nano Transistors by Selective Deposition of a Gate Dielectric around Carbon Nanotubes. ACS Appl. Mater. Interfaces 2015, 7, 24094–24102. [Google Scholar] [CrossRef] [PubMed]
- Nam, G.-H.; Lee, J.-H.; Zahari, N.E.M.; Khalid, N.C.; Kang, W.-S.; Kim, J.-H. Single-Bundle Carbon-Nanotube-Bridged Nanorod Devices with Control of Gap Length. J. Phys. Chem. C 2014, 118, 10463–10471. [Google Scholar] [CrossRef]
- Ostermann, R.; Li, D.; Yin, Y.; McCann, J.T.; Xia, Y. V2O5 Nanorods on TiO2 Nanofibers: A New Class of Hierarchical Nanostructures Enabled by Electrospinning and Calcination. Nano Lett. 2006, 6, 1297–1302. [Google Scholar] [CrossRef]
- Huynh, W.U.; Peng, X.; Alivisatosen, A.P. CdSe Nanocrystal Rods/Poly(3-hexylthiophene) Composite Photovoltaic Devices. Adv. Mater. 1999, 11, 923–927. [Google Scholar] [CrossRef]
- Zhang, J.; Zhong, L.; Sun, Y.; Li, A.; Huang, J.; Meng, F.; Chandran, B.K.; Li, S.; Jiang, L.; Chen, X. Enhanced Photoresponse of Conductive Polymer Nanowires Embedded with Au Nanoparticles. Adv. Mater. 2016, 28, 2978–2982. [Google Scholar] [CrossRef]
- Park, S.; Lim, J.-H.; Chung, S.-W.; Mirkin, C.A. Self-Assembly of Mesoscopic Metal-Polymer Amphiphiles. Science 2004, 303, 348–351. [Google Scholar] [CrossRef] [Green Version]
- Chen, N.; Qian, X.; Lin, H.; Liu, H.; Li, Y.; Li, Y. Synthesis and characterization of axial heterojunction inorganic–organic semiconductor nanowire arrays. Dalton Trans. 2011, 40, 10804–10808. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Ji, R.; Gösele, U.; Nielsch, K. Fast fabrication of long-range ordered porous alumina membranes by hard anodization. Nat. Mater. 2006, 5, 741–747. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.; Maiz, J.; Sacristan, J.; Mijangos, C. Tailored polymer-based nanorods and nanotubes by “template synthesis”: From preparation to applications. Polymer 2012, 53, 1149–1166. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.; Kwon, N.; Hong, J.; Chung, I. Fabrication and characterization of metal-semiconductor-metal nanorod using template synthesis. J. Vac. Sci. Technol. A 2009, 27, 808–812. [Google Scholar] [CrossRef]
- Spirin, M.G.; Brichkin, S.B.; Razumov, V.F. The solvent effect on luminescent properties of cadmium selenide quantum dots. High Energy Chem. 2015, 49, 193–198. [Google Scholar] [CrossRef]
- Raksawong, P.; Nurerk, P.; Chullasat, K.; Kanatharana, P.; Bunkoed, O. A polypyrrole doped with fluorescent CdTe quantum dots and incorporated into molecularly imprinted silica for fluorometric determination of ampicillin. Mikrochim. Acta 2019, 186, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.-Y.; Lin, I.-H. Characterization of Polypyrrole-CdSe/CdTe Nanocomposite Films Prepared with an All Electrochemical Deposition Process. J. Phys. Chem. B 2003, 107, 6974–6978. [Google Scholar] [CrossRef]
- Xie, Y.; Du, H. Electrochemical capacitance of a carbon quantum dots–polypyrrole/titania nanotube hybrid. RSC Adv. 2015, 5, 89689–89697. [Google Scholar] [CrossRef]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, W.-S.; Oh, T.; Nam, G.-H.; Kim, H.-S.; Kim, K.-S.; Park, S.-H.; Kim, J.-H.; Lee, J.-H. Template-Assisted Electrochemical Synthesis of CdSe Quantum Dots—Polypyrrole Composite Nanorods. Appl. Sci. 2020, 10, 5966. https://doi.org/10.3390/app10175966
Kang W-S, Oh T, Nam G-H, Kim H-S, Kim K-S, Park S-H, Kim J-H, Lee J-H. Template-Assisted Electrochemical Synthesis of CdSe Quantum Dots—Polypyrrole Composite Nanorods. Applied Sciences. 2020; 10(17):5966. https://doi.org/10.3390/app10175966
Chicago/Turabian StyleKang, Won-Seok, Taegon Oh, Gwang-Hyeon Nam, Hyo-Sop Kim, Ki-Suk Kim, Sun-Hyun Park, Jae-Ho Kim, and Jae-Hyeok Lee. 2020. "Template-Assisted Electrochemical Synthesis of CdSe Quantum Dots—Polypyrrole Composite Nanorods" Applied Sciences 10, no. 17: 5966. https://doi.org/10.3390/app10175966
APA StyleKang, W. -S., Oh, T., Nam, G. -H., Kim, H. -S., Kim, K. -S., Park, S. -H., Kim, J. -H., & Lee, J. -H. (2020). Template-Assisted Electrochemical Synthesis of CdSe Quantum Dots—Polypyrrole Composite Nanorods. Applied Sciences, 10(17), 5966. https://doi.org/10.3390/app10175966