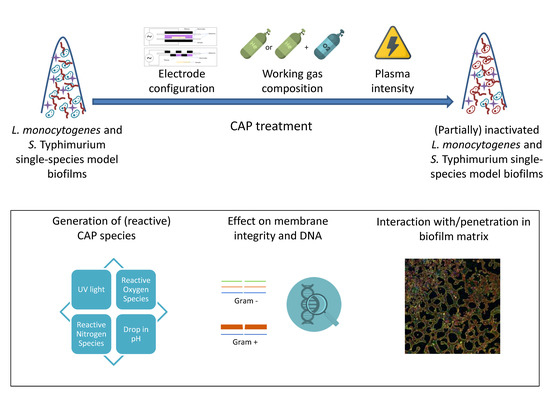
Influence of Plasma Characteristics on the Inactivation Mechanism of Cold Atmospheric Plasma (CAP) for Listeria monocytogenes and Salmonella Typhimurium Biofilms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Microorganisms, Pre-Culture Conditions, and Model Biofilm Development
2.3. CAP Equipment and Inactivation Procedure
2.4. Quantification of the Generated (Reactive) CAP Species
2.4.1. Detection of the Generation of UV Light
2.4.2. Chemical Composition and pH of CAP Treated Water
2.4.3. Generation of Intracellular Reactive Oxygen Species (ROS)
2.5. Effect of the Generated (Reactive) CAP Species on the Biofilms
2.5.1. Viable Cell Density Reduction due to UV Light
2.5.2. Membrane Integrity
2.5.3. DNA Damage
2.5.4. Depth of Penetration of the Generated CAP Species
2.6. Statistical Analysis
3. Results
3.1. Quantification of the Generated (Reactive) CAP Species
3.1.1. Detection of the Generation of UV Light
3.1.2. Chemical Composition and pH of CAP Treated Water
3.1.3. Generation of Intracellular Reactive Oxygen Species (ROS)
3.2. Effect of the Generated (Reactive) CAP Species on the Biofilms
3.2.1. Viable Cell Density Reduction Due to UV Light
3.2.2. Membrane Integrity
3.2.3. DNA Damage
3.2.4. Confocal Laser Scanning Microscopy (CLSM)
4. Discussion
4.1. Quantification of the Generated (Reactive) CAP Species
4.1.1. Detection of the Generation of UV Light
4.1.2. Generation of ROS/RNS
4.2. Effect of the Generated (Reactive) CAP Species on the Biofilms
4.2.1. Impact of the Generated UV Light
4.2.2. Membrane Integrity
4.2.3. DNA Damage
4.2.4. Penetration of CAP Species into the Biofilms and Their Effect on the Biofilm Structure
4.3. Summary
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bakke, R.; Trulear, M.G.; Robinson, J.A.; Characklis, W.G. Activity of Pseudomonas aeruginosa in biofilms: Steady state. Biotechnol. Bioeng. 1984, 26, 1418–1424. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Cheng, K.J.; Geesey, G.G.; Ladd, T.I.; Nickel, J.C.; Dasgupta, M.; Marrie, T.J. Bacterial biofilms in nature and disease. Annu. Rev. Microbiol. 1987, 41, 435–464. [Google Scholar] [CrossRef] [PubMed]
- Kumar, C.G.; Anand, S.K. Significance of microbial biofilms in food industry: A review. Int. J. Food Microbiol. 1998, 42, 9–27. [Google Scholar] [CrossRef]
- Jefferson, K.K. What drives bacteria to produce a biofilm? FEMS Microbiol. Lett. 2004, 236, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Garrett, T.R.; Bhakoo, M.; Zhang, Z. Bacterial adhesion and biofilms on surfaces. Prog. Nat. Sci-Mater. 2008, 18, 1049–1056. [Google Scholar] [CrossRef]
- Giaouris, E.; Heir, E.; Hébraud, M.; Chorianopoulos, N.; Langsrud, S.; Møretrø, T.; Habimana, O.; Desvaux, M.; Renier, S.; Nychas, G.-J. Attachment and biofilm formation by foodborne bacteria in meat processing environments: Causes, implications, role of bacterial interactions and control by alternative novel methods. Meat Sci. 2014, 97, 298–309. [Google Scholar] [CrossRef]
- Tendero, C.; Tixier, C.; Tristant, P.; Desmaison, J.; Leprince, P. Atmospheric pressure plasmas: A review. Spectrochim. Acta Part B 2006, 61, 2–30. [Google Scholar] [CrossRef]
- Misra, N.N.; Tiwari, B.K.; Raghavarao, K.S.M.S.; Cullen, P.J. Nonthermal Plasma Inactivation of Food-Borne Pathogens. Food Eng. Rev. 2011, 3, 159–170. [Google Scholar] [CrossRef] [Green Version]
- Banu, M.S.; Sasikala, P.; Dhanapal, A.; Kavitha, V.; Yazhini, G.; Rajamani, L. Cold plasma as a novel food processing technology. IFSET 2012, 4, 803–818. [Google Scholar]
- Fernández, A.; Thompson, A. The inactivation of Salmonella by cold atmospheric plasma treatment. Food Res. Int. 2012, 45, 678–684. [Google Scholar] [CrossRef]
- Patil, S.; Bourke, P.; Cullen, P.J. Principles of Nonthermal Plasma Decontamination. In Cold Plasma in Food and Agriculture—Fundamentals and Applications; Misra, N.N., Schlüter, O.K., Cullen, P.J., Eds.; Academic Press: Cambridge, MA, USA; Elsevier Inc.: London, UK, 2016; pp. 143–178. [Google Scholar]
- Vleugels, M.; Shama, G.; Deng, X.T.; Greenacre, E.; Brocklehurst, T.; Kong, M.G. Atmospheric Plasma Inactivation of Biofilm-Forming Bacteria for Food Safety Control. IEEE Trans. Plasma Sci. 2005, 33, 824–828. [Google Scholar] [CrossRef] [Green Version]
- Niemira, B.A.; Boyd, G.; Sites, J. Cold Plasma Rapid Decontamination of Food Contact Surfaces Contaminated With Salmonella Biofilms. J. Food Sci. 2014, 79, M917–M922. [Google Scholar] [CrossRef] [PubMed]
- Ziuzina, D.; Han, L.; Cullen, P.J.; Bourke, P. Cold plasma inactivation of internalised bacteria and biofilms for Salmonella enterica serovar Typhimurium, Listeria monocytogenes and Escherichia coli. Int. J. Food Microbiol. 2015, 210, 53–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govaert, M.; Smet, C.; Baka, M.; Ećimović, B.; Walsh, J.L.; Van Impe, J. Resistance of L. monocytogenes and S. Typhimurium towards Cold Atmospheric Plasma as function of biofilm age. Appl. Sci. 2018, 8, 2702. [Google Scholar] [CrossRef] [Green Version]
- Niemira, B.A.; Boyd, G.; Sites, J. Cold Plasma Inactivation of Escherichia coli O17:H7 Biofilms. Front. Sust. Food Syst. 2018, 2, 47. [Google Scholar] [CrossRef] [Green Version]
- Govaert, M.; Smet, C.; Vergauwen, L.; Ećimović, B.; Walsh, J.L.; Baka, M.; Van Impe, J. Influence of plasma characteristics on the efficacy of Cold Atmospheric Plasma (CAP) for inactivation of Listeria monocytogenes and Salmonella Typhimurium biofilms. IFSET 2019, 52, 376–386. [Google Scholar] [CrossRef]
- Laroussi, M.; Leipold, F. Evaluation of the roles of reactive species, heat, and UV radiation in the inactivation of bacterial cells by air plasmas at atmospheric pressure. Int. J. Mass Spectrom. 2004, 233, 81–86. [Google Scholar] [CrossRef]
- Surowsky, B.; Schlüter, O.; Knorr, D. Interactions of Non-Thermal Atmospheric Pressure Plasma with Solid and Liquid Food Systems: A Review. Food Eng. Rev. 2015, 7, 82–108. [Google Scholar] [CrossRef]
- Puligundla, P.; Mok, C. Potential applications of nonthermal plasmas against biofilm-associated micro-organisms in vitro. J. Appl. Microbiol. 2017, 122, 1134–1148. [Google Scholar] [CrossRef] [Green Version]
- Lerouge, S.; Wertheimer, M.R.; Yahia, L. Plasma Sterilization: A Review of Parameters, Mechanisms, and Limitations. Plasmas Polym. 2001, 6, 175–188. [Google Scholar] [CrossRef]
- Rød, S.K.; Hansen, F.; Leipold, F.; Knøchel, S. Cold atmospheric pressure plasma treatment of ready-to-eat meat: Inactivation of Listeria innocua and changes in product quality. Food Microbiol. 2012, 30, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Bourke, P.; Ziuzina, D.; Han, L.; Cullen, P.J.; Gilmore, B.F. Microbiological interactions with cold plasma. J. Appl. Microbiol. 2017, 123, 308–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, R.J.W.; Johnston, M.D. The effect of interfering substances on the disinfection process: A mathematical model. J. Appl. Microbiol. 2001, 91, 548–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- BCCM. Belgian Co-ordinated Collections of Micro-organisms. Available online: http://bccm.belspo.be/ (accessed on 10 January 2017).
- Govaert, M.; Smet, C.; Baka, M.; Janssens, T.; Van Impe, J. Influence of incubation conditions on the formation of model biofilms by Listeria monocytogenes and Salmonella Typhimurium on abiotic surfaces. J. Appl. Microbiol. 2018, 125, 1890–1990. [Google Scholar] [CrossRef]
- Lu, P.; Boehm, D.; Bourke, P.; Cullen, P.J. Achieving reactive species specificity within plasma-activated water through selective generation using air spark and glow discharges. Plasma Process. Polym. 2017, 14, 1600207. [Google Scholar] [CrossRef] [Green Version]
- Han, L.; Patil, S.; Boehm, D.; Milosavljević, V.; Cullen, P.J.; Bourke, P. Mechanisms of Inactivation by High-Voltage Atmospheric Cold Plasma Differ for Escherichia coli and Staphylococcus aureus. Appl. Environ. Microbiol. 2015, 82, 450–458. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, H.W.; Henins, I.; Park, J.; Selwyn, G.S. Decontamination of chemical and biological warfare (CBW) agents using an atmospheric pressure plasma jet (APPJ). Phys. Plasmas 1999, 6, 2284–2289. [Google Scholar] [CrossRef] [Green Version]
- Mendis, D.A.; Rosenberg, M.; Azam, F. A note on the possible electrostatic disruption of bacteria. IEEE Trans. Plasma Sci. 2000, 28, 1304–1306. [Google Scholar] [CrossRef]
- Hong, Y.F.; Kang, J.G.; Lee, H.-Y.; Uhm, H.S.; Moon, E.; Park, Y.H. Sterilization effect of atmospheric plasma on Escherichia coli and Bacillus subtilis endospores. Lett. Appl. Microbiol. 2009, 48, 33–37. [Google Scholar] [CrossRef]
- Qiagen. DNeasy Blood & Tissue Handbook. Qiagen, 2006. Available online: https://www.qiagen.com/us/resources/resourcedetail?id=6b09dfb8-6319-464d-996c-79e8c7045a50&lang=en (accessed on 5 January 2020).
- Stewart, P.S.; Murga, R.; Srinivasan, R.; de Beer, D. Biofilm structural heterogeneity visualized by three microscopic methods. Water Res. 1995, 29, 2006–2009. [Google Scholar] [CrossRef]
- Boulos, L.; Prévost, M.; Barbeau, B.; Coallier, J.; Desjardins, R. LIVE/DEAD® BacLight™: Application of a new rapid staining method for direct enumeration of viable and total bacteria in drinking water. J. Microbiol. Meth. 1999, 37, 77–86. [Google Scholar] [CrossRef]
- Philips, J.; Rabaey, K.; Lovley, D.R.; Vargas, M. Biofilm Formation by Clostridium Ijungdahlii Is Induced by Sodium Chloride Stress: Experimental Evaluation and Transcriptome Analysis. PLoS ONE 2017, 12, e0170406. [Google Scholar] [CrossRef]
- Murakami, T.; Niemi, K.; Gans, T.; O’Connell, D.; Graham, W.G. Afterglow chemistry of atmospheric-pressure helium–oxygen plasmas with humid air impurity. Plasma Sources Sci. Technol. 2014, 23, 025005. [Google Scholar] [CrossRef]
- Boxhammer, V.; Morfill, G.E.; Jokipii, J.R.; Shimizu, T.; Klämpfl, T.; Li, Y.-F.; Köritzer, J.; Schlegel, J.; Zimmermann, J.L. Bactericidal action of cold atmospheric plasma in solution. New J. Phys. 2012, 14, 113042. [Google Scholar] [CrossRef]
- Govaert, M.; Smet, C.; Graeffe, A.; Walsh, J.L.; Van Impe, J. Inactivation of L. monocytogenes and S. typhimurium biofilms by means of an air-based Cold Atmospheric Plasma (CAP) system. Foods 2020, 9, 157. [Google Scholar] [CrossRef] [Green Version]
- Lukes, P.; Dolezalova, E.; Sisrova, I.; Clupek, M. Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: Evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2 and HNO2. Plasma Sources Sci. Technol. 2014, 23, 015019. [Google Scholar] [CrossRef]
- Chauvin, J.; Judée, F.; Yousfi, M.; Vicendo, P.; Merbahi, N. Analysis of reactive oxygen and nitrogen species generated in three liquid media by low temperature helium plasma jet. Sci. Rep. 2017, 7, 4562. [Google Scholar] [CrossRef]
- Gilmore, B.; Flynn, P.B.; O’Brien, S.; Hickok, N.; Freeman, T.; Bourke, P. Cold Plasma for Biofilm Control: Opportunities and Challenges. Trends Biotechnol. 2018, 36, 627–638. [Google Scholar] [CrossRef]
- Duan, J.; Lu, X.; He, G. On the penetration depth of reactive oxygen and nitrogen species generated by a plasma jet through real biological tissue. Phys. Plasmas 2017, 24, 073506. [Google Scholar] [CrossRef]
- Laroussi, M. Low Temperature Plasma-Based Sterilization: Overview and State-of-the-Art. Plasma Process. Polym. 2005, 2, 391–400. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Clauson, M.; Hong, J.; Murphy, A.B. Gram positive and Gram negative bacteria differ in their sensitivity to cold plasma. Sci. Rep. 2016, 6, 38610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitehead, J.C. The Chemistry of Cold Plasma. In Cold Plasma in Food and Agriculture—Fundamentals and Applications; Misra, N.N., Schlüter, O.K., Cullen, P.J., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 53–81. [Google Scholar]
- Hojnik, N.; Modic, M.; Ni, Y.; Filipič, G.; Cvelbar, U.; Walsh, J.L. Effective Fungal Spore Inactivation with an Environmentally Friendly Approach Based on Atmospheric Pressure Air Plasma. Environ. Sci. Technol. 2019, 53, 1893–1904. [Google Scholar] [CrossRef] [PubMed]
- Judée, F.; Simon, S.; Bailly, C.; Dufour, T. Plasma-activation of tap water using DBD for agronomy applications: Identification and quantification of long lifetime chemical species production/consumption mechanisms. Water Res. 2018, 133, 47–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barker, C.; Park, S.F. Sensitization of Listeria monocytogenes to Low pH, Organic Acids, and Osmotic Stress by Ethanol. Appl. Environ. Microbiol. 2001, 67, 1594–1600. [Google Scholar] [CrossRef] [Green Version]
- Marriott, N.G.; Gravani, R.B. Principles of Food Sanitation, 5th ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Food Safety Authority of Ireland. Listeria Monocytogenes Factsheet. Available online: https://www.fsai.ie/search-results.html?searchString=Listeria (accessed on 10 January 2019).
- Lawrence, E. How Salmonella survive the stomach. Nature 1998. [Google Scholar] [CrossRef]
- Albrecht, J.A. Salmonella. Institute of Agriculture and Natural Resources. Available online: https://food.unl.edu/salmonella (accessed on 10 January 2019).
- Zhang, Q.; Ma, R.; Tian, Y.; Su, B.; Wang, K.; Yu, S.; Zhang, J.; Fang, J. Sterilization Efficiency of a Novel Electrochemical Disinfectant against Staphylococcus aureus. Environ. Sci. Technol. 2016, 50, 3184–3192. [Google Scholar] [CrossRef]
- Caminiti, I.M.; Palgan, I.; Muñoz, A.; Noci, F.; Whyte, P.; Morgan, D.J.; Cronin, D.A.; Lyng, J.G. The Effect of Ultraviolet Light on Microbial Inactivation and Quality Attributes of Apple Juice. Food Bioprocess Technol. 2010, 5, 680–686. [Google Scholar] [CrossRef]
- BMG Labtech. UV Absorbance DNA Quantification. BMG Labtech. Available online: http://www.bmglabtech.com/uv-absorbance-dna-quantification (accessed on 2 March 2020).
- Smet, C.; Baka, M.; Dickenson, A.; Walsh, J.L.; Valdramidis, V.P.; Van Impe, J. Antimicrobial efficacy of cold atmospheric plasma for different intrinsic and extrinsic parameters. Plasma Process. Polym. 2017, 15, e1700048. [Google Scholar] [CrossRef]
- Maisch, T.; Shimizu, T.; Mitra, A.; Heinlin, J.; Karrer, S.; Li, Y.F.; Morfill, G.; Zimmermann, J.L. Contact-free cold atmospheric plasma treatment of Deinococcus radiodurans. J. Ind. Microbiol. Biotechnol. 2012, 39, 1367–1375. [Google Scholar] [CrossRef]
- Angarano, V.; Smet, C.; Akkermans, S.; Watt, C.; Chieffe, A.; Van Impe, J.F.M. Impact of LED light on Pseudomonas fluorescens and Staphylococcus epidermidis biofilms. In Proceedings of the FOODSIM 2020, Ghent, Belgium, 5–9 April 2020; Van Impe, J., Polanska, M., Eds.; EUROSIS-ETI: Ostend, Belgium, 2020. in press. [Google Scholar]
- Ghimire, B.; Szili, E.J.; Lamichhane, P.; Short, R.D.; Sup Lim, J.; Attri, P.; Masur, K.; Weltmann, K.-D.; Hong, S.-H.; Ha Choi, E. The role of UV photolysis and molecular transport in the generation of reactive species in a tissue model with a cold atmospheric pressure plasma jet. Appl. Phys. Lett. 2019, 114, 093701. [Google Scholar] [CrossRef] [Green Version]
- Stoffels, E.; Sakiyama, Y.; Graves, D.B. Cold Atmospheric Plasma: Charged Species and their Interactions with Cells and Tissues. IEEE Trans. Plasma Sci. 2008, 36, 1441–1457. [Google Scholar] [CrossRef]
- Laroussi, M. Low-temperature plasma for medicine? IEEE Trans. Plasma Sci. 2009, 37, 714–725. [Google Scholar] [CrossRef]
- Ciofu, O.; Tolker-Nielsen, T. Antibiotic Tolerance and Resistance in Biofilms. In Biofilms Infections; Bjarnsholt, T., Jensen, P.Ø., Moser, C., Høiby, N., Eds.; Springer: New York, NY, USA, 2011; pp. 215–229. [Google Scholar]
- Harm, W. Biological Effects of Ultraviolet Radiation; Cambridge University Press: Cambridge, UK, 1980. [Google Scholar]
- Traba, C.; Liang, J.F. Susceptibility of Staphylococcus aureus biofilms to reactive discharge gases. Biofouling 2011, 27, 763–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govaert, M.; Smet, C.; Verheyen, D.; Walsh, J.L.; Van Impe, J. Combined effect of Cold Atmospheric Plasma and hydrogen peroxide treatment on mature Listeria monocytogenes and Salmonella Typhimurium biofilms. Front. Microbiol. 2019, 10, 2674. [Google Scholar] [CrossRef] [PubMed] [Green Version]













| Electrode-Oxygen Level % (v/v) | Discharge Power/Input Voltage | Peak-to-Peak Output Voltage (kV) | Dissipated Power (W) |
|---|---|---|---|
| DBD-0% | 13.88 | 4.5 ± 0.2 | 2.6 ± 0.1 |
| 21.88 | 6.4 ± 0.0 | 7.0 ± 0.3 | |
| DBD-1% | 13.88 | 6.3 ± 0.1 | 1.9 ± 0.2 |
| 21.88 | 7.8 ± 0.1 | 5.9 ± 0.2 | |
| SBD-0% | 13.88 | 2.4 ± 0.1 | 1.6 ± 0.0 |
| 21.88 | 3.5 ± 0.1 | 3.2 ± 0.1 | |
| SBD-1% | 13.88 | 2.8 ± 0.1 | 1.9 ± 0.0 |
| 21.88 | 3.9 ± 0.1 | 3.8 ± 0.1 |
| DBD-0%-21.88 V | DBD-1%-21.88 V | DBD-0%-13.88 V | DBD-1%-13.88 V | SBD-0%-21.88 V | SBD-1%-21.88 V | SBD-0%-13.88 V | SBD-1%-13.88 V | |
|---|---|---|---|---|---|---|---|---|
| UV photons detected using UV-TEC strips | No | Yes | No | No | No | No | No | No |
| H2O2 | Yes | Yes | No | No | No | No | No | No |
| Nitrite | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Nitrate | Yes | Yes | Yes | Yes | No | No | Yes | No |
| pH drop | Yes | Yes | Yes | Yes | Yes | Yes | No | No |
| Listeria monocytogenes | ||||||||
| DBD-0%-21.88 V | DBD-1%-21.88 V | DBD-0%-13.88 V | DBD-1%-13.88 V | SBD-0%-21.88 V | SBD-1%-21.88 V | SBD-0%-13.88 V | SBD-1%-13.88 V | |
| Intracellular ROS | No | Yes | Yes | Yes | No | Yes | No | No |
| Log-reductions due to UV light (>1 log10 (CFU/cm2)) | Yes | Yes | No | No | No | No | Yes | Yes |
| Membrane damage | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No |
| DNA damage | No | Yes | Yes | No | Yes | Yes | Yes | No |
| Clear indication of an increased porosity | No | Yes | Yes | No | Yes | No | No | Yes |
| Salmonella Typhimurium | ||||||||
| DBD-0%-21.88 V | DBD-1%-21.88 V | DBD-0%-13.88 V | DBD-1%-13.88 V | SBD-0%-21.88 V | SBD-1%-21.88 V | SBD-0%-13.88 V | SBD-1%-13.88 V | |
| Intracellular ROS | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes |
| Log-reductions due to UV light (>1 log10 (CFU/cm2)) | Yes | Yes | No | Yes | No | No | No | No |
| Membrane damage | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| DNA damage | No | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Clear indication of an increased porosity | No | No | No | No | No | No | No | No |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Govaert, M.; Smet, C.; L. Walsh, J.; Van Impe, J.F.M. Influence of Plasma Characteristics on the Inactivation Mechanism of Cold Atmospheric Plasma (CAP) for Listeria monocytogenes and Salmonella Typhimurium Biofilms. Appl. Sci. 2020, 10, 3198. https://doi.org/10.3390/app10093198
Govaert M, Smet C, L. Walsh J, Van Impe JFM. Influence of Plasma Characteristics on the Inactivation Mechanism of Cold Atmospheric Plasma (CAP) for Listeria monocytogenes and Salmonella Typhimurium Biofilms. Applied Sciences. 2020; 10(9):3198. https://doi.org/10.3390/app10093198
Chicago/Turabian StyleGovaert, Marlies, Cindy Smet, James L. Walsh, and Jan F. M. Van Impe. 2020. "Influence of Plasma Characteristics on the Inactivation Mechanism of Cold Atmospheric Plasma (CAP) for Listeria monocytogenes and Salmonella Typhimurium Biofilms" Applied Sciences 10, no. 9: 3198. https://doi.org/10.3390/app10093198
APA StyleGovaert, M., Smet, C., L. Walsh, J., & Van Impe, J. F. M. (2020). Influence of Plasma Characteristics on the Inactivation Mechanism of Cold Atmospheric Plasma (CAP) for Listeria monocytogenes and Salmonella Typhimurium Biofilms. Applied Sciences, 10(9), 3198. https://doi.org/10.3390/app10093198