Effect of Semi-Continuous Anaerobic Digestion on the Substrate Solubilisation of Lignin-Rich Steam-Exploded Ludwigia grandiflora
Abstract
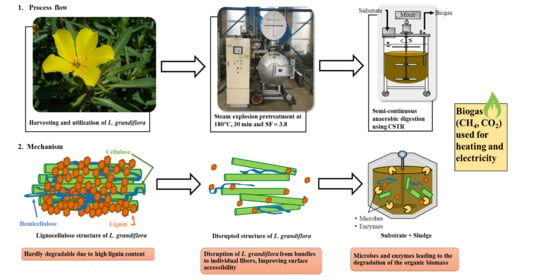
:1. Introduction
2. Material and Methods
2.1. Substrate and Inoculum
2.2. SE Pretreatment
2.3. AD of L. grandiflora Using a CSTR
2.4. Analytical Parameters
3. Results and Discussions
3.1. Mass Balance and Chemical Composition of the Untreated and Pretreated Substrate and Effluent
3.2. The Effluent pH, HRT and OLR during the AD Process
3.3. Biogas Production Yield and Methane Content in the CSTR Reactor
3.4. Carbon Mass Balance during the AD Process
3.5. Comparison of the Differently Pretreated Lignocellulosic Biomass in the AD Process
3.6. Future Research Perspectives and Challenges
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forster-Carneiro, T.; Pérez, M.; Romero, L.I. Thermophilic anaerobic digestion of source-sorted organic fraction of municipal solid waste. Bioresour. Technol. 2008, 99, 6763–6770. [Google Scholar] [CrossRef]
- Carlsson, M.; Lagerkvist, A.; Morgan-Sagastume, F. The effects of substrate pre-treatment on anaerobic digestion systems: A review. Waste Manag. 2012, 32, 1634–1650. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.A.; Nipaney, P.C.; Schaumberg, G.D. Bioenergy potential of eight common aquatic weeds. Biol. Wastes 1990, 34, 359–366. [Google Scholar] [CrossRef]
- Das, A.; Mondal, C.; Roy, S. Pretreatment methods of ligno-cellulosic biomass: A review. J. Eng. Sci. Technol. Rev. 2015, 8, 141–165. [Google Scholar] [CrossRef]
- Sun, J.X.; Sun, R.C.; Sun, X.F.; Su, Y.Q. Fractional and physico-chemical characterization of hemicelluloses from ultrasonic irradiated sugarcane bagasse. Carbohydr. Res. 2004, 339, 291–300. [Google Scholar] [CrossRef]
- Saratale, G.D.; Jung, M.Y.; Oh, M.K. Reutilization of green liquor chemicals for pretreatment of whole rice waste biomass and its application to 2,3-butanediol production. Bioresour. Technol. 2016, 205, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, P.; Fujiwara, M.; Ban, S.; Toda, T. Effect of steam explosion pre-treatment on methane generation from Ludwigia grandiflora. Biomass Bioenergy 2020, 142, 105771. [Google Scholar] [CrossRef]
- Dandelot, S.; Verlaque, R.; Dutartre, A.; Cazaubon, A. Ecological, dynamic and taxonomic problems due to Ludwigia (Onagraceae) in France. Hydrobiologia 2005, 551, 131–136. [Google Scholar] [CrossRef]
- Thouvenot, L.; Puech, C.; Martinez, L.; Haury, J.; Thiébaut, G. Strategies of the invasive macrophyte Ludwigia grandiflora in its introduced range: Competition, facilitation or coexistence with native and exotic species? Aquat. Bot. 2013, 107, 8–16. [Google Scholar] [CrossRef]
- Benner, R.; Maccubbin, A.E.; Hodson, R.E. Anaerobic biodegradation of the lignin and polysaccharide components of lignocellulose and synthetic lignin by sediment microflora. Appl. Environ. Microbiol. 1984, 47, 998–1004. [Google Scholar] [CrossRef] [Green Version]
- Galbe, M.; Zacchi, G. Pretreatment: The key to efficient utilization of lignocellulosic materials. Biomass Bioenergy 2012, 46, 70–78. [Google Scholar] [CrossRef]
- Sawatdeenarunat, C.; Surendra, K.C.; Takara, D.; Oechsner, H.; Khanal, S.K. Anaerobic digestion of lignocellulosic biomass: Challenges and opportunities. Bioresour. Technol. 2015, 178, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Koyama, M.; Yamamoto, S.; Ishikawa, K.; Ban, S.; Toda, T. Enhancing anaerobic digestibility of lignin-rich submerged macrophyte using thermochemical pre-treatment. Biochem. Eng. J. 2015, 99, 124–130. [Google Scholar] [CrossRef]
- Koyama, M.; Yamamoto, S.; Ishikawa, K.; Ban, S.; Toda, T. Inhibition of anaerobic digestion by dissolved lignin derived from alkaline pre-treatment of an aquatic macrophyte. Chem. Eng. J. 2017, 311, 55–62. [Google Scholar] [CrossRef]
- Auxenfans, T.; Crônier, D.; Chabbert, B.; Paës, G. Understanding the structural and chemical changes of plant biomass following steam explosion pretreatment. Biotechnol. Biofuels 2017, 10, 36. [Google Scholar] [CrossRef] [Green Version]
- Biswas, R.; Uellendahl, H.; Ahring, B.K. Wet Explosion: A Universal and Efficient Pretreatment Process for Lignocellulosic Biorefineries. Bioenergy Res. 2015, 8, 1101–1116. [Google Scholar] [CrossRef]
- Weber, B.; Estrada-Maya, A.; Sandoval-Moctezuma, A.C.; Martínez-Cienfuegos, I.G. Anaerobic digestion of extracts from steam exploded Agave tequilana bagasse. J. Environ. Manag. 2019, 245, 489–495. [Google Scholar] [CrossRef]
- Hendriks, A.T.W.M.; Zeeman, G. Pretreatments to enhance the digestibility of lignocellulosic biomass. Bioresour. Technol. 2009, 100, 10–18. [Google Scholar] [CrossRef]
- Xu, J.; Chen, Y.; Cheng, J.J.; Sharma-Shivappa, R.R.; Burns, J.C. Delignification of switchgrass cultivars for bioethanol production. BioResources 2011, 6, 707–720. [Google Scholar] [CrossRef]
- Bauer, A.; Lizasoain, J.; Theuretzbacher, F.; Agger, J.W.; Rincón, M.; Menardo, S.; Saylor, M.K.; Enguídanos, R.; Nielsen, P.J.; Potthast, A.; et al. Steam explosion pretreatment for enhancing biogas production of late harvested hay. Bioresour. Technol. 2014, 166, 403–410. [Google Scholar] [CrossRef]
- Li, C.; Liu, G.; Nges, I.A.; Liu, J. Enhanced biomethane production from Miscanthus lutarioriparius using steam explosion pretreatment. Fuel 2016, 179, 267–273. [Google Scholar] [CrossRef]
- Lizasoain, J.; Rincón, M.; Theuretzbacher, F.; Enguídanos, R.; Nielsen, P.J.; Potthast, A.; Zweckmair, T.; Gronauer, A.; Bauer, A. Biogas production from reed biomass: Effect of pretreatment using different steam explosion conditions. Biomass Bioenergy 2016, 95, 84–91. [Google Scholar] [CrossRef]
- Menardo, S.; Bauer, A.; Theuretzbacher, F.; Piringer, G.; Nilsen, P.J.; Balsari, P.; Pavliska, O.; Amon, T. Biogas Production from Steam-Exploded Miscanthus and Utilization of Biogas Energy and CO2 in Greenhouses. Bioenergy Res. 2013, 6, 620–630. [Google Scholar] [CrossRef]
- Take, H.; Andou, Y.; Nakamura, Y.; Kobayashi, F.; Kurimoto, Y.; Kuwahara, M. Production of methane gas from Japanese cedar chips pretreated by various delignification methods. Biochem. Eng. J. 2006, 28, 30–35. [Google Scholar] [CrossRef]
- El Achkar, J.H.; Lendormi, T.; Hobaika, Z.; Salameh, D.; Louka, N.; Maroun, R.G.; Lanoisellé, J.L. Anaerobic digestion of grape pomace: Biochemical characterization of the fractions and methane production in batch and continuous digesters. Waste Manag. 2016, 50, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Forgács, G.; Pourbafrani, M.; Niklasson, C.; Taherzadeh, M.J.; Hováth, I.S. Methane production from citrus wastes: Process development and cost estimation. J. Chem. Technol. Biotechnol. 2012, 87, 250–255. [Google Scholar] [CrossRef]
- Serrano, A.; Fermoso, F.G.; Alonso-Fariñas, B.; Rodríguez-Gutiérrez, G.; López, S.; Fernandez-Bolaños, J.; Borja, R. Long-Term Evaluation of Mesophilic Semi-Continuous Anaerobic Digestion of Olive Mill Solid Waste Pretreated with Steam-Explosion. Energies 2019, 12, 2222. [Google Scholar] [CrossRef] [Green Version]
- Kumar, G.; Dharmaraja, J.; Arvindnarayan, S.; Shoban, S.; Bakonyi, P.; Saratale, G.D.; Nemestóthy, N.; Bélafi-Bakó, K.; Yoon, J.J.; Kim, S.H. A comprehensive review on thermochemical, biological, biochemical and hybrid conversion methods of bio-derived lignocellulosic molecules into renewable fuels. Fuel 2019, 251, 352–367. [Google Scholar] [CrossRef]
- Kim, D. Physico-chemical conversion of lignocellulose: Inhibitor effects and detoxification strategies: A mini review. Molecules 2018, 23, 309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capolupo, L.; Faraco, V. Green methods of lignocellulose pretreatment for biorefinery development. Appl. Microbiol. Biotechnol. 2016, 100, 9451–9467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafiei, M.; Kabir, M.M.; Zilouei, H.; Sárvári Horváth, I.; Karimi, K. Techno-economical study of biogas production improved by steam explosion pretreatment. Bioresour. Technol. 2013, 148, 53–60. [Google Scholar] [CrossRef]
- Tucker, C.S.; Debusk, T.A. Seasonal growth of Eichhornia crassipes (Mart.) solms: Relationship to protein, fiber, and available carbohydrate content. Aquat. Bot. 1981, 11, 137–141. [Google Scholar] [CrossRef]
- Jančík, F.; Homolka, P.; Čermák, B.; Lád, F. Determination of indigestible neutral detergent fibre contents of grasses and its prediction from chemical composition. Czech J. Anim. Sci. 2008, 53, 128–135. [Google Scholar] [CrossRef] [Green Version]
- Koyama, M.; Watanabe, K.; Kurosawa, N.; Ishikawa, K.; Ban, S.; Toda, T. Effect of alkaline pretreatment on mesophilic and thermophilic anaerobic digestion of a submerged macrophyte: Inhibition and recovery against dissolved lignin during semi-continuous operation. Bioresour. Technol. 2017, 238, 666–674. [Google Scholar] [CrossRef] [PubMed]
- Richard, T.L. Municipal solid waste composting: Physical and biological processing. Biomass Bioenergy 1992, 3, 163–180. [Google Scholar] [CrossRef]
- Saragih, F.N.A.; Priadi, C.R.; Adityosulindro, S.; Abdillah, A.; Islami, B.B. The effectiveness of anaerobic digestion process by thermal pre-treatment on food waste as a substrate. In IOP Conference Series: Earth and Environmental Science; Institute of Physics Publishing: Tangerang, Indonesia, 2019; Volume 251, Available online: https://iopscience.iop.org/article/10.1088/1755-1315/251/1/012014/meta (accessed on 13 May 2021).
- Van, D.P.; Fujiwara, T.; Tho, B.L.; Toan, P.P.S.; Minh, G.H. A review of anaerobic digestion systems for biodegradable waste: Configurations, operating parameters, and current trends. Environ. Eng. Res. 2020, 25, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Theuretzbacher, F.; Lizasoain, J.; Lefever, C.; Saylor, M.K.; Enguidanos, R.; Weran, N.; Gronauer, A.; Bauer, A. Steam explosion pretreatment of wheat straw to improve methane yields: Investigation of the degradation kinetics of structural compounds during anaerobic digestion. Bioresour. Technol. 2015, 179, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.S.; Dong, J.J.; Yu, J.H.; Yin, H.; Hu, S.M.; Huang, S.X.; Yuan, X.Z. Effect of Hydraulic Retention Time on Anaerobic Digestion of Wheat Straw in the Semicontinuous Continuous Stirred-Tank Reactors. BioMed Res. Int. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Park, S.Y.; Zhu, J. Solid-state anaerobic digestion for methane production from organic waste. Renew. Sustain. Energy Rev. 2011, 15, 821–826. [Google Scholar] [CrossRef]
- Jain, S.; Jain, S.; Wolf, I.T.; Lee, J.; Tong, Y.W. A comprehensive review on operating parameters and different pretreatment methodologies for anaerobic digestion of municipal solid waste. Renew. Sustain. Energy Rev. 2015, 52, 142–154. [Google Scholar] [CrossRef]
- Tuomela, M.; Vikman, M.; Hatakka, A.; Itävaara, M. Biodegradation of lignin in a compost environment: A review. Bioresour. Technol. 2000, 72, 169–183. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, R.; Liu, X.; Chen, C.; Xiao, X.; Feng, L.; He, Y.; Liu, G. Evaluating methane production from anaerobic mono- and co-digestion of kitchen waste, corn stover, and chicken manure. Energy Fuels 2013, 27, 2085–2091. [Google Scholar] [CrossRef]
- Vivekanand, V.; Olsen, E.F.; Eijsink, V.G.H.; Horn, S.J. Effect of different steam explosion conditions on methane potential and enzymatic saccharification of birch. Bioresour. Technol. 2013, 127, 343–349. [Google Scholar] [CrossRef]
- Vivekanand, V.; Olsen, E.F.; Eijsink, V.G.H.; Horn, S.J. Methane Potential and Enzymatic Saccharification of Steam-exploded Bagasse. BioResources 2014, 9. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroyen, M.; Vervaeren, H.; Raes, K.; Van Hulle, S.W.H. Modelling and simulation of anaerobic digestion of various lignocellulosic substrates in batch reactors: Influence of lignin content and phenolic compounds II. Biochem. Eng. J. 2018, 134, 80–87. [Google Scholar] [CrossRef]
- Chen, Y.; Cheng, J.J.; Creamer, K.S. Inhibition of anaerobic digestion process: A review. Bioresour. Technol. 2008, 99, 4044–4064. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Arshad, M.; Anjum, M.; Mahmood, T.; Dawson, L. The anaerobic digestion of solid organic waste. Waste Manag. 2011, 31, 1737–1744. [Google Scholar] [CrossRef]
- Cotana, F.; Cavalaglio, G.; Gelosia, M.; Nicolini, A.; Coccia, V.; Petrozzi, A. Production of Bioethanol in a Second Generation Prototype from Pine Wood Chips. Energy Procedia 2014, 45, 42–51. [Google Scholar] [CrossRef] [Green Version]
- Michelin, M.; Ximenes, E.; de Lourdes Teixeira de Moraes Polizeli, M.; Ladisch, M.R. Effect of phenolic compounds from pretreated sugarcane bagasse on cellulolytic and hemicellulolytic activities. Bioresour. Technol. 2016, 199, 275–278. [Google Scholar] [CrossRef]
- Bajaj, M.; Gallert, C.; Winter, J. Treatment of phenolic wastewater in an anaerobic fixed bed reactor (AFBR)-Recovery after shock loading. J. Hazard. Mater. 2009, 162, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Ma, X.; Chen, L.; Cao, S.; Nasrallah, J. Lignin extraction and recovery in hydrothermal pretreatment of bamboo. J. Bioresour. Bioprod. 2016, 1, 145–151. [Google Scholar] [CrossRef]
- Ajiboye, A.V.; Lasisi, K.H.; Babatola, J.O. Evaluation of the effect of sodium hydroxide solution on biogas yield of anaerobic digestion of poultry waste and the digestate. Int. J. Energy Water Resour. 2018, 2, 23–31. [Google Scholar] [CrossRef]
- Awe, O.W.; Lu, J.; Wu, S.; Zhao, Y.; Nzihou, A.; Lyczko, N.; Minh, D.P. Effect of Oil Content on Biogas Production, Process Performance and Stability of Food Waste Anaerobic Digestion. Waste Biomass Valorization 2018, 9, 2295–2306. [Google Scholar] [CrossRef] [Green Version]
- Widyarani; Victor, Y.; Sriwuryandari, L.; Priantoro, E.A.; Sembiring, T.; Sintawardani, N. Influence of pH on biogas production in a batch anaerobic process of tofu wastewater. In IOP Conference Series: Earth and Environmental Science; Institute of Physics Publishing: Jakarta, Indonesia, 2018; Volume 160, p. 012014. Available online: https://iopscience.iop.org/article/10.1088/1755-1315/160/1/012014/meta (accessed on 13 May 2021).
- Kim, M.; Ahn, Y.H.; Speece, R.E. Comparative process stability and efficiency of anaerobic digestion; mesophilic vs. thermophilic. Water Res. 2002, 36, 4369–4385. [Google Scholar] [CrossRef]
- Sambusiti, C.; Ficara, E.; Malpei, F.; Steyer, J.P.; Carrère, H. Benefit of sodium hydroxide pretreatment of ensiled sorghum forage on the anaerobic reactor stability and methane production. Bioresour. Technol. 2013, 144, 149–155. [Google Scholar] [CrossRef] [PubMed]
- Michele, P.; Giuliana, D.; Carlo, M.; Sergio, S.; Fabrizio, A. Optimization of solid state anaerobic digestion of the OFMSW by digestate recirculation: A new approach. Waste Manag. 2015, 35, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.K.; Bhui, I.; Banerjee, S.N.; Goswami, R.; Chakraborty, A.K.; Shome, A.; Balachandran, S.; Chaudhury, S. Biogas production from locally available aquatic weeds of Santiniketan through anaerobic digestion. Clean Technol. Environ. Policy 2015, 17, 1681–1688. [Google Scholar] [CrossRef]
- Constantinescu, M.; Bucura, F.; Ionete, E.I.; Ion-Ebrasu, D.; Sandru, C.; Zaharioiu, A.; Marin, F.; Miricioiu, M.G.; Niculescu, V.C.; Oancea, S.; et al. From plastic to fuel—New challenges. Mater. Plast. 2019, 56, 721–729. [Google Scholar] [CrossRef]
- Zaharioiu, A.; Bucura, F.; Ionete, E.I.; Ionete, R.E.; Ebrasu, D.; Sandru, C.; Marin, F.; Oancea, S.; Niculescu, V.; Miricioiu, M.G.; et al. Thermochemical decomposition of sewage sludge—An eco-friendly solution for a sustainable energy future by using wastes. Rev. Chim. 2020, 71, 171–181. [Google Scholar] [CrossRef]
- Koyama, M.; Yamamoto, S.; Ishikawa, K.; Ban, S.; Toda, T. Anaerobic digestion of submerged macrophytes: Chemical composition and anaerobic digestibility. Ecol. Eng. 2014, 69, 304–309. [Google Scholar] [CrossRef]
- Bengtsson, A.; Bengtsson, J.; Sedin, M.; Sjöholm, E. Carbon Fibers from Lignin-Cellulose Precursors: Effect of Stabilization Conditions. ACS Sustain. Chem. Eng. 2019, 7, 8440–8448. [Google Scholar] [CrossRef] [Green Version]
- Serrano, A.; Fermoso, F.G.; Alonso-Fariñas, B.; Rodríguez-Gutiérrez, G.; López, S.; Fernandez-Bolaños, J.; Borja, R. Performance evaluation of mesophilic semi-continuous anaerobic digestion of high-temperature thermally pre-treated olive mill solid waste. Waste Manag. 2019, 87, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Ouazzane, H.; Laajine, F.; Yamani, M.E.; Hilaly, J.E.; Rharrabti, Y.; Amarouch, M.; Mazouzi, D. Olive Mill Solid Waste Characterization and Recycling opportunities: A review. J. Mater. Environ. Sci. 2017, 8, 2632–2650. [Google Scholar]




| Parameter | Values |
|---|---|
| Solid fraction (kg-wwt) | 70.34 |
| Liquid fraction (kg-wwt) | 175.14 |
| Solid/Liquid | 0.40 |
| pH of liquid fraction | 4.64 |
| Parameter | Substrate | Effluent | |||
|---|---|---|---|---|---|
| Untreated | Steam Exploded | ||||
| Solid | Solid | Liquid | Day 0 | Day 98 | |
| TS (g L−1) | 194.50 | 187.10 | 8.60 | 28.50 | 20.50 |
| VS (g L−1) | 166.70 | 162.10 | 5.40 | 15.80 | 15.70 |
| Cellulose (%TS) | 32.83 ± 0.5 | 28.02 ± 0.2 | − | − | 5.03 ± 0.8 |
| Hemicellulose (%TS) | ND | ND | − | − | ND |
| Lignin (%TS) | 25.22 ± 4.6 | 29.15 ± 0.5 | − | − | 17.0 ± 1.0 |
| C/N (%TS) | 22.12 ± 1.1 | 8.24 ± 0.9 | 10.82 ± 0.2 | 7.32 ± 0.1 | 14.3 ± 0.8 |
| Carbon (%TS) | 40.51 ± 0.0 | 42.90 ± 0.5 | 28.03 ± 0.5 | 29.63 ± 0.0 | 37.33 ± 0.9 |
| Nitrogen (%TS) | 1.83 ± 0.1 | 5.28 ± 0.7 | 2.60 ± 0.0 | 4.04 ± 0.0 | 2.61 ± 0.1 |
| Protein (%TS) | 11.48 ± 0.6 | 33.0 ± 4.3 | 16.19 ± 0.0 | 25.3 ± 0.2 | 16.3 ± 0.7 |
| NH4+ (g L−1) | − | − | 0.1 ± 0.0 | 1.0 ± 0.0 | 0.1 ± 0.0 |
| Phenolic compounds (g L−1) | − | − | 0.03 ± 0.0 | 0.07 ± 0.0 | 0.18 ± 0.0 |
| Dissolvedlignin (g L−1) | − | − | 0.64 | 0.27 | 0.40 |
| Operational Day | Fed Carbon (kg) | Biogas Carbon (kg) | Effluent Carbon (kg) | Residual Carbon (kg) | Residual/Fed Carbon (%) |
|---|---|---|---|---|---|
| 0 | 0.12 | 0 | 0 | 0.12 | 100 |
| 7 | 1.96 | 0.15 | 0.14 | 1.67 | 85.20 |
| 21 | 14.64 | 0.40 | 0.39 | 13.85 | 94.60 |
| 35 | 39.32 | 0.66 | 0.60 | 38.06 | 96.79 |
| 42 | 53.88 | 0.76 | 0.69 | 52.43 | 97.30 |
| 58 | 76.51 | 0.87 | 0.77 | 74.87 | 97.85 |
| 70 | 107.33 | 0.94 | 0.86 | 105.54 | 98.33 |
| 84 | 159.22 | 1.10 | 1.02 | 157.10 | 98.67 |
| 91 | 188.66 | 1.19 | 1.10 | 186.37 | 98.79 |
| 98 | 211.10 | 1.29 | 1.18 | 208.63 | 98.88 |
| Feedstock/Lignin Content | Pretreatment/Operation Type | Operating Condition and Gas Production | Reference |
|---|---|---|---|
| Potamogeton maackianus Type: Aquatic Lignin = 20.7%TS | Alkaline: 80 °C 3 h 0.2 g NaOH/1 g TS Semi-continuous | b CH4 = 219.2 (HRT: 40 d) a OLR: 1 | [34] |
| Wheat straw Type: Terrestrial Lignin = 7.5 ± 0.3 * | Sun dried Semi-continuous | Biogas, OLR: 8%TS b R1 = 55.2 (HRT: 20 d) b R2 = 94.3 (HRT: 40 d) b R3 = 105.2 (HRT: 60 d) | [39] * [38] |
| Olive mill solid waste Type: Terrestrial Lignin = 20.3 * | Thermal 170 °C, 60 min Semi-continuous | HRT: 25 d Day 0–75 = b CH4: 119 ± 30, a OLR: 2 Day 75–175 = 172 ± 60, a OLR: 1 Day 175–275 = 2, aOLR: 2 | [65] * [66] |
| Ensiled sorghum forage Type: Terrestrial Lignin = 20−23%TS | Alkaline: 40 °C 24 h 10 g NaOH/100 g TS Semi-continuous | CH4 = 346 ± 9 (HRT: 21 d) a OLR: 1.8 | [58] |
| Olive mill solid waste Type: Terrestrial Lignin = 20.3 * | Steam explosion 200 °C, 5 min Semi-continuous | HRT: 25 d Day 0–75 = b CH4: 151 ± 21, a OLR: 2 Day 75–175 = 163 ± 28, a OLR: 1 Day 175–275 = 85 ± 59, a OLR: 2 | [27] |
| Ludwigia grandiflora Type: Aquatic Lignin = 25.22 ± 4.6%TS | Steam explosion 180 °C, 30 min Semi-continuous | b CH4 = 133.2 Day 0–42 = 148.24, a OLR: 0.9 (HRT: 30 d) Day 42–49 = 144.32, a OLR: 0.5 (HRT: 50 d) Day 49–98 = 113.0, a OLR:0.7(HRT: 40 d) | This study |
| Steam-exploded citrus waste, municipal solid wastes Lignin = Not specified | Steam explosion: 150 °C, 20 min Semi-continuous co-digestion | b CH4 = 560 ± 15 (HRT: 21 d) a OLR: 3 | [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhatia, P.; Fujiwara, M.; Salangsang, M.C.D.; Qian, J.; Liu, X.; Ban, S.; Myojin, M.; Toda, T. Effect of Semi-Continuous Anaerobic Digestion on the Substrate Solubilisation of Lignin-Rich Steam-Exploded Ludwigia grandiflora. Appl. Sci. 2021, 11, 4452. https://doi.org/10.3390/app11104452
Bhatia P, Fujiwara M, Salangsang MCD, Qian J, Liu X, Ban S, Myojin M, Toda T. Effect of Semi-Continuous Anaerobic Digestion on the Substrate Solubilisation of Lignin-Rich Steam-Exploded Ludwigia grandiflora. Applied Sciences. 2021; 11(10):4452. https://doi.org/10.3390/app11104452
Chicago/Turabian StyleBhatia, Pranshu, Masaaki Fujiwara, Maria Cecilia D. Salangsang, Jun Qian, Xin Liu, Syuhei Ban, Mitsuyuki Myojin, and Tatsuki Toda. 2021. "Effect of Semi-Continuous Anaerobic Digestion on the Substrate Solubilisation of Lignin-Rich Steam-Exploded Ludwigia grandiflora" Applied Sciences 11, no. 10: 4452. https://doi.org/10.3390/app11104452
APA StyleBhatia, P., Fujiwara, M., Salangsang, M. C. D., Qian, J., Liu, X., Ban, S., Myojin, M., & Toda, T. (2021). Effect of Semi-Continuous Anaerobic Digestion on the Substrate Solubilisation of Lignin-Rich Steam-Exploded Ludwigia grandiflora. Applied Sciences, 11(10), 4452. https://doi.org/10.3390/app11104452