Effect of Plant Biostimulants on Nutritional and Chemical Profiles of Almond and Hazelnut
Abstract
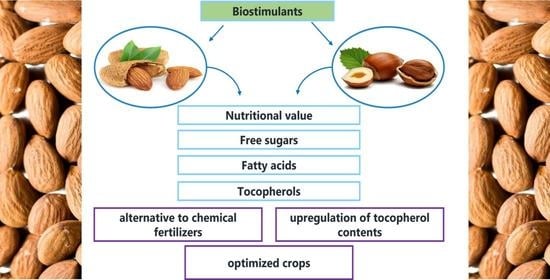
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Nutritional Value
2.3. Free Sugars
2.4. Fatty Acids
2.5. Tocopherols
2.6. Statistical Analysis
3. Results and Discussion
3.1. Nutritional Value
3.2. Fatty Acids
3.3. Tocopherols
3.4. Linear Discriminant Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Fideghelli, C.; De Salvador, F.R. World hazelnut situation and perspectives. Acta Hortic. 2009, 845, 39–52. [Google Scholar] [CrossRef]
- Islam, A. Hazelnut cultivation in Turkey. Akad. Ziraat Derg. 2018, 7, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Sang, S.; Cheng, X.; Fu, H.-Y.; Shieh, D.-E.; Bai, N.; Lapsley, K.; Stark, R.E.; Rosen, R.T.; Ho, C.-T. New type sesquiterpene lactone from almond hulls (Prunus amygdalus Batsch). Tetrahedron Lett. 2002, 43, 2547–2549. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Bento, A.; Arrobas, M. Â Amendoeira: Estado Da Produção; Voz do Campo Lda: Castelo Branco, Portugal, 2018. [Google Scholar]
- Maguire, L.S.; O’Sullivan, S.M.; Galvin, K.; O’Connor, T.P.; O’Brien, N.M. Fatty acid profile, tocopherol, squalene and phytosterol content of walnuts, almonds, peanuts, hazelnuts and the macadamia nut. Int. J. Food Sci. Nutr. 2004, 55, 171–178. [Google Scholar] [CrossRef]
- Savage, G.P.; McNeil, D.L.; Dutta, P.C. Lipid composition and oxidative stability of oils in hazelnuts (Corylus avellana L.) grown in New Zealand. JAOCS J. Am. Oil Chem. Soc. 1997, 74, 755–759. [Google Scholar] [CrossRef]
- Alasalvar, C.; Shahidi, F.; Ohshima, T.; Wanasundara, U.; Yurttas, H.C.; Liyanapathirana, C.M.; Rodrigues, F.B. Turkish Tombul hazelnut (Corylus avellana L.). 2. Lipid characteristics and oxidative stability. J. Agric. Food Chem. 2003, 51, 3797–3805. [Google Scholar] [CrossRef]
- Amaral, J.S.; Casal, S.; Citová, I.; Santos, A.; Seabra, R.M.; Oliveira, B.P.P. Characterization of several hazelnut (Corylus avellana L.) cultivars based in chemical, fatty acid and sterol composition. Eur. Food Res. Technol. 2006, 222, 274–280. [Google Scholar] [CrossRef] [Green Version]
- Alasalvar, C.; Salvadó, J.-S.; Ros, E. Bioactives and health benefits of nuts and dried fruits. Food Chem. 2020, 314, 126192. [Google Scholar] [CrossRef]
- Alasalvar, C.; Pelvan, E. Fat-soluble bioactives in nuts. Eur. J. Lipid Sci. Technol. 2011, 113, 943–949. [Google Scholar] [CrossRef]
- Alasalvar, C.; Bolling, B.W. Review of nut phytochemicals, fat-soluble bioactives, antioxidant components and health effects. Br. J. Nutr. 2015, 113, S68–S78. [Google Scholar] [CrossRef]
- Venkatachalan, M.; Sathe, S.K. Chemical composition of selected edible nut seeds. J. Agric. Food Chem. 2006, 54, 4705–4714. [Google Scholar] [CrossRef] [PubMed]
- Horrobin, D.F.; Manku, M.S. How do polyunsaturated fatty acids lower plasma cholesterol levels? Lipids 1983, 18, 558–562. [Google Scholar] [CrossRef]
- Feldman, E.B. The scientific evidence for a beneficial health relationship between walnuts and coronary heart disease. J. Nutr. 2002, 132, 1062S–1101S. [Google Scholar] [CrossRef]
- Jones, P.; MacDougall, D.E.; Ntanios, F.; Vanstone, C.A. Dietary phytosterols as cholesterol-lowering agents in humans. Can. J. Physiol. Pharmacol. 1997, 75, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Weststrate, J.A.; Meijer, G.W. Plant sterol-enriched margarines and reduction of plasma total- and LDL-cholesterol concentrations in normocholesterolaemic and mildly hypercholesterolaemic subjects. Eur. J. Clin. Nutr. 1998, 52, 334–343. [Google Scholar] [CrossRef] [Green Version]
- Coates, A.; Hill, A.; Tan, S. Nuts and Cardiovascular Disease Prevention. Curr. Atheroscler. Rep. 2018, 20, 1–9. [Google Scholar] [CrossRef]
- McGuire, M.; Beerman, K.A. Nutritional Sciences: From Fundamentals to Food; Wadsworth Cengage Learning: Boston, MA, USA, 2011; ISBN 9780324598643. [Google Scholar]
- Fang, Y.Z.; Yang, S.; Wu, G. Free radicals, antioxidants, and nutrition. Nutrition 2002, 18, 872–879. [Google Scholar] [CrossRef]
- Arranz, S.; Cert, R.; Pérez-Jiménez, J.; Cert, A.; Saura-Calixto, F. Comparison between free radical scavenging capacity and oxidative stability of nut oils. Food Chem. 2008, 110, 985–990. [Google Scholar] [CrossRef] [Green Version]
- Tahan, G.; Aytac, E.; Aytekin, H.; Gunduz, F.; Dogusoy, G.; Aydin, S.; Tahan, V.; Uzun, H. Vitamin e has a dual effect of anti-inflammatory and antioxidant activities in acetic acid-induced ulcerative colitis in rats. Can. J. Surg. 2011, 54, 333–338. [Google Scholar] [CrossRef] [Green Version]
- Valverde, M.; Madrid, R.; García, A.L. Effect of the irrigation regime, type of fertilization, and culture year on the physical properties of almond (cv. Guara). J. Food Eng. 2006, 76, 584–593. [Google Scholar] [CrossRef]
- Mondragón-Valero, A.; Lopéz-Cortés, I.; Salazar, D.M.; de Córdova, P.F. Physical mechanisms produced in the development of nursery almond trees (Prunus dulcis Miller) as a response to the plant adaptation to different substrates. Rhizosphere 2017, 3, 44–49. [Google Scholar] [CrossRef]
- Blunden, G. Agricultural Uses of Seaweeds and Seaweed Extracts; John Wiley & Sons: Chichester, UK, 1991; ISBN 0-471-92947-6. [Google Scholar]
- Crouch, I.J.; Van Staden, J. Commercial Seaweed Products as Biostimulants in Horticulture. J. Home Consum. Hortic. 1993, 1, 19–76. [Google Scholar] [CrossRef]
- Mooney, P.A.; van Staden, J. Algae and Cytokinins. J. Plant Physiol. 1986, 123, 1–21. [Google Scholar] [CrossRef]
- Kauffman, G.L.; Kneivel, D.P.; Watschke, T.L. Effects of a Biostimulant on the Heat Tolerance Associated with Photosynthetic Capacity, Membrane Thermostability, and Polyphenol Production of Perennial Ryegrass. Crop Sci. 2007, 47, 261–267. [Google Scholar] [CrossRef]
- Zhang, X.; Ervin, E.H.; Schmidt, R.E. Plant Growth Regulators Can Enhance the Recovery of Kentucky Bluegrass Sod from Heat Injury. Crop Sci. 2003, 43, 952. [Google Scholar] [CrossRef]
- Zhang, X.; Schmidt, R.E. Hormone—Containing Products’ Impact on Antioxidant Status of Tall Fescue and Creeping Bentgrass Subjected to Drought. Crop Sci. 2000, 40, 1344–1349. [Google Scholar] [CrossRef]
- Turan, M.; Köse, C. Seaweed extracts improve copper uptake of grapevine. Acta Agric. Scand. Sect. B Soil Plant. Sci. 2004, 54, 213–220. [Google Scholar] [CrossRef]
- Verkleij, F.N. Seaweed extracts in agriculture and horticulture: A review. Biol. Agric. Hortic. 1992, 8, 309–324. [Google Scholar] [CrossRef]
- Rathore, S.S.; Chaudhary, D.R.; Boricha, G.N.; Ghosh, A.; Bhatt, B.P.; Zodape, S.T.; Patolia, J.S. Effect of seaweed extract on the growth, yield and nutrient uptake of soybean (Glycine max) under rainfed conditions. S. Afr. J. Bot. 2009, 75, 351–355. [Google Scholar] [CrossRef] [Green Version]
- Amaral, J.S.; Cunha, S.C.; Santos, A.; Alves, M.R.; Seabra, R.M.; Oliveira, B.P.P. Influence of cultivar and environmental conditions on the triacylglycerol profile of hazelnut (Corylus avellana L.). J. Agric. Food Chem. 2006, 54, 449–456. [Google Scholar] [CrossRef]
- O’Brien, R. Fats and Oils; CRC Press: New York, NY, USA, 2003; ISBN 978-0-8493-1599-2. [Google Scholar]
- AOAC. Official Methods of Analysis of AOAC International, 20th ed.; Latimer, G.W., Jr., Ed.; AOAC: Rockville, MD, USA, 2016; ISBN 0935584870. [Google Scholar]
- Harumi Iyda, J.; Fernandes, Â.; Calhelha, R.C.; Alves, M.J.; Ferreira, F.D.; Barros, L.; Amaral, J.S.; Ferreira, I.C.F.R. Nutritional composition and bioactivity of Umbilicus rupestris (Salisb.)Dandy: An underexploited edible wild plant. Food Chem. 2019, 295, 341–349. [Google Scholar] [CrossRef] [Green Version]
- Barros, L.; Pereira, E.; Calhelha, R.C.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Bioactivity and chemical characterization in hydrophilic and lipophilic compounds of Chenopodium ambrosioides L. J. Funct. Foods 2013, 5, 1732–1740. [Google Scholar] [CrossRef]
- Pinela, J.; Barros, L.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C.F.R. Antioxidant activity, ascorbic acid, phenolic compounds and sugars of wild and commercial Tuberaria lignosa samples: Effects of drying and oral preparation methods. Food Chem. 2012, 135, 1028–1035. [Google Scholar] [CrossRef]
- Botta, R.; Gianotti, C.; Richardson, D.; Suwanagul, A.; Sanz, C.L. Hazelnut Variety Organic Acids, Sugars, And Total Lipid Fatty Acids. Acta Hortic. 1994, 351, 693–699. [Google Scholar] [CrossRef]
- Alasalvar, C.; Shahidi, F.; Liyanapathirana, C.M.; Ohshima, T. Turkish Tombul hazelnut (Corylus avellana L.). 1. Compositional characteristics. J. Agric. Food Chem. 2003, 51, 3790–3796. [Google Scholar] [CrossRef]
- Alasalvar, C.; Amaral, J.S.; Shahidi, F. Functional lipid characteristics of Turkish Tombul hazelnut (Corylus avellana L.). J. Agric. Food Chem. 2006, 54, 10177–10183. [Google Scholar] [CrossRef]
- Derewiaka, D.; Szwed, E.; Wołosiak, R. Physicochemical Properties and Composition of Lipid Fraction of Selected Edible Nuts. Pak. J. Bot. 2014, 46, 337–343. [Google Scholar]
- Karaman, S.; Karasu, S.; Tornuk, F.; Toker, O.S.; Geçgel, Ü.; Sagdic, O.; Ozcan, N.; Gül, O. Recovery potential of cold press byproducts obtained from the edible oil industry: Physicochemical, bioactive, and antimicrobial properties. J. Agric. Food Chem. 2015, 63, 2305–2313. [Google Scholar] [CrossRef] [PubMed]
- Memon, N.N.; Kanwal, S.; Talpur, F.N.; Afridi, H.I.; Memon, G.Z.; Samejo, M.Q.; Memon, J.R.; Khan, H.A. Nutritional characteristics (fatty acid profile, proximate composition and dietary feature) of selected nuts available in local market. Pak. J. Anal. Environ. Chem. 2019, 20, 39–46. [Google Scholar] [CrossRef]
- Kodad, O.; Estopañán, G.; Juan, T.; Alonso, J.M.; Espiau, M.T.; Socias i Company, R. Oil content, fatty acid composition and tocopherol concentration in the Spanish almond genebank collection. Sci. Hortic. 2014, 177, 99–107. [Google Scholar] [CrossRef]
- Özdemir, K.S.; Yılmaz, C.; Durmaz, G.; Gökmen, V. Corrigendum to “Hazelnut skin powder: A new brown colored functional ingredient” [Food Research International 65 (2014) 291-297]. Food Res. Int. 2015, 71, 184. [Google Scholar] [CrossRef]
- Zulfiqar, F.; Casadesús, A.; Brockman, H.; Munné-Bosch, S. An overview of plant-based natural biostimulants for sustainable horticulture with a particular focus on moringa leaf extracts. Plant Sci. 2020, 295, 110194. [Google Scholar] [CrossRef] [PubMed]
- Alasalvar, C.; Amaral, J.S.; Satır, G.; Shahidi, F. Lipid characteristics and essential minerals of native Turkish hazelnut varieties (Corylus avellana L.). Food Chem. 2009, 113, 919–925. [Google Scholar] [CrossRef]
- Benitez-Sánchez, P.L.; León-Camacho, M.; Aparicio, R. A comprehensive study of hazelnut oil composition with comparisons to other vegetable oils, particularly olive oil. Eur. Food Res. Technol. 2003, 218, 13–19. [Google Scholar] [CrossRef]
- Taş, N.G.; Gökmen, V. Profiling triacylglycerols, fatty acids and tocopherols in hazelnut varieties grown in Turkey. J. Food Compos. Anal. 2015, 44, 115–121. [Google Scholar] [CrossRef]


| Biostimulant | Water | Fat | Protein | Ash | Carbohydrates | Sucrose | Energy |
|---|---|---|---|---|---|---|---|
| Almond (Vairo cultivar) | |||||||
| Control | 3.3 ± 0.1 b | 56 ± 1.3 a | 15.1 ± 0.2 a | 2.5 ± 0.2 | 27 ± 1.3 c | 11.8 ± 0.2 c | 669 ± 6.4 a |
| Phytoalgae | 3.5 ± 0.1 a | 54 ± 1.1 c | 14.6 ± 0.5 b | 2.5 ± 0.2 | 29 ± 1.5 a | 12.8 ± 0.5 b | 660 ± 4.9 c |
| Tradebor® | 3.1 ± 0.1 c | 55 ± 2.1 bc | 15.2 ± 0.5 a | 2.4 ± 0.2 | 28 ± 2.4 bc | 13.7 ± 0.5 a | 664 ± 7.8 bc |
| Sprint Plus® | 3.3 ± 0.2 b | 55 ± 0.7 ab | 14.6 ± 0.3 b | 2.5 ± 0.1 | 28 ± 1.5 b | 11.4 ± 0.2 d | 665 ± 7.2 ab |
| Neobor® | 3.3 ± 0.1 b | 55 ± 1.7 ab | 14.7 ± 0.4 b | 2.4 ± 0.2 | 28 ± 2.2 b | 12.1 ± 0.5 c | 665 ± 8.0 ab |
| ANOVA p-value (n = 45) 1 | <0.001 | <0.001 | <0.001 | 0.225 | <0.001 | <0.001 | <0.001 |
| Hazelnut (Ennis cultivar) | |||||||
| Control | 2.4 ± 0.1 d | 57 ± 1.0 a | 15.0 ± 0.5 d | 2.8 ± 0.1 c | 25 ± 1.3 c | 14.4 ± 0.5 d | 675 ± 2.7 a |
| NPK | 2.9 ± 0.1 b | 53 ± 1.5 c | 16.8 ± 0.5 a | 3.4 ± 0.2 a | 27 ± 1.3 ab | 15.7 ± 0.3 c | 651 ± 6.1 c |
| NPK + phytoalgae | 3.1 ± 0.1 a | 53 ± 3.0 c | 15.5 ± 0.1 c | 3.1 ± 0.2 b | 28 ± 3.3 a | 18.8 ± 0.5 a | 655 ± 14.6 c |
| Sprint Plus® | 2.4 ± 0.1 d | 56 ± 2.1 ab | 16.0 ± 0.2 b | 3.1 ± 0.2 b | 25 ± 2.4 c | 17.1 ± 0.5 b | 668 ± 7.8 b |
| NPK + Sprint Plus® | 2.6 ± 0.1 c | 55 ± 1.4 b | 16.2 ± 0.5 b | 2.8 ± 0.1 c | 26 ± 2.4 bc | 17.0 ± 0.5 b | 665 ± 5.9 b |
| Phytoalgae | 2.7 ± 0.1 c | 56 ± 1.4 b | 15.1 ± 0.2 d | 3.1 ± 0.1 b | 26 ± 1.5 b | 15.4 ± 0.4 c | 665 ± 4.6 b |
| ANOVA p-value (n = 54) 1 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Biostimulant | C16:0 | C18:0 | C18:1n9c | C18:2n6c |
|---|---|---|---|---|
| Almond (Vairo cultivar) | ||||
| Control | 8.8 ± 0.5 a | 2.0 ± 0.1 b | 71 ± 0.6 b | 17.2 ± 0.5 a |
| Phytoalgae | 8.7 ± 0.5 a | 2.0 ± 0.1 b | 71 ± 0.9 ab | 16.9 ± 0.3 b |
| Tradebor® | 8.1 ± 0.5 b | 2.1 ± 0.1 a | 72 ± 0.8 a | 17.1 ± 0.4 ab |
| Sprint Plus® | 8.8 ± 0.3 a | 2.0 ± 0.1 b | 71 ± 1.0 b | 17.2 ± 0.5 a |
| Neobor® | 9.1 ± 0.5 a | 1.9 ± 0.1 b | 71 ± 0.8 b | 17.2 ± 0.1 a |
| ANOVA p-value (n = 45) 1 | <0.001 | <0.001 | 0.002 | 0.001 |
| Hazelnut (Ennis cultivar) | ||||
| Control | 8.8 ± 0.3 d | 2.5 ± 0.1 b | 76 ± 1.0 a | 11.7 ± 0.3 d |
| NPK | 9.1 ± 0.4 cd | 2.1 ± 0.1 d | 72 ± 2.0 de | 15.1 ± 0.5 a |
| NPK + phytoalgae | 8.9 ± 0.3 cd | 2.3 ± 0.1 c | 73 ± 1.0 c | 14.3 ± 0.4 b |
| Sprint Plus® | 9.6 ± 0.4 a | 2.8 ± 0.1 a | 72 ± 1.1 e | 14.5 ± 0.4 b |
| NPK + Sprint Plus® | 9.5 ± 0.5 ab | 2.1 ± 0.1 d | 73 ± 1.0 cd | 14.3 ± 0.3 b |
| Phytoalgae | 9.2 ± 0.5 bc | 2.4 ± 0.2 b | 74 ± 1.0 b | 12.7 ± 0.3 c |
| ANOVA p-value (n = 54) 1 | <0.001 | <0.001 | <0.001 | <0.001 |
| Biostimulant | α-Tocopherol | β-Tocopherol | γ-Tocopherol | Tocopherols |
|---|---|---|---|---|
| Almond (Vairo cultivar) | ||||
| Control | 46 ± 1.0 b | 0.8 ± 0.1 ab | 3.6 ± 0.3 b | 50 ± 1.1 b |
| Phytoalgae | 49 ± 2.7 a | 0.8 ± 0.1 a | 4.4 ± 0.2 a | 55 ± 3.1 a |
| Tradebor® | 43 ± 1.6 c | 0.6 ± 0.1 c | 3.1 ± 0.2 d | 47 ± 2.5 c |
| Sprint Plus® | 45 ± 3.0 b | 0.8 ± 0.1 b | 3.5 ± 0.2 c | 49 ± 3.0 b |
| Neobor® | 41 ± 1.0 d | 0.8 ± 0.1 b | 3.6 ± 0.2 bc | 46 ± 1.0 c |
| ANOVA p-value (n = 45) 1 | <0.001 | <0.001 | <0.001 | <0.001 |
| Hazelnut (Ennis cultivar) | ||||
| Control | 22 ± 1.5 c | 0.57 ± 0.03 e | 0.75 ± 0.02 d | 23 ± 1.5 c |
| NPK | 22 ± 2.0 c | 0.72 ± 0.03 c | 0.83 ± 0.05 ab | 24 ± 2.1 c |
| NPK + phytoalgae | 26 ± 1.1 a | 0.76 ± 0.05 b | 0.79 ± 0.05 c | 28 ± 1.2 a |
| Sprint Plus® | 26 ± 2.2 a | 0.88 ± 0.03 a | 0.84 ± 0.03 ab | 27 ± 2.2 a |
| NPK + Sprint Plus® | 24 ± 0.6 b | 0.67 ± 0.03 d | 0.80 ± 0.05 bc | 26 ± 0.7 b |
| Phytoalgae | 22 ± 1.4 c | 0.74 ± 0.04 bc | 0.87 ± 0.04 a | 24 ± 1.4 c |
| ANOVA p-value (n = 54) 1 | <0.001 | <0.001 | <0.001 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascoalino, L.A.; Reis, F.S.; Barros, L.; Rodrigues, M.Â.; Correia, C.M.; Vieira, A.L.; Ferreira, I.C.F.R.; Barreira, J.C.M. Effect of Plant Biostimulants on Nutritional and Chemical Profiles of Almond and Hazelnut. Appl. Sci. 2021, 11, 7778. https://doi.org/10.3390/app11177778
Pascoalino LA, Reis FS, Barros L, Rodrigues MÂ, Correia CM, Vieira AL, Ferreira ICFR, Barreira JCM. Effect of Plant Biostimulants on Nutritional and Chemical Profiles of Almond and Hazelnut. Applied Sciences. 2021; 11(17):7778. https://doi.org/10.3390/app11177778
Chicago/Turabian StylePascoalino, Liege A., Filipa S. Reis, Lillian Barros, Manuel Ângelo Rodrigues, Carlos M. Correia, Admilson L. Vieira, Isabel C. F. R. Ferreira, and João C. M. Barreira. 2021. "Effect of Plant Biostimulants on Nutritional and Chemical Profiles of Almond and Hazelnut" Applied Sciences 11, no. 17: 7778. https://doi.org/10.3390/app11177778
APA StylePascoalino, L. A., Reis, F. S., Barros, L., Rodrigues, M. Â., Correia, C. M., Vieira, A. L., Ferreira, I. C. F. R., & Barreira, J. C. M. (2021). Effect of Plant Biostimulants on Nutritional and Chemical Profiles of Almond and Hazelnut. Applied Sciences, 11(17), 7778. https://doi.org/10.3390/app11177778