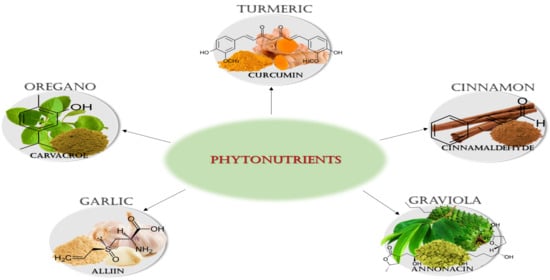
Biomedical Effects of the Phytonutrients Turmeric, Garlic, Cinnamon, Graviola, and Oregano: A Comprehensive Review
Abstract
:Featured Application
Abstract
1. Introduction
2. Phytonutrients
2.1. Turmeric
2.1.1. Botanical Description
2.1.2. Phytochemicals
2.1.3. Biomedical effects
Anticancer
Antioxidant
Antimicrobial
- Antiviral
- Antibacterial
- Antifungal
Anti-Inflammatory
Immunomodulatory
2.1.4. Contraindications
2.2. Garlic
2.2.1. Botanical Description
2.2.2. Phytochemicals
2.2.3. Biomedical effects
Anticancer
Antioxidant
Antimicrobial
- Antiviral
- Antibacterial
- Antifungal
Anti-Inflammatory
Immunomodulatory
2.2.4. Contraindications
2.3. Cinnamon
2.3.1. Botanical Description
2.3.2. Phytochemicals
2.3.3. Biomedical effects
Anticancer
Antioxidant
Antimicrobial
- Antiviral
- Antibacterial
- Antifungal
Anti-Inflammatory
Immunomodulatory
2.3.4. Contraindications
2.4. Graviola
2.4.1. Botanical Description
2.4.2. Phytochemicals
2.4.3. Biomedical Effects
Anticancer
Antioxidant
Antimicrobial
- Antiviral
- Antibacterial
- Antifungal
Anti-Inflammatory
Immunomodulatory
2.4.4. Contraindications
2.5. Oregano
2.5.1. Botanical Description
2.5.2. Phytochemicals
2.5.3. Biomedical Effects
Anticancer
Antioxidant
Antimicrobial
- Antiviral
- Antibacterial
- Antifungal
Anti-Inflammatory
Immunomodulatory
2.5.4. Contraindications
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Doosti, F.; Dashti, S.; Tabatabai, S.M.; Hosseinzadeh, H. Traditional Chinese and Indian medicine in the treatment of opioid-dependence: A review. Avicenna J. Phytomed. 2013, 3, 205–215. [Google Scholar] [PubMed]
- Pan, S.Y.; Litscher, G.; Gao, S.H.; Zhou, S.F.; Yu, Z.L.; Chen, H.Q.; Zhang, S.F.; Tang, M.K.; Sun, J.N.; Ko, K.M. Historical perspective of traditional indigenous medical practices: The current renaissance and conservation of herbal resources. Evid. Based Complement Altern. Med. 2014, 2014, 525340. [Google Scholar] [CrossRef]
- Senti, S.; Habicht, M.E.; Rayo, E.; Eppenberger, P.E.; Rühli, F.J.; Galassi, F.M. Egyptian canopic jars at the crossroad of medi-cine and archaeology: Overview of 100 years of research and future scientific expectations. Pathobiology 2018, 85, 267–275. [Google Scholar] [CrossRef] [Green Version]
- Molyneux, R.J.; Lee, S.T.; Gardner, D.R.; Panter, K.E.; James, L.F. Phytochemicals: The good, the bad and the ugly? Phytochemistry 2007, 68, 2973–2985. [Google Scholar] [CrossRef] [PubMed]
- Koche, D.; Shirsat, R.; Kawale, M. An overview of major classes of phytochemicals: Their types and role in disease prevention. Hislopia J. 2016, 9, 1–11. [Google Scholar]
- Giada, M.D.L.R. Food Phenolic Compounds: Main Classes, Sources and Their Antioxidant Power. Oxidative Stress Chronic Degener. Dis. A Role Antioxid. 2013. [Google Scholar] [CrossRef] [Green Version]
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Müller, M.J.; Oberritter, H.; Schulze, M.B.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef] [Green Version]
- De Lima, R.M.T.; Dos Reis, A.C.; de Menezes, A.P.M.; Santos, J.V.O.; Filho, J.; Ferreira, J.R.O.; de Alencar, M.V.O.B.; da Mata, A.M.O.F.; Khan, I.N.; Islam, A.; et al. Protective and therapeutic potential of ginger (zingiber officinale) extract and [6]-gingerol in cancer: A comprehensive review. Phytother. Res. 2018, 32, 1885–1907. [Google Scholar] [CrossRef]
- Kibe, M.N.; Konyole, S.O.; Oloo, M.O.; Ochieng, N.G.; Kathure, D. The role of phytochemicals in prevention and control of chronic diseases. Int. J. Curr. Res. 2017, 9, 62540–62543. [Google Scholar]
- Napolitano, G.; Fasciolo, G.; Di Meo, S.; Venditti, P. Vitamin e supplementation and mitochondria in experimental and fun-ctional hyperthyroidism: A mini-review. Nutrients 2019, 11, 2900. [Google Scholar] [CrossRef] [Green Version]
- Reddavide, R.; Rotolo, O.; Caruso, M.G.; Stasi, E.; Notarnicola, M.; Miraglia, C.; Nouvenne, A.; Meschi, T.; Angelis, G.L.D.; Di Mario, F.; et al. The role of diet in the prevention and treatment of Inflammatory Bowel Diseases. Acta Biomed. 2018, 89, 60–75. [Google Scholar] [CrossRef]
- Salas-Salvadó, J.; Becerra-Tomás, N.; Papandreou, C.; Bulló, M. Dietary Patterns Emphasizing the Consumption of Plant Foods in the Management of Type 2 Diabetes: A Narrative Review. Adv. Nutr. 2019, 10, S320–S331. [Google Scholar] [CrossRef] [Green Version]
- Kourouma, V.; Mu, T.-H.; Zhang, M.; Sun, H.-N. Effects of cooking process on carotenoids and antioxidant activity of or ange-fleshed sweet potato. LWT 2019, 104, 134–141. [Google Scholar] [CrossRef]
- Palermo, M.; Pellegrini, N.; Fogliano, V. The effect of cooking on the phytochemical content of vegetables. J. Sci. Food Agric. 2014, 94, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Wen, Q.; Jiang, J.; Li, H.L.; Tan, Y.F.; Li, Y.H.; Zeng, N.K. Could the gut microbiota reconcile the oral bioavailability conundrum of traditional herbs? J. Ethnopharmacol. 2016, 179, 253–264. [Google Scholar] [CrossRef]
- Zimmermann, G.R.; Lehár, J.; Keith, C.T. Multi-target therapeutics: When the whole is greater than the sum of the parts. Drug Discov. Today 2007, 12, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wen, M.; Yu, J.; Zhang, Q.; Polyakov, N.E.; Dushkin, A.V.; Su, W. Mechanochemical preparation of kaempferol intermolecular complexes for enhancing the solubility and bioavailability. Drug Dev. Ind. Pharm. 2018, 44, 1924–1932. [Google Scholar] [CrossRef]
- Mouhid, L.; Corzo-Martínez, M.; Torres, C.; Vázquez, L.; Reglero, G.; Fornari, T.; de Ramírez Molina, A. Improving in vivo efficacy of bioactive molecules: An overview of potentially antitumor phytochemicals and currently available lipid-based de-livery systems. J. Oncol. 2017, 2017, 7351976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McClements, D.J. Advances in nanoparticle and microparticle delivery systems for increasing the dispersibility, stability, and bioactivity of phytochemicals. Biotechnol. Adv. 2020, 38, 107287. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.P.; Jaganathan, S.K.; Manikandan, A.; Pandiaraj, K.N.; Gomathi, N.; Supriyanto, E. Recent trends in nano-based drug delivery systems for efficient delivery of phytochemicals in chemotherapy. RSC Adv. 2016, 6, 48294–48314. [Google Scholar] [CrossRef]
- G Peixoto, M.P.; Treter, J.; de Resende, P.E.; da Silveira, N.P.; Ortega, G.G.; Lawrence, M.J.; Dreiss, C.A. Wormlike micellar aggregates of saponins from ilex paraguariensis a. St. Hil. (mate): A characterisation by cryo-tem, rheology, light scattering and small-angle neutron scattering. J. Pharm. Sci. 2011, 100, 536–546. [Google Scholar] [CrossRef]
- Kregiel, D.; Berlowska, J.; Witonska, I.; Antolak, H.; Proestos, C.; Babic, M.; Babic, L.; Zhang, B. Saponin-based, biological-active surfactants from plants. Appl. Charact. Surfactants 2017, 183, 184–205. [Google Scholar]
- Zhao, Q.; Luan, X.; Zheng, M.; Tian, X.-H.; Zhao, J.; Zhang, W.-D.; Ma, B.-L. Synergistic mechanisms of constituents in herbal extracts during intestinal absorption: Focus on natural occurring nanoparticles. Pharmaceutics 2020, 12, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, I.A.; Smillie, T. Implementing a “Quality by Design” Approach to Assure the Safety and Integrity of Botanical Dietary Supplements. J. Nat. Prod. 2012, 75, 1665–1673. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 31 May 2021).
- ChemSpider | Search and share chemistry. 2021. Available online: http://www.chemspider.com/ (accessed on 4 March 2021).
- PhysChem, ADME/Tox Calculations | ACD/Labs Percepta Software. 2021. Available online: https://www.acdlabs.com/products/percepta/index.php (accessed on 9 March 2021).
- US EPA. EPI Suite™-Estimation Program Interface | US EPA. 2021. Available online: https://www.epa.gov/tsca-screening-tools/epi-suitetm-estimation-program-interface (accessed on 12 March 2021).
- Jablonsky, M.; Haz, A.; Burčová, Z.; Kreps, F.; Jablonsky, J. Pharmacokinetic properties of biomass-extracted substances iso-lated by green solvents. Bioresources 2019, 14, 6294–6303. [Google Scholar]
- Leong-Škorničková, J.; Šída, O.; Wijesundara, S.; Marhold, K. On the identity of turmeric: The typification of Curcuma longa L. (Zingiberaceae). Bot. J. Linn. Soc. 2008, 157, 37–46. [Google Scholar] [CrossRef]
- Chatzinasiou, L.; Booker, A.; MacLennan, E.; Mackonochie, M.; Heinrich, M. Turmeric (Curcuma longa L.) products: What quality differences exist? J. Herb. Med. 2019, 17-18, 100281. [Google Scholar] [CrossRef]
- Rao, P.S.; Ramanjaneyulu, Y.S.; Prisk, V.R.; Schurgers, L.J. A Combination of Tamarindus indica seeds and Curcuma longa Rhizome Extracts Improves Knee Joint Function and Alleviates Pain in Non-Arthritic Adults Following Physical Activity. Int. J. Med. Sci. 2019, 16, 845–853. [Google Scholar] [CrossRef] [Green Version]
- Hossain, M.A.; Ishimine, Y. Growth, yield and quality of turmeric (curcuma longa l.) cultivated on dark-red soil, gray soil and red soil in okinawa, japan. Plant Prod. Sci. 2005, 8, 482–486. [Google Scholar] [CrossRef]
- Dhakal, S.; Schmidt, W.F.; Kim, M.; Tang, X.; Peng, Y.; Chao, K. Detection of additives and chemical contaminants in turmeric powder using FT-IR spectroscopy. Foods 2019, 8, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amalraj, A.; Pius, A.; Gopi, S.; Gopi, S. Biological activities of curcuminoids, other biomolecules from turmeric and their de-rivatives—A review. J. Tradit. Complement. Med. 2016, 7, 205–233. [Google Scholar] [CrossRef] [Green Version]
- Czernicka, L.; Grzegorczyk, A.; Marzec, Z.; Antosiewicz, B.; Malm, A.; Kukula-Koch, W. Antimicrobial Potential of Single Metabolites of Curcuma longa Assessed in the Total Extract by Thin-Layer Chromatography-Based Bioautography and Image Analysis. Int. J. Mol. Sci. 2019, 20, 898. [Google Scholar] [CrossRef] [Green Version]
- Irshad, S.; Muazzam, A.; Shahid, Z.; Dalrymple, M.B. Curcuma longa (Turmeric): An auspicious spice for antibacterial, phytochemical and antioxidant activities. Pak. J. Pharm. Sci. 2018, 31, 2689–2696. [Google Scholar]
- Choi, Y.; Ban, I.; Lee, H.; Baik, M.-Y.; Kim, W. Puffing as a Novel Process to Enhance the Antioxidant and Anti-Inflammatory Properties of Curcuma longa L. (Turmeric). Antioxidants 2019, 8, 506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez Castaño, P.; Parween, S.; Pandey, A.V. Bioactivity of curcumin on the cytochrome p450 enzymes of the steroido-genic pathway. Int. J. Mol. Sci. 2019, 20, 4606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toden, S.; Theiss, A.; Wang, X.; Goel, A. Essential turmeric oils enhance anti-inflammatory efficacy of curcumin in dextran sulfate sodium-induced colitis. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, Y.; Shen, Q.; Lai, Y.; Park, S.Y.; Ou, X.; Lin, D.; Jin, M.; Zhang, W. Anti-inflammatory Effects of Curcumin in Microglial Cells. Front. Pharmacol. 2018, 9, 386. [Google Scholar] [CrossRef] [Green Version]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, Y.; Lee, R.J.; Xiang, G. Nano Encapsulated Curcumin: And Its Potential for Biomedical Applications. Int. J. Nanomed. 2020, ume 15, 3099–3120. [Google Scholar] [CrossRef]
- Agarwal, D.K.; Agarwal, S. Current Trends in Drug Delivery System of Curcumin and its Therapeutic Applications. Mini Rev. Med. Chem. 2020, 20, 1190–1232. [Google Scholar] [CrossRef]
- Kim, J.H.; Gupta, S.C.; Park, B.; Yadav, V.R.; Aggarwal, B.B. Turmeric (curcuma longa) inhibits inflammatory nuclear fac-tor (nf)-κb and nf-κb-regulated gene products and induces death receptors leading to suppressed proliferation, induced chemosensitization, and suppressed osteoclastogenesis. Mol. Nutr. Food Res. 2012, 56, 454–465. [Google Scholar] [CrossRef] [Green Version]
- Rizeq, B.; Gupta, I.; Ilesanmi, J.; Alsafran, M.; Rahman, M.; Ouhtit, A. The Power of Phytochemicals Combination in Cancer Chemoprevention. J. Cancer 2020, 11, 4521–4533. [Google Scholar] [CrossRef]
- Siviero, A.; Gallo, E.; Maggini, V.; Gori, L.; Mugelli, A.; Firenzuoli, F.; Vannacci, A. Curcumin, a golden spice with a low bio-availability. J. Herb. Med. 2015, 5, 57–70. [Google Scholar] [CrossRef]
- Kuttan, R.; Bhanumathy, P.; Nirmala, K.; George, M. Potential anticancer activity of turmeric (Curcuma longa). Cancer Lett. 1985, 29, 197–202. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar] [PubMed]
- Kunnumakkara, A.B.; Bordoloi, D.; Harsha, C.; Banik, K.; Gupta, S.C.; Aggarwal, B.B. Curcumin mediates anticancer effects by modulating multiple cell signaling pathways. Clin. Sci. 2017, 131, 1781–1799. [Google Scholar] [CrossRef] [PubMed]
- Goel, A.; Kunnumakkara, A.B.; Aggarwal, B.B. Curcumin as “curecumin”: From kitchen to clinic. Biochem. Pharm. 2008, 75, 787–809. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Yue, G.G.-L.; Tsui, S.K.-W.; Fung, K.-P.; Lau, C.B.-S. Turmeric extract, with absorbable curcumin, has potent anti-metastatic effect in vitro and in vivo. Phytomedicine 2018, 46, 131–141. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, N.S.; Srivastava, R.A.K. Curcumin and quercetin synergistically inhibit cancer cell proliferation in multiple cancer cells and modulate Wnt/β-catenin signaling and apoptotic pathways in A375 cells. Phytomedicine 2019, 52, 117–128. [Google Scholar] [CrossRef]
- Almutairi, F.M.; el Rabey, H.A.; Tayel, A.A.; Alalawy, A.I.; Al-Duais, M.A.; Sakran, M.I.; Zidan, N.S. Augmented anti-cancer activity of curcumin loaded fungal chitosan nanoparticles. Int. J. Biol. Macromol. 2020, 155, 861–867. [Google Scholar] [CrossRef]
- Kasi, P.D.; Tamilselvam, R.; Skalicka-Woźniak, K.; Nabavi, S.M.; Daglia, M.; Bishayee, A.; Pazoki-Toroudi, H. Molecular targets of curcumin for cancer therapy: An updated review. Tumor Biol. 2016, 37, 13017–13028. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, H.; Makabel, B.; Cui, Q.; Li, J.; Su, C.; Ashby, C.R., Jr.; Chen, Z.; Zhang, J. The targeting of non-coding RNAs by curcumin: Facts and hopes for cancer therapy (Review). Oncol. Rep. 2019, 42, 20–34. [Google Scholar] [CrossRef]
- Song, X.; Zhang, M.; Dai, E.; Luo, Y. Molecular targets of curcumin in breast cancer (Review). Mol. Med. Rep. 2019, 19, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhang, K.; Liu, J.; Yang, J.; Tian, Y.; Yang, C.; Li, Y.; Shao, M.; Su, W.; Song, N. Curcumin regulates cancer pro-gression: Focus on ncrnas and molecular signaling pathways. Front. Oncol. 2021, 11, 660712. [Google Scholar] [CrossRef]
- Wang, M.; Jiang, S.; Zhou, L.; Yu, F.; Ding, H.; Li, P.; Zhou, M.; Wang, K. Potential Mechanisms of Action of Curcumin for Cancer Prevention: Focus on Cellular Signaling Pathways and miRNAs. Int. J. Biol. Sci. 2019, 15, 1200–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanvir, E.M.; Hossen, M.S.; Hossain, M.F.; Afroz, R.; Gan, S.H.; Khalil, M.I.; Karim, N. Antioxidant properties of popular turmeric (curcuma longa) varieties from bangladesh. J. Food Qual. 2017, 8471785. [Google Scholar] [CrossRef] [Green Version]
- Ak, T.; Gülçin, I. Antioxidant and radical scavenging properties of curcumin. Chem. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef]
- Yuliani, S.; Mustofa; Partadiredja, G. Turmeric (Curcuma longa L.) extract may prevent the deterioration of spatial memory and the deficit of estimated total number of hippocampal pyramidal cells of trimethyltin-exposed rats. Drug Chem. Toxicol. 2017, 41, 62–71. [Google Scholar] [CrossRef] [PubMed]
- Hossen, S.; Tanvir, E.M.; Prince, M.B.; Paul, S.; Saha, M.; Ali, Y.; Gan, S.H.; Khalil, I.; Karim, N. Protective mechanism of turmeric (Curcuma longa) on carbofuran-induced hematological and hepatic toxicities in a rat model. Pharm. Biol. 2017, 55, 1937–1945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Praditya, D.; Kirchhoff, L.; Brüning, J.; Rachmawati, H.; Steinmann, J.; Steinmann, E. Anti-infective Properties of the Golden Spice Curcumin. Front. Microbiol. 2019, 10, 912. [Google Scholar] [CrossRef] [Green Version]
- Jennings, M.; Parks, R. Curcumin as an Antiviral Agent. Viruses 2020, 12, 1242. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.L.; Mizushina, Y.; Wang, S.Y.; Chuang, D.Y.; Nadar, M.; Hsu, W.L. Structure-activity relationship analysis of curcumin analogues on anti-influenza virus activity. FEBS J. 2013, 280, 5829–5840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treml, J.; Gazdová, M.; Šmejkal, K.; Šudomová, M.; Kubatka, P.; Hassan, S.T.S. Natural Products-Derived Chemicals: Breaking Barriers to Novel Anti-HSV Drug Development. Viruses 2020, 12, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Wang, J.; Liu, Y.; Luo, X.; Lei, W.; Xie, L. Antiviral and virucidal effects of curcumin on transmissible gastroenteritis virus in vitro. J. Gen. Virol. 2020, 101, 1079–1084. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Rana, V.; Parama, D.; Banik, K.; Girisa, S.; Henamayee, S.; Thakur, K.K.; Dutta, U.; Garodia, P.; Gupta, S.C.; et al. COVID-19, cytokines, inflammation, and spices: How are they related? Life Sci. 2021, 119201. [Google Scholar] [CrossRef] [PubMed]
- Thimmulappa, R.K.; Mudnakudu-Nagaraju, K.K.; Shivamallu, C.; Subramaniam, K.J.T.; Radhakrishanan, A.; Bhojraj, S.; Kuppusamy, G. Antivarial and immunomodulatory activity of curcumin: A case for prophylatic therapy for COVID-19. Heliyon 2021, 7, e06350. [Google Scholar] [CrossRef] [PubMed]
- Bangun, H.; Arianto, A.; Bangun, Y.S.; Nainggolan, M. Antibacterial Activity of Mucoadhesive Gastroretentive Drug Delivery System of Alginate Beads Containing Turmeric Extract-PVP Solid Dispersion. Open Access Maced. J. Med. Sci. 2019, 7, 3868–3873. [Google Scholar] [CrossRef] [Green Version]
- Shakeri, A.; Panahi, Y.; Johnston, T.P.; Sahebkar, A. Biological properties of metal complexes of curcumin. BioFactors 2019, 45, 304–317. [Google Scholar] [CrossRef]
- Chen, C.; Long, L.; Zhang, F.; Chen, Q.; Chen, C.; Yu, X.; Liu, Q.; Bao, J.; Long, Z. Antifungal activity, main active compo-nents and mechanism of curcuma longa extract against fusarium graminearum. PLoS ONE 2018, 13, e0194284. [Google Scholar]
- Murugesh, J.; Annigeri, R.G.; Mangala, G.K.; Mythily, P.H.; Chandrakala, J. Evaluation of the antifungal efficacy of differ-ent concentrations of curcuma longa on candida albicans: An in vitro study. JOMFP 2019, 23, 305. [Google Scholar]
- Jamali, N.; Adib-Hajbaghery, M.; Soleimani, A. The effect of curcumin ointment on knee pain in older adults with osteoar-thritis: A randomized placebo trial. BMC Complement. Med. Ther. 2020, 20, 305. [Google Scholar] [CrossRef] [PubMed]
- Jurenka, J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: A review of preclinical and clinical research. Altern. Med. Rev. J. Clin. Ther. 2009, 14, 141–153. [Google Scholar]
- Lee, S.-Y.; Cho, S.-S.; Li, Y.; Bae, C.-S.; Park, K.M.; Park, D.-H. Anti-inflammatory effect of curcuma longa and allium hook-eri co-treatment via nf-κb and cox-2 pathways. Sci. Rep. 2020, 10, 5718. [Google Scholar] [CrossRef]
- Velusami, C.C.; Bethapudi, B.; Murugan, S.; Illuri, R.; Mundkinajeddu, D. Bioactive turmerosaccharides from Curcuma longa Extract (NR-INF-02): Potential ameliorating effect on osteoarthritis pain. Pharmacogn. Mag. 2017, 13, 623–S627. [Google Scholar] [CrossRef]
- Nicoliche, T.; Maldonado, D.C.; Faber, J.; Silva, M.C.P. Evaluation of the articular cartilage in the knees of rats with induced arthritis treated with curcumin. PLoS ONE 2020, 15, e0230228. [Google Scholar] [CrossRef]
- Jacob, A.; Wu, R.; Zhou, M.; Wang, P. Mechanism of the Anti-inflammatory Effect of Curcumin: PPAR-γActivation. PPAR Res. 2007, 2007, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, M.J.; Moon, S.J.; Lee, S.H.; Yang, E.J.; Min, J.K.; Cho, S.G.; Yang, C.W.; Park, S.H.; Kim, H.Y.; Cho, M.L. Curcu-min attenuates acute graft-versus-host disease severity via in vivo regulations on th1, th17 and regulatory t cells. PLoS ONE 2013, 8, e67171. [Google Scholar]
- Jiang, Z.; Wan, Y.; Li, P.; Xue, Y.; Cui, W.; Chen, Q.; Chen, J.; Wang, F.; Mao, D. Effect of Curcumin Supplement in Summer Diet on Blood Metabolites, Antioxidant Status, Immune Response, and Testicular Gene Expression in Hu Sheep. Animals 2019, 9, 720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdollahi, E.; Momtazi, A.A.; Johnston, T.P.; Sahebkar, A. Therapeutic effects of curcumin in inflammatory and im-mune-mediated diseases: A nature-made jack-of-all-trades? J. Cell Physiol. 2018, 233, 830–848. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic Roles of Curcumin: Lessons Learned from Clinical Trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef] [Green Version]
- Asher, G.N.; Spelman, K. Clinical utility of curcumin extract. Altern. Ther. Health Med. 2013, 19, 20–22. [Google Scholar] [PubMed]
- Lee, S.-W.; Nah, S.-S.; Byon, J.-S.; Ko, H.J.; Park, S.-H.; Lee, S.-J.; Shin, W.-Y.; Jin, D.-K. Transient complete atrioventricular block associated with curcumin intake. Int. J. Cardiol. 2011, 150, e50–e52. [Google Scholar] [CrossRef] [PubMed]
- Heck, A.M.; DeWitt, B.A.; Lukes, A.L. Potential interactions between alternative therapies and warfarin. Am. J. Health Pharm. 2000, 57, 1221–1227. [Google Scholar] [CrossRef]
- Pivari, F.; Mingione, A.; Brasacchio, C.; Soldati, L. Curcumin and Type 2 Diabetes Mellitus: Prevention and Treatment. Nutrients 2019, 11, 1837. [Google Scholar] [CrossRef] [Green Version]
- Jiao, Y.; Wilkinson, J.; Di, X.; Wang, W.; Hatcher, H.; Kock, N.D.; D’Agostino, R., Jr.; Knovich, M.A.; Torti, F.M.; Torti, S.V. Curcumin, a cancer chemopreventive and chemotherapeutic agent, is a biologically active iron chelator. Blood 2009, 113, 462–469. [Google Scholar] [CrossRef] [Green Version]
- Great Plains Herbaria-Allium Sativum. 2021. Available online: https://ngpherbaria.org/portal/taxa/index.php?taxon=71501&cl=VPlants (accessed on 2 April 2021).
- Wisconsin Horticulture. Garlic, Allium Sativum. 2021. Available online: https://hort.extension.wisc.edu/articles/garlic-allium-sativum/ (accessed on 8 April 2021).
- Mikaili, P.; Maadirad, S.; Moloudizargari, M.; Aghajanshakeri, S.; Sarahroodi, S. Therapeutic Uses and Pharmacological Properties of Garlic, Shallot, and Their Biologically Active Compounds. Iran. J. Basic Med. Sci 2013, 16, 1031–1048. [Google Scholar] [PubMed]
- Bayan, L.; Koulivand, P.H.; Gorji, A. Garlic: A review of potential therapeutic effects. Avicenna J. Phytomed. 2014, 4, 1–14. [Google Scholar] [PubMed]
- Bratt, D.A. Garlic and Other Alliums. The Lore and the Science. R. Soc. Chem. 2010, 49, 7162. [Google Scholar] [CrossRef]
- Rivlin, R.S. Historical Perspective on the Use of Garlic. J. Nutr. 2001, 131, 951S–954S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Divya, B.J.; Suman, B.; Venkataswamy, M.; Thyagaraju, K. A STUDY ON PHYTOCHEMICALS, FUNCTIONAL GROUPS AND MINERAL COMPOSITION OF ALLIUM SATIVUM (GARLIC) CLOVES. Int. J. Curr. Pharm. Res. 2017, 9, 42. [Google Scholar] [CrossRef] [Green Version]
- Saif, S.; Hanif, M.A.; Rehman, R.; Riaz, M. Garlic. In Medicinal Plants of South Asia; Hanif, M.A., Nawaz, H., Khan, M.M., Byrne, H.J., Eds.; Elsevier: New York, NY, USA, 2020; Chapter 23; pp. 301–315. [Google Scholar]
- El-Saber Batiha, G.; Magdy Beshbishy, A.; G Wasef, L.; Elewa, Y.H.A.; A Al-Sagan, A.; Abd El-Hack, M.E.; Taha, A.E.; M Abd-Elhakim, Y.; Prasad Devkota, H. Chemical constituents and pharmacological activities of garlic (allium sativum l.): A review. Nutrients 2020, 12, 872. [Google Scholar] [CrossRef] [Green Version]
- Shang, A.; Cao, S.-Y.; Xu, X.-Y.; Gan, R.-Y.; Tang, G.-Y.; Corke, H.; Mavumengwana, V.; Li, H.-B. Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef] [Green Version]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.H.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and biological prop-erties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef] [Green Version]
- Amagase, H.; Petesch, B.L.; Matsuura, H.; Kasuga, S.; Itakura, Y. Intake of Garlic and Its Bioactive Components. J. Nutr. 2001, 131, 955S–962S. [Google Scholar] [CrossRef]
- Lawson, L.D.; Hunsaker, S.M. Allicin Bioavailability and Bioequivalence from Garlic Supplements and Garlic Foods. Nutrients 2018, 10, 812. [Google Scholar] [CrossRef] [Green Version]
- Kaschula, C.H.; Hunter, R.; Cotton, J.; Tuveri, R.; Ngarande, E.; Dzobo, K.; Schäfer, G.; Siyo, V.; Lang, D.; Kusza, D.A.; et al. The garlic compound ajoene targets protein folding in the endoplasmic reticulum of cancer cells. Mol. Carcinog. 2016, 55, 1213–1228. [Google Scholar] [CrossRef] [PubMed]
- Palazzolo, C.T.; De Paola, M.; Quesada, I.; Camargo, A.; Castro, C. 2-Vinyl-4H-1,3-Dithiin, a Bioavailable Compound from Garlic, Inhibits Vascular Smooth Muscle Cells Proliferation and Migration by Reducing Oxidative Stress. Plant Foods Hum. Nutr. 2020, 75, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yang, J.-H.; Hsieh, S.-C.; Sheen, L.-Y. Allyl Sulfides Inhibit Cell Growth of Skin Cancer Cells through Induction of DNA Damage Mediated G2/M Arrest and Apoptosis. J. Agric. Food Chem. 2010, 58, 7096–7103. [Google Scholar] [CrossRef] [PubMed]
- Țigu, A.B.; Toma, V.-A.; Moț, A.C.; Jurj, A.; Moldovan, C.S.; Fischer-Fodor, E.; Berindan-Neagoe, I.; Pârvu, M. The syner-gistic antitumor effect of 5-fluorouracil combined with allicin against lung and colorectal carcinoma cells. Molecules 2020, 25, 1947. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, V.; Nepal, A.; Olaisen, C.; Bachke, S.; Hira, J.; Søgaard, C.K.; Røst, L.M.; Misund, K.; Andreassen, T.; Melø, T.M.; et al. Anti-cancer potential of homemade fresh garlic extract is related to increased endo-plasmic reticulum stress. Nutrients 2018, 10, 450. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, S.; Haruma, K.; Kunihiro, M.; Nagata, S.; Kitadai, Y.; Manabe, N.; Sumii, M.; Yoshihara, M.; Kajiyama, G.; Chayama, K. Effects of aged garlic extract (AGE) on colorectal adenomas: A double-blinded study. Hiroshima J. Med. Sci. 2004, 53, 39–45. [Google Scholar]
- Zhou, X.; Qian, H.; Zhang, D.; Zeng, L. Garlic intake and the risk of colorectal cancer: A meta-analysis. Medicine 2020, 99, e18575. [Google Scholar] [CrossRef]
- Fleischauer, A.T.; Poole, C.; Arab, L. Garlic consumption and cancer prevention: Meta-analyses of colorectal and stomach cancers. Am. J. Clin. Nutr. 2000, 72, 1047–1052. [Google Scholar] [CrossRef] [Green Version]
- Chiavarini, M.; Minelli, L.; Fabiani, R. Garlic consumption and colorectal cancer risk in man: A systematic review and me-ta-analysis. Public. Health Nutr. 2016, 19, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.; Patel, V. Antioxidant activity of garlic using conventional extraction and in vitro gastrointestinal digestion. Free. Radic. Antioxid. 2013, 3, 30–34. [Google Scholar] [CrossRef] [Green Version]
- Lei, M.; Xu, M.; Zhang, Z.; Zhang, M.; Gao, Y. The Analysis of Saccharide in Black Garlic and its Antioxidant Activity. Adv. J. Food Sci. Technol. 2014, 6, 755–760. [Google Scholar] [CrossRef]
- Moosavian, S.P.; Paknahad, Z.; Habibagahi, Z. A randomized, double-blind, placebo-controlled clinical trial, evaluating the garlic supplement effects on some serum biomarkers of oxidative stress, and quality of life in women with rheumatoid arthritis. Int. J. Clin. Pr. 2020, 74, e13498. [Google Scholar] [CrossRef] [PubMed]
- Rouf, R.; Uddin, S.J.; Sarker, D.K.; Islam, M.T.; Ali, E.S.; Shilpi, J.A.; Nahar, L.; Tiralongo, E.; Sarker, S.D. Antiviral po-tential of garlic (allium sativum) and its organosulfur compounds: A systematic update of pre-clinical and clinical data. Trends Food Sci. Technol. 2020, 104, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Khanal, S.; Ghimire, P.; Dhamoon, A.S. The Repertoire of Adenovirus in Human Disease: The Innocuous to the Deadly. Biomed 2018, 6, 30. [Google Scholar] [CrossRef] [Green Version]
- Mehrbod, P.; Aini, I.; Amini, E.; Eslami, M.; Torabi, A.; Bande, F.; Kheiri, M.T. Assessment of direct immunofluorescence assay in detection of antiviral effect of garlic extract on influenza virus. Afr. J. Microbiol. Res. 2013, 7, 2608–2612. [Google Scholar] [CrossRef]
- Alejandria, M.M. Dengue haemorrhagic fever or dengue shock syndrome in children. BMJ Clin. Evid. 2015, 2015, 0917. [Google Scholar]
- Straface, G.; Selmin, A.; Zanardo, V.; De Santis, M.; Ercoli, A.; Scambia, G. Herpes Simplex Virus Infection in Pregnancy. Infect. Dis. Obstet. Gynecol. 2012, 2012, 1–6. [Google Scholar] [CrossRef]
- Chavan, R.D.; Shinde, P.; Girkar, K.; Madage, R.; Chowdhary, A. Assessment of anti-influenza activity and hemagglutina-tion inhibition of plumbago indica and allium sativum extracts. Pharm. Res. 2016, 8, 105. [Google Scholar]
- Klenk, H.-D.; Matrosovich, M.; Stech, J. Chapter 6-Avian influenza: Molecular mechanisms of pathogenesis and host range. In Animal Viruses: Molecular Biology, 1st ed.; Mettenleiter, T.C., Sobrino, F., Eds.; Caister Academic Press: Poole, UK, 2008; pp. 253–301. [Google Scholar]
- Matheny, S.C.; Kingery, J.E. Hepatitis A. Am. Fam. Physician 2012, 86, 1027–1034. [Google Scholar]
- Wang, L.; Jiao, H.; Zhao, J.; Wang, X.; Sun, S.; Lin, H. Allicin alleviates reticuloendotheliosis virus-induced immunosuppres-sion via erk/mitogen-activated protein kinase pathway in specific pathogen-free chickens. Front. Immunol. 2017, 8, 1856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vlasova, A.N.; Amimo, J.O.; Saif, L.J. Porcine Rotaviruses: Epidemiology, Immune Responses and Control Strategies. Viruses 2017, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Thuy, B.; My, T.; Hai, N.; Hieu, L.; Hoa, T.; Thi Phuong Loan, H.; Triet, N.; Anh, T.; Quy, P.; Tat, P.; et al. Investigation into SARS-CoV-2 resistance of compounds in garlic essential oil. ACS Omega 2020, 5, 8312–8320. [Google Scholar] [CrossRef]
- Trivedi, N.A.; Yadav, S.; Bhatt, J.D. Antimicrobial activity of fresh garlic juice: An in vitro study. AYU 2015, 36, 203–207. [Google Scholar] [CrossRef] [Green Version]
- Venâncio, P.C.; Figueroba, S.R.; Nani, B.D.; Ferreira, L.; Muniz, B.V.; Fiol, F.D.S.D.; Sartoratto, A.; Rosa, E.; Groppo, F.C. Antimicrobial Activity of Two Garlic Species (Allium Sativum and A. Tuberosum) Against Staphylococci Infection. In Vivo Study in Rats. Adv. Pharm. Bull. 2017, 7, 115–121. [Google Scholar] [CrossRef]
- Farrag, H.A.; Hosny, A.E.-D.M.; Hawas, A.M.; Hagras, S.A.; Helmy, O.M. Potential efficacy of garlic lock therapy in combating biofilm and catheter-associated infections; experimental studies on an animal model with focus on toxicological aspects. Saudi Pharm. J. 2019, 27, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Zardast, M.; Namakin, K.; Kaho, J.E.; Hashemi, S.S. Assessment of antibacterial effect of garlic in patients infected with Helicobacter pylori using urease breath test. Avicenna J. Phytomed. 2016, 6, 495–501. [Google Scholar] [PubMed]
- Li, W.-R.; Shi, Q.-S.; Dai, H.-Q.; Liang, Q.; Xie, X.-B.; Huang, X.-M.; Zhao, G.-Z.; Zhang, L.-X. Antifungal activity, kinetics and molecular mechanism of action of garlic oil against Candida albicans. Sci. Rep. 2016, 6, 22805. [Google Scholar] [CrossRef]
- Aala, F.; Yusuf, U.K.; Nulit, R.; Rezaie, S. Inhibitory effect of allicin and garlic extracts on growth of cultured hyphae. Iran. J. Basic Med. Sci 2014, 17, 150–154. [Google Scholar]
- Li, W.R.; Shi, Q.S.; Liang, Q.; Huang, X.M.; Chen, Y.B. Antifungal effect and mechanism of garlic oil on penicillium funic-ulosum. Appl. Microbiol. Biotechnol. 2014, 98, 8337–8346. [Google Scholar] [CrossRef]
- Lee, D.Y.; Li, H.; Lim, H.J.; Lee, H.J.; Jeon, R.; Ryu, J.-H. Anti-Inflammatory Activity of Sulfur-Containing Compounds from Garlic. J. Med. Food 2012, 15, 992–999. [Google Scholar] [CrossRef] [Green Version]
- Zare, E.; Alirezaei, A.; Bakhtiyari, M.; Mansouri, A. Evaluating the effect of garlic extract on serum inflammatory markers of peritoneal dialysis patients: A randomized double-blind clinical trial study. BMC Nephrol. 2019, 20, 26. [Google Scholar] [CrossRef]
- Rahman, K.; Lowe, G.M. Garlic and Cardiovascular Disease: A Critical Review. J. Nutr. 2006, 136, 736S–740S. [Google Scholar] [CrossRef]
- Feng, Y.; Zhu, X.; Wang, Q.; Jiang, Y.; Shang, H.; Cui, L.; Cao, Y. Allicin enhances host pro-inflammatory immune responses and protects against acute murine malaria infection. Malar. J. 2012, 11, 268. [Google Scholar] [CrossRef] [Green Version]
- Bruck, R.; Aeed, H.; Brazovsky, E.; Noor, T.; Hershkoviz, R. Allicin, the active component of garlic, prevents immune-mediated, concanavalin A-induced hepatic injury in mice. Liver Int. 2005, 25, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Mathews, A.E.; Rodrigues, C.; Eudy, B.J.; Rowe, C.A.; O’Donoughue, A.; Percival, S.S. Aged garlic extract supple-mentation modifies inflammation and immunity of adults with obesity: A randomized, double-blind, placebo-controlled clin-ical trial. Clin. Nutr. ESPEN 2018, 24, 148–155. [Google Scholar] [CrossRef] [Green Version]
- Nantz, M.P.; Rowe, C.A.; Muller, C.E.; Creasy, R.A.; Stanilka, J.M.; Percival, S.S. Supplementation with aged garlic ex-tract improves both nk and γδ-t cell function and reduces the severity of cold and flu symptoms: A randomized, dou-ble-blind, placebo-controlled nutrition intervention. Clin. Nutr. 2012, 31, 337–344. [Google Scholar] [CrossRef]
- Schafer, G.; Kaschula, C.H. The immunomodulation and anti-inflammatory effects of garlic oregano sulfur compounds in cancer chemoprevention. Anticancer Agents Med. Chem. 2014, 14, 233–240. [Google Scholar] [CrossRef]
- Shaikh, S.A.; Tischer, S.; Choi, E.K.; Fontana, R.J. Good for the lung but bad for the liver? Garlic-induced hepatotoxicity following liver transplantation. J. Clin. Pharm. Ther. 2017, 42, 646–648. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, T.; Kashimoto, N.; Kasuga, S. Effects of garlic preparations on the gastrointestinal mucosa. J. Nutr. 2001, 131, 1109S–1113S. [Google Scholar] [CrossRef]
- Suzuki, Y.; Saito, J.; Misa, K.; Fukuhara, N.; Fukuhara, A.; Munakata, M. A case of black garlic-induced pneumonia as an adverse reaction. Allergol. Int. 2016, 65, 353–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubey, H.; Singh, A.; Patole, A.M.; Tenpe, C.R. Antihypertensive effect of allicin in dexamethasone-induced hypertensive rats. Integr. Med. Res. 2017, 6, 60–65. [Google Scholar] [CrossRef]
- Barceloux, D.G. Cinnamon (cinnamomum species). Dis. Mon. 2009, 55, 327–335. [Google Scholar] [CrossRef]
- Thomas, J.; Kuruvilla, K. Cinnamon. In Handbook of Herbs and Spices; Elsevier: Amsterdam, The Netherlands, 2012; pp. 182–196. [Google Scholar]
- Ramazani, E.; Yazdfazeli, M.; Emami, S.A.; Mohtashami, L.; Javadi, B.; Asili, J.; Tayarani-Najaran, Z. Protective effects of Cinnamomum verum, Cinnamomum cassia and cinnamaldehyde against 6-OHDA-induced apoptosis in PC12 cells. Mol. Biol. Rep. 2020, 47, 2437–2445. [Google Scholar] [CrossRef] [PubMed]
- Behbahani, B.A.; Falah, F.; Arab, F.L.; Vasiee, M.; Yazdi, F.T. Chemical Composition and Antioxidant, Antimicrobial, and Antiproliferative Activities of Cinnamomum zeylanicum Bark Essential Oil. Evid. Based Complement. Altern. Med. 2020, 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.V.; Gan, S.H. Cinnamon: A Multifaceted Medicinal Plant. Evid. Based Complement. Altern. Med. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ananthakrishnan, R.; Chandra, P.; Kumar, B.; Rameshkumar, K.B. Quantification of coumarin and related phenolics in cinnamon samples from south india using UHPLC-ESI-QqQLIT-MS/MS. Int. J. Food Prop. 2018, 21, 50–57. [Google Scholar] [CrossRef] [Green Version]
- Khuwijitjaru, P.; Sayputikasikorn, N.; Samuhasaneetoo, S.; Penroj, P.; Siriwongwilaichat, P.; Adachi, S. Subcritical water extraction of flavoring and phenolic compounds from cinnamon bark (Cinnamomum zeylanicum). J. Oleo Sci. 2012, 61, 349–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klejdus, B.; Kováčik, J. Quantification of phenols in cinnamon: A special focus on “total phenols” and phenolic acids including desi-orbitrap MS detection. Ind. Crops Prod. 2016, 83, 774–780. [Google Scholar] [CrossRef]
- Yang, X.-Q.; Zheng, H.; Ye, Q.; Li, R.-Y.; Chen, Y. Essential oil of Cinnamon exerts anti-cancer activity against head and neck squamous cell carcinoma via attenuating epidermal growth factor receptor-tyrosine kinase. J. BUON 2016, 20, 1518–1525. [Google Scholar]
- Koppikar, S.J.; Choudhari, A.S.; Suryavanshi, S.A.; Kumari, S.; Chattopadhyay, S.; Kaul-Ghanekar, R. Aqueous Cinnamon Extract (ACE-c) from the bark of Cinnamomum cassia causes apoptosis in human cervical cancer cell line (SiHa) through loss of mitochondrial membrane potential. BMC Cancer 2010, 10, 210. [Google Scholar] [CrossRef] [Green Version]
- Cherng, J.-M.; Perng, D.-S.; Tsai, Y.-H.; Cherng, J.; Wang, J.-S.; Chou, K.-S.; Shih, C.-W. Discovery of a novel anticancer agent with both anti-topoisomerase I and II activities in hepatocellular carcinoma SK-Hep-1 cells in vitro and in vivo: Cinnamomum verum component 2-methoxycinnamaldehyde. Drug Des. Dev. Ther. 2016, 10, 141–153. [Google Scholar] [CrossRef] [Green Version]
- Mateen, S.; Rehman, T.; Shahzad, S.; Naeem, S.S.; Faizy, A.F.; Khan, A.Q.; Khan, M.S.; Husain, F.M.; Moin, S. Anti-oxidant and anti-inflammatory effects of cinnamaldehyde and eugenol on mononuclear cells of rheumatoid arthritis patients. Eur. J. Pharmacol. 2019, 852, 14–24. [Google Scholar] [CrossRef]
- Davaatseren, M.; Jo, Y.-J.; Hong, G.-P.; Hur, H.J.; Park, S.; Choi, M.-J. Studies on the Anti-Oxidative Function of trans-Cinnamaldehyde-Included β-Cyclodextrin Complex. Molecules 2017, 22, 1868. [Google Scholar] [CrossRef] [Green Version]
- Borzoei, A.; Rafraf, M.; Niromanesh, S.; Farzadi, L.; Narimani, F.; Doostan, F. Effects of cinnamon supplementation on anti-oxidant status and serum lipids in women with polycystic ovary syndrome. J. Tradit. Complement. Med. 2018, 8, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Setzer, W. Essential oils as complementary and alternative medicines for the treatment of influenza. Am. J. Essent. Oil Nat. Prod. 2016, 4, 16–22. [Google Scholar]
- Lavaee, F.; Moshaverinia, M.; Rastegarfar, M.; Moattari, A. Evaluation of the effect of hydro alcoholic extract of cinnamon on herpes simplex virus-1. Dent. Res. J. 2020, 17, 114. [Google Scholar] [CrossRef]
- Kulkarni, S.A.; Nagarajan, S.K.; Ramesh, V.; Palaniyandi, V.; Selvam, S.P.; Madhavan, T. Computational evaluation of major components from plant essential oils as potent inhibitors of SARS-CoV-2 spike protein. J. Mol. Struct. 2020, 1221, 128823. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.M.; Ramadhani, A.M.; Erwa, I.Y.; Ishag, O.A.O.; Saeed, M.B. Phytochemical screening, chemical composition and antimicrobial activity of cinnamon verum bark. Int. Res. J. Pure Appl. Chem. 2020, 36–43. [Google Scholar] [CrossRef]
- Faikoh, E.N.; Hong, Y.-H.; Hu, S.-Y. Liposome-encapsulated cinnamaldehyde enhances zebrafish (Danio rerio) immunity and survival when challenged with Vibrio vulnificus and Streptococcus agalactiae. Fish. Shellfish. Immunol. 2014, 38, 15–24. [Google Scholar] [CrossRef]
- Kowalska, J.; Tyburski, J.; Krzymińska, J.; Jakubowska, M. Cinnamon powder: An in vitro and in vivo evaluation of anti-fungal and plant growth promoting activity. Eur. J. Plant. Pathol. 2020, 156, 237–243. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.S.; Deng, J.H.; Ma, Y.H.; Shi, M.; Li, B. Mechanisms, clinically curative effects, and antifungal activities of cinnamon oil and pogostemon oil complex against three species of candida. J. Tradit. Chin. Med. 2012, 32, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Ayatollahi Mousavi, S.A.; Kazemi, A. In vitro and in vivo antidermatophytic activities of some iranian medicinal plants. Med. Mycol. 2015, 53, 852–859. [Google Scholar] [CrossRef] [Green Version]
- Han, X.; Parker, T.L. Antiinflammatory Activity of Cinnamon (Cinnamomum zeylanicum) Bark Essential Oil in a Human Skin Disease Model. Phytother. Res. 2017, 31, 1034–1038. [Google Scholar] [CrossRef] [Green Version]
- Gunawardena, D.; Karunaweera, N.; Lee, S.; van Der Kooy, F.; Harman, D.; Raju, R.; Bennett, L.; Gyengesi, E.; Sucher, N.; Münch, G. Anti-inflammatory activity of cinnamon (C. zeylanicum and C. cassia) extracts—Identification of E-cinnamaldehyde and o-methoxy cinnamaldehyde as the most potent bioactive compounds. Food Funct. 2015, 6, 910–919. [Google Scholar] [CrossRef]
- Hagenlocher, Y.; Satzinger, S.; Civelek, M.; Feilhauer, K.; Köninger, J.; Bischoff, S.C.; Lorentz, A. Cinnamon reduces in-flammatory response in intestinal fibroblasts in vitro and in colitis in vivo leading to decreased fibrosis. Mol. Nutr. Food Res. 2017, 61, 1601085. [Google Scholar] [CrossRef]
- Hagenlocher, Y.; Kiessling, K.; Schäffer, M.; Bischoff, S.C.; Lorentz, A. Cinnamaldehyde is the main mediator of cinnamon extract in mast cell inhibition. Eur. J. Nutr. 2014, 54, 1297–1309. [Google Scholar] [CrossRef]
- Ose, R.; Tu, J.; Schink, A.; Maxeiner, J.; Schuster, P.; Lucas, K.; Saloga, J.; Bellinghausen, I. Cinnamon extract inhibits aller-gen-specific immune responses in human and murine allergy models. Clin. Exp. Allergy 2020, 50, 41–50. [Google Scholar] [CrossRef]
- Lucas, K.; Fröhlich-Nowoisky, J.; Oppitz, N.; Ackermann, M. Cinnamon and Hop Extracts as Potential Immunomodulators for Severe COVID-19 Cases. Front. Plant Sci. 2021, 12, 589783. [Google Scholar] [CrossRef]
- Grant-Alfieri, A.; Schaechter, J.; Lipshultz, S.E. Ingesting and Aspirating Dry Cinnamon by Children and Adolescents: The “Cinnamon Challenge”. Pediatrics 2013, 131, 833–835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roth-Walter, F.; Moskovskich, A.; Gomez-Casado, C.; Diaz-Perales, A.; Oida, K.; Singer, J.; Kinaciyan, T.; Fuchs, H.C.; Jensen-Jarolim, E. Immune Suppressive Effect of Cinnamaldehyde Due to Inhibition of Proliferation and Induction of Apoptosis in Immune Cells: Implications in Cancer. PLoS ONE 2014, 9, e108402. [Google Scholar] [CrossRef]
- Ernst, E. Herbal medicinal products during pregnancy: Are they safe? BJOG Int. J. Obstet. Gynaecol. 2002, 109, 227–235. [Google Scholar] [CrossRef]
- Hsieh, C.J.; Sun, M.; Osborne, G.; Ricker, K.; Tsai, F.C.; Li, K.; Tomar, R.; Phuong, J.; Schmitz, R.; Sandy, M.S. Cancer hazard identification integrating human variability: The case of coumarin. Int. J. Toxicol. 2019, 38, 501–552. [Google Scholar] [CrossRef] [PubMed]
- Rethinam, P.; Sundararaj, P. Annona muricata l., soursop (graviola)-nature’s gift to mankind with amazing medicinal benefits. Int. J. Innov. Horticult. 2016, 5, 73–80. [Google Scholar]
- Chatrou, L.W.; Pirie, M.; Erkens, R.; Couvreur, T.; Neubig, K.M.; Abbott, J.R.; Mols, J.B.; Maas, J.W.; Saunders, R.; Chase, M.W. A new subfamilial and tribal classification of the pantropical flowering plant family Annonaceae informed by molecular phylogenetics. Bot. J. Linn. Soc. 2012, 169, 5–40. [Google Scholar] [CrossRef] [Green Version]
- Coria-Téllez, A.V.; Montalvo-Gónzalez, E.; Yahia, E.M.; Obledo-Vázquez, E.N. Annona muricata: A comprehensive review on its traditional medicinal uses, phytochemicals, pharmacological activities, mechanisms of action and toxicity. Arab. J. Chem. 2018, 11, 662–691. [Google Scholar] [CrossRef] [Green Version]
- Annona muricata Sour Sop PFAF Plant Database. 2021. Available online: https://pfaf.org/user/Plant.aspx?LatinName=Annona+muricata (accessed on 7 July 2021).
- Agu, K.C.; Okolie, P.N. Proximate composition, phytochemical analysis, and in vitro antioxidant potentials of extracts of Annona muricata(Soursop). Food Sci. Nutr. 2017, 5, 1029–1036. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viraraghavan, S.S.N. Phytochemical screening of hydroalcohol fruit extract of annona muricata. Int. J. Adv. Res. Dev. 2018, 3, 681–685. [Google Scholar]
- Anaya Esparza, L.M.; Montalvo-González, E. Bioactive compounds of soursop (annona muricata l.) fruit. In Bioactive Com-Pounds in Underutilized Fruits and Nuts; Murthy, H.N., Bapat, V.A., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 175–189. [Google Scholar]
- Rady, I.; Bloch, M.; Chamcheu, R.-C.N.; Mbeumi, S.B.; Anwar, R.; Mohamed, H.; Babatunde, A.S.; Kuiate, J.-R.; Noubissi, F.K.; El Sayed, K.A.; et al. Anticancer Properties of Graviola (Annona muricata): A Comprehensive Mechanistic Review. Oxidative Med. Cell. Longev. 2018, 2018, 1–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simpson, D.; Amos, S. Other plant metabolites. In Pharmacognos; Badal, S., Delgoda, R., Eds.; Academic Press: Boston, MA, USA, 2017; Chapter 12; pp. 267–280. [Google Scholar]
- Jan, A.T.; Kamli, M.R.; Murtaza, I.; Singh, J.B.; Ali, A.; Haq, Q. Dietary Flavonoid Quercetin and Associated Health Benefits—An Overview. Food Rev. Int. 2010, 26, 302–317. [Google Scholar] [CrossRef]
- Kahkeshani, N.; Farzaei, F.; Fotouhi, M.; Alavi, S.S.; Bahramsoltani, R.; Naseri, R.; Momtaz, S.; Abbasabadi, Z.; Rahimi, R.; Farzaei, M.H.; et al. Pharmacological effects of gallic acid in health and disease: A mechanistic review. Iran. J. Basic Med. Sci. 2019, 22, 225–237. [Google Scholar] [CrossRef]
- Seo, D.J.; Choi, C. Viral disease and use of polyphenolic compounds. In Polyphenols: Prevention and Treatment of Human Disease, 2nd ed.; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: Cambridge, MA, USA, 2018; Chapter 25; pp. 301–312. [Google Scholar]
- Enweani, I.; Obroku, J.; Enahoro, T.; Omoifo, C. The biochemical analysis of soursop (annona muricata l.) and sweetsop (a.Squamosa l.) and their potential use as oral rehydration therapy. J. Food Agric. Environ. 2004, 22, 39–43. [Google Scholar]
- Gyesi, J.N.; Opoku, R.; Borquaye, L.S. Chemical Composition, Total Phenolic Content, and Antioxidant Activities of the Essential Oils of the Leaves and Fruit Pulp of Annona muricata L. (Soursop) from Ghana. Biochem. Res. Int. 2019, 2019, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Lannuzel, A.; Michel, P.P.; Caparros-Lefebvre, D.; Abaul, J.; Hocquemiller, R.; Ruberg, M. Toxicity of Annonaceae for do-paminergic neurons: Potential role in atypical parkinsonism in Guadeloupe. Mov. Disord. 2002, 17, 84–90. [Google Scholar] [CrossRef]
- Deep, G.; Kumar, R.; Jain, A.K.; Dhar, D.; Panigrahi, G.K.; Hussain, A.; Agarwal, C.; El-Elimat, T.; Sica, V.P.; Oberlies, N.H.; et al. Graviola inhibits hypoxia-induced NADPH oxidase activity in prostate cancer cells reducing their prolifera-tion and clonogenicity. Sci. Rep. 2016, 6, 23135. [Google Scholar] [CrossRef] [Green Version]
- Qazi, A.K.; A Siddiqui, J.; Jahan, R.; Chaudhary, S.; A Walker, L.; Sayed, Z.; Jones, D.T.; Batra, S.K.; A Macha, M. Emerging therapeutic potential of graviola and its constituents in cancers. Carcinogenesis 2018, 39, 522–533. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, J.L. Paw Paw and Cancer: Annonaceous Acetogenins from Discovery to Commercial Products. J. Nat. Prod. 2008, 71, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.P.; Rachagani, S.; Purohit, V.; Pandey, P.; Joshi, S.; Moore, E.D.; Johansson, S.L.; Singh, P.K.; Ganti, A.K.; Batra, S.K. Graviola: A novel promising natural-derived drug that inhibits tumorigenicity and metastasis of pancreatic cancer cells in vitro and in vivo through altering cell metabolism. Cancer Lett. 2012, 323, 29–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, I.; Yap, C.V.; Subramaniam, K.S.; Khor, S.W. Annonacin exerts antitumor activity through induction of apoptosis and extracellular signal-regulated kinase inhibition. Pharmacogn. Res. 2017, 9, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Yiallouris, A.; Patrikios, I.; Johnson, E.O.; Sereti, E.; Dimas, K.; de Ford, C.; Fedosova, N.U.; Graier, W.F.; Sokratous, K.; Kyriakou, K.; et al. Annonacin promotes selective cancer cell death via nka-dependent and serca-dependent pathways. Cell Death Dis. 2018, 9, 764. [Google Scholar] [CrossRef] [PubMed]
- Byun, E.-B.; Song, H.-Y.; Kim, W.S. Polysaccharides from Annona muricata leaves protect normal human epidermal keratinocytes and mice skin from radiation-induced injuries. Radiat. Phys. Chem. 2020, 170, 108672. [Google Scholar] [CrossRef]
- George, V.C.; Kumar, D.; Rajkumar, V.; Suresh, P.; Kumar, R.A. Quantitative assessment of the relative antineoplastic potential of the n-butanolic leaf extract of annona muricata linn. In normal and immortalized human cell lines. Asian Pac. J. Cancer Prev. 2012, 13, 699–704. [Google Scholar] [CrossRef]
- Zamudio-Cuevas, Y.; Díaz-Sobac, R.; Vázquez-Luna, A.; Landa-Solís, C.; Cruz-Ramos, M.; Santamaría-Olmedo, M.; Martínez-Flores, K.; Fuentes-Gómez, A.J.; López-Reyes, A. The antioxidant activity of soursop decreases the expression of a member of the NAPDH oxidase family. Food Funct. 2014, 5, 303–309. [Google Scholar] [CrossRef]
- Baskar, R.; Rajeswari, V.; Sathishkumar, T. In vitro antioxidant studies in leaves of Annona species. Indian J. Exp. Boil. 2007, 45, 480–485. [Google Scholar]
- Biba, V.; Amily, A.; Sangeetha, S.; Remani, P. Anticancer, antioxidant and antimicrobial activity of annonaceae family. World J. Pharm. Pharm. Sci. 2014, 3, 1595–1604. [Google Scholar]
- Padma, P.; Pramod, N.P.; Thyagarajan, S.P.; Khosa, R.L. Effect of the extract of annona muricata and petunia nyctagini-flora on herpes simplex virus. J. Ethnopharmacol. 1998, 61, 81–83. [Google Scholar] [CrossRef]
- Wahab, N.Z.A.; Ibrahim, N.; Kamarudin, M.K.A.; Lananan, F.; Juahir, H.; Ghazali, A.; Ireana Yusra, A.F. Cytotoxicity and antiviral activity of annona muricata aqueous leaves extract against dengue virus type. J. Fundam. Appl. Sci. 2018, 10, 580–589. [Google Scholar]
- Le Donne, M.; Lentini, M.; Alibrandi, A.; Salimbeni, V.; Giuffre’, G.; Mazzeo, F.; Triolo, O.; D’Anna, R. Antiviral activity of Ellagic acid and Annona Muricata in cervical HPV related pre-neoplastic lesions: A randomized trial. J. Funct. Foods 2017, 35, 549–554. [Google Scholar] [CrossRef]
- Elmi, A.; Sayem, S.A.-J.; Ahmed, M.; Abdoul-Latif, F. NATURAL COMPOUNDS FROM DJIBOUTIAN MEDICINAL PLANTS AS INHIBITORS OF COVID-19 BY IN SILICO INVESTIGATIONS. Int. J. Curr. Pharm. Res. 2020, 52–57. [Google Scholar] [CrossRef]
- Jianfeng, Y.; Shengxi, S.; Bin, L.; Zhihao, W.; Yi-Zhou, J.; Yunqing, L.; Feng, C.; Bing, L. Emergency antiviral drug discovery during a pandemic-a case study on the application of natural compounds to treat covid-19. ChemiRxiv 2020. [Google Scholar] [CrossRef]
- Pai, B.M.; Rajesh, G.; Shenoy, R.; Rao, A. Anti-microbial efficacy of soursop leaf extract (annona muricata) on oral patho-gens: An in-vitro study. JCDR 2016, 10, ZC01–ZC04. [Google Scholar]
- Rodriguez-Perez, J.L.; A Millones-Gomez, P. Antibacterial Effect of Annona muricata L. Leaves on Streptococcus mutans ATCC 25175 Strains. J. Clin. Diagn. Res. 2019, 13. [Google Scholar] [CrossRef]
- Dada, E.O.; Faleye, O.S. In-vitro and in-vivo Activities of the Ethanol Pulp Extract of Annona muricata (Linn) Fruit in Albino Rats Infected with Salmonella typhi. J. Adv. Med. Pharm. Sci. 2016, 5, 1–12. [Google Scholar] [CrossRef]
- Iyanda-Joel, W.O.; Omonigbehin, E.A.; Iweala, E.E.J.; Chinedu, S.N. Antibacterial studies on fruit-skin and leaf extracts of Annona muricata in Ota, Nigeria. IOP Conf. Ser. Earth Environ. Sci. 2019, 331, 012029. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.-J.; Liu, M.; Dawuti, G.; Dou, Q.; Ma, Y.; Liu, H.-G.; Aibai, S. Antifungal Activity of Gallic Acid In Vitro and In Vivo. Phytother. Res. 2017, 31, 1039–1045. [Google Scholar] [CrossRef]
- Kwun, M.S.; Lee, D.G. Quercetin-induced yeast apoptosis through mitochondrial dysfunction under the accumulation of magnesium in Candida albicans. Fungal Biol. 2020, 124, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Wahab, S.M.A.; Jantan, I.; Haque, A.; Arshad, L. Exploring the Leaves of Annona muricata L. as a Source of Potential Anti-inflammatory and Anticancer Agents. Front. Pharmacol. 2018, 9, 661. [Google Scholar] [CrossRef] [PubMed]
- Cercato, L.M.; Araújo, J.M.D.; Oliveira, A.S.; Melo, A.J.O.; Lima, B.S.; dos Santos, E.W.P.; Dos S Neto, A.G.; de Albu-querque-Júnior, R.L.C.; Duarte, M.C.; Araujo, A.A.S. Reduced cutaneous inflammation associated with antioxidant action after topical application of the aqueous extract of annona muri-cata leaves. Inflammopharmacology 2021, 29, 307–315. [Google Scholar] [CrossRef]
- Laksmitawati, D.R.; Prasanti, A.P.; Larasinta, N.; Syauta, G.A.; Hilda, R.; Ramadaniati, H.U.; Widyastuti, A.; Karami, N.; Afni, M.; Rihibiha, D.D.; et al. Anti-Inflammatory Potential of Gandarusa (Gendarussa vulgaris Nees) and Soursoup (Annona muricata L) Extracts in LPS Stimulated-Macrophage Cell (RAW264.7). J. Nat. Remedies 2016, 16, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Choi, M.; Kang, Y.-R.; Zu, H.D.; Lim, I.-S.; Jung, S.K.; Chang, Y.H. Effects of Time on Phenolics and in vitro Bioactivity in Autoclave Extraction of Graviola (Annona muricata) Leaf. Biotechnol. Bioprocess. Eng. 2020, 25, 9–15. [Google Scholar] [CrossRef]
- Ishola, I.O.; Awodele, O.; Olusayero, A.M.; Ochieng, C.O. Mechanisms of Analgesic and Anti-Inflammatory Properties of Annona muricata Linn. (Annonaceae) Fruit Extract in Rodents. J. Med. Food 2014, 17, 1375–1382. [Google Scholar] [CrossRef] [Green Version]
- Umayra, A.N.; Qasim, M.J.; Al-Yasari, A.K.S.; Dakheel, M.H. Immunological Role of Annona muricata (Dietary Supplement of gravula) Against Cypermethrin’s Toxic Effects in Female Rats. Ann. Trop. Med. Public Health 2020, 23, 103–109. [Google Scholar] [CrossRef]
- Kim, G.-T.; Tran, N.K.S.; Choi, E.-H.; Song, Y.-J.; Song, J.-H.; Shim, S.-M.; Park, T.-S. Immunomodulatory efficacy of stand-ardized annona muricata (graviola) leaf extract via activation of mitogen-activated protein kinase pathways in raw 264.7 macrophages. Evid. Based Complementary Altern. Med. 2016, 2016, 2905127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baser, K.H.C. Biological and Pharmacological Activities of Carvacrol and Carvacrol Bearing Essential Oils. Curr. Pharm. Des. 2008, 14, 3106–3119. [Google Scholar] [CrossRef]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential oils of oregano: Biological activity be-yond their antimicrobial properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef] [Green Version]
- Nostro, A.; Papalia, T. Antimicrobial Activity of Carvacrol: Current Progress and Future Prospectives. Recent Pat. Anti-Infect. Drug Discov. 2012, 7, 28–35. [Google Scholar] [CrossRef]
- Grbović, F.; Stanković, M.S.; Ćurčić, M.; Đorđević, N.; Šeklić, D.; Topuzović, M.; Marković, S. In vitro cytotoxic activity of origanum vulgare l. On hct-116 and mda-mb-231 cell lines. Plants 2013, 2, 371–378. [Google Scholar]
- Grevsen, K.; Fretté, X.; Christensen, L.P. Content and composition of volatile terpenes, flavonoids and phenolic acids in greek oregano (origanum vulgare l. Ssp hirtum) at different development stages during cultivation in cool temperate climate. Eur. J. Horticult. Sci. 2009, 74, 193–203. [Google Scholar]
- Oregano | Diseases and Pests, Description, Uses, Propagation. 2021. Available online: https://plantvillage.psu.edu/topics/oregano/infos/diseases_and_pests_description_uses_propagation (accessed on 20 May 2021).
- Gutiérrez-Grijalva, E.P.; Picos-Salas, M.A.; Leyva-López, N.; Criollo-Mendoza, M.S.; Vazquez-Olivo, G.; Heredia, J.B. Fla-vonoids and phenolic acids from oregano: Occurrence, biological activity and health benefits. Plants 2017, 7, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elpiniki, S.; Alexandra, D.S.; Nicholaos, G.D. Ecology, cultivation and utilization of the aromatic greek oregano (origanum vulgare l.): A review. Not. Bot. Horti Agrobot. Cluj-Napoca 2019, 47, 545–552. [Google Scholar]
- Guarda, A.; Rubilar, J.F.; Miltz, J.; Galotto, M.J. The antimicrobial activity of microencapsulated thymol and carvacrol. Int. J. Food Microbiol. 2011, 146, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; Maria, D.M.C.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M.; et al. Carvacrol and human health: A comprehensive review. Phytother. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Chen, Y.; Fang, J.; Wang, Z.; Xie, C.; Hou, B.; Chen, W.; Xu, F. Solubility and solution thermodynamics of thymol in six pure organic solvents. J. Chem. Thermodyn. 2016, 92, 198–206. [Google Scholar] [CrossRef]
- Ruiz Reyes, E.; Moreira Castro, J. Secondary metabolites in medicinal plants to heal for gastrointestinal problems. A review of Ecuadorian ancestral medicine. Rev. Bases Cienc. 2017, 2, 1–16. [Google Scholar]
- Pozzatti, P.; Scheid, L.A.; Spader, T.B.; Atayde, M.L.; Santurio, J.M.; Alves, S.H. In vitro activity of essential oils extracted from plants used as spices against fluconazole-resistant and fluconazole-susceptible Candida spp. Can. J. Microbiol. 2008, 54, 950–956. [Google Scholar] [CrossRef]
- Food and Drug Administration Code of Federal Regulations. 2021. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=172.515&SearchTerm=thymol (accessed on 28 March 2021).
- Spyridopoulou, K.; Fitsiou, E.; Bouloukosta, E.; Tiptiri-Kourpeti, A.; Vamvakias, M.; Oreopoulou, A.; Papavassilopoulou, E.; Pappa, A.; Chlichlia, K. Extraction, Chemical Composition, and Anticancer Potential of Origanum onites L. Essential Oil. Molecules 2019, 24, 2612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coccimiglio, J.; Alipour, M.; Jiang, Z.-H.; Gottardo, C.; Suntres, Z. Antioxidant, Antibacterial, and Cytotoxic Activities of the Ethanolic Origanum vulgare Extract and Its Major Constituents. Oxidative Med. Cell. Longev. 2016, 2016, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llana-Ruiz-Cabello, M.; Gutiérrez-Praena, D.; Pichardo, S.; Moreno, F.J.; Bermúdez, J.M.; Aucejo, S.; Cameán, A.M. Cyto-toxicity and morphological effects induced by carvacrol and thymol on the human cell line caco-2. Food. Chem. Toxicol. 2014, 64, 281–290. [Google Scholar] [CrossRef]
- Potočnjak, I.; Gobin, I.; Domitrović, R. Carvacrol induces cytotoxicity in human cervical cancer cells but causes cisplatin re-sistance: Involvement of mek-erk activation. Phytother. Res. 2018, 32, 1090–1097. [Google Scholar] [CrossRef]
- Zeng, Q.; Che, Y.; Zhang, Y.; Chen, M.; Guo, Q.; Zhang, W.J.D.D. Thymol isolated from thymus vulgaris l. Inhibits colorectal cancer cell growth and metastasis by suppressing the wnt/β-catenin pathway. Drug Des. Dev. Ther. 2020, 14, 2535. [Google Scholar] [CrossRef]
- Khan, I.; Bahuguna, A.; Kumar, P.; Bajpai, V.K.; Kang, S.C. In vitro and in vivo antitumor potential of carvacrol nanoemulsion against human lung adenocarcinoma a549 cells via mitochondrial mediated apoptosis. Sci. Rep. 2018, 8, 144. [Google Scholar] [CrossRef] [Green Version]
- Günes-Bayir, A.; Kocyigit, A.; Güler, E.M.; Bilgin, M.G.; Ergün, İ.S.; Dadak, A. Effects of carvacrol on human fibroblast (ws-1) and gastric adenocarcinoma (ags) cells in vitro and on wistar rats in vivo. Mol. Cell. Biochem. 2018, 448, 237–249. [Google Scholar] [CrossRef]
- Gavaric, N.; Mozina, S.S.; Kladar, N.; Bozin, B. Chemical profile, antioxidant and antibacterial activity of thyme and oregano essential oils, thymol and carvacrol and their possible synergism. J. Essent. Oil Bear Plants 2015, 18, 1013–1021. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Gutiérrez-Praena, D.; Puerto, M.; Pichardo, S.; Jos, Á.; Cameán, A.M. In vitro pro-oxidant/antioxidant role of carvacrol, thymol and their mixture in the intestinal Caco-2 cell line. Toxicol. Vitr. 2015, 29, 647–656. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Salehi, B.; Schnitzler, P.; Ayatollahi, S.A.; Kobarfard, F.; Fathi, M.; Eisazadeh, M.; Sharifi-Rad, M. Susceptibil-ity of herpes simplex virus type 1 to monoterpenes thymol, carvacrol, p-cymene and essential oils of sinapis arvensis l., lallemantia royleana benth. and pulicaria vulgaris gaertn. Cell Mol. Biol. 2017, 63, 42–47. [Google Scholar] [CrossRef]
- Sánchez, G.; Aznar, R. Evaluation of Natural Compounds of Plant Origin for Inactivation of Enteric Viruses. Food Environ. Virol. 2015, 7, 183–187. [Google Scholar] [CrossRef]
- Zarrini, G.; Delgosha, Z.B.; Moghaddam, K.M.; Shahverdi, A.R. Post-antibacterial effect of thymol. Pharm. Biol. 2010, 48, 633–636. [Google Scholar] [CrossRef]
- Gniewosz, M.; Synowiec, A. Antibacterial activity of pullulan films containing thymol. Flavour Fragr. J. 2011, 26, 389–395. [Google Scholar] [CrossRef]
- Du, E.; Gan, L.; Li, Z.; Wang, W.; Liu, D.; Guo, Y. In vitro antibacterial activity of thymol and carvacrol and their effects on broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2015, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Lu, M.; Dai, T.; Murray, C.K.; Wu, M.X. Bactericidal Property of Oregano Oil against Multidrug-Resistant Clinical Isolates. Front. Microbiol. 2018, 9, 2329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magi, G.; Marini, E.; Facinelli, B. Antimicrobial activity of essential oils and carvacrol, and synergy of carvacrol and erythro-mycin, against clinical, erythromycin-resistant group a streptococci. Front. Microbiol. 2015, 6, 165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Numpaque, M.A.; Oviedo, L.A.; Gil, J.H.; García, C.M.; Durango, D.L. Thymol and carvacrol: Biotransformation and antifungal activity against the plant pathogenic fungi colletotrichum acutatum and botryodiplodia theobromae. Trop. Plant. Pathol. 2011, 36, 3–13. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Alfonso, C.O.; Martínez-Romero, D.; Zapata, P.J.; Serrano, M.; Valero, D.; Castillo, S. The effects of essential oils car-vacrol and thymol on growth of penicillium digitatum and p. Italicum involved in lemon decay. Int. J. Food Microbiol. 2012, 158, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadeh, S.; Sharifzadeh, A.; Shokri, H.; Khosravi, A.R.; Abbaszadeh, A. Antifungal efficacy of thymol, carvacrol, eugenol and menthol as alternative agents to control the growth of food-relevant fungi. J. Mycol. Med. 2014, 24, e51–e56. [Google Scholar] [CrossRef] [PubMed]
- Suwanamornlert, P.; Sangchote, S.; Chinsirikul, W.; Sane, A.; Chonhenchob, V. Antifungal activity of plant-derived compounds and their synergism against major postharvest pathogens of longan fruit in vitro. Int. J. Food Microbiol. 2018, 271, 8–14. [Google Scholar] [CrossRef]
- Ochoa-Velasco, C.E.; Navarro-Cruz, A.R.; Vera-López, O.; Palou, E.; Avila-Sosa, R. Growth modeling to control (in vitro) Fusarium verticillioides and Rhizopus stolonifer with thymol and carvacrol. Revista Argentina de Microbiología 2018, 50, 70–74. [Google Scholar] [CrossRef]
- Shu, C.; Sun, L.; Zhang, W. Thymol has antifungal activity against candida albicans during infection and maintains the innate immune response required for function of the p38 mapk signaling pathway in caenorhabditis elegans. Immunol. Res. 2016, 64, 1013–1024. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Parker, T.L. Anti-inflammatory, tissue remodeling, immunomodulatory, and anticancer activities of oregano (origanum vulgare) essential oil in a human skin disease model. Biochim. Open. 2017, 4, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Jiang, K.; Yin, N.; Ma, X.; Zhao, G.; Qiu, C.; Deng, G. Thymol mitigates lipopolysaccharide-induced endometritis by regulating the tlr4- and ros-mediated nf-κb signaling pathways. Oncotarget 2017, 8, 20042–20055. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Li, F.; Fu, Y.; Cao, Y.; Song, X.; Wang, T.; Wang, W.; Guo, M.; Zhou, E.; Li, D.; et al. Thymol inhibits LPS-stimulated inflammatory response via down-regulation of NF-kB and MAPK signaling pathways in mouse mammary epithelial cells. Inflammation 2014, 37, 214–222. [Google Scholar] [CrossRef]
- de Santis, F.; Poerio, N.; Gismondi, A.; Nanni, V.; di Marco, G.; Nisini, R.; Thaller, M.C.; Canini, A.; Fraziano, M. Hydroal-coholic extract from origanum vulgare induces a combined anti-mycobacterial and anti-inflammatory response in innate immune cells. PLoS ONE 2019, 14, e0213150. [Google Scholar] [CrossRef] [Green Version]
- Gholijani, N.; Amirghofran, Z. Effects of thymol and carvacrol on T-helper cell subset cytokines and their main transcription factors in ovalbumin-immunized mice. J. Immunotoxicol. 2016, 13, 729–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schönknecht, K.; Krauss, H.; Jambor, J.; Fal, A. Treatment of cough in respiratory tract infections-the effect of combining the natural active compounds with thymol. Wiadomości Lekarskie 2017, 69, 791–798. [Google Scholar]
- Ris, M.M.; Deitrich, R.A.; Von Wartburg, J.-P. Inhibition of aldehyde reductase isoenzymes in human and rat brain. Biochem. Pharmacol. 1975, 24, 1865–1869. [Google Scholar] [CrossRef]
- Tinworth, C.P.; Young, R.J. Facts, Patterns, and Principles in Drug Discovery: Appraising the Rule of 5 with Measured Physicochemical Data. J. Med. Chem. 2020, 63, 10091–10108. [Google Scholar] [CrossRef]
- Pollastri, M. Overview on the Rule of Five. Curr. Protoc. Pharmacol. 2010, 49, 9.12.1–9.12.8. [Google Scholar] [CrossRef] [PubMed]
- Benet, L.Z.; Hosey, C.M.; Ursu, O.; Oprea, T. BDDCS, the Rule of 5 and drugability. Adv. Drug Deliv. Rev. 2016, 101, 89–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Phytochemical Compound Name | Empirical Formula/Structure | MW (Da) | HBA/HBD/RB | Log P | Log D | A (cm3) | PSA (Å2) | GI Absorption/L-RO5, GF, and VR Violations |
|---|---|---|---|---|---|---|---|---|
| Turmeric | ||||||||
| Curcumin |  | 368.4 | 6/3/8 | 2.9 | 2.6 | 106 | 93.1 | High/0 |
| Demethoxycurcumin |  | 338.3 | 5/2/7 | 3.2 | 2.6 | 97 | 83.8 | High/0 |
| Bisdemethoxycurcumin |  | 308.3 | 4/2/6 | 3.4 | 2.8 | 91 | 74.6 | High/0 |
| α-Turmerone |  | 218.3 | 1/0/4 | 4.4 | 4.1 | 69 | 17 | High/0 |
| Garlic | ||||||||
| Alliin |  | 177.2 | 4/3/5 | −0.5 | −3.3 | 44 | 99.6 | High/0 Negative LogD |
| Allicin |  | 162.3 | 1/0/5 | 1.2 | 1.4 | 46 | 61.6 | Low/ TNA < 20 |
| Diallylsulfide |  | 114.2 | 0/0/4 | 2.6 | 2.9 | 37 | 25.3 | Low/ TNA < 20 MW < 160 A < 40 |
| Z-Ajoene |  | 234.4 | 1/0/8 | 3.1 | 2.8 | 68 | 86.9 | High/0 |
| 2-Vinyl-4H-1,3-dithiin |  | 144.3 | 0/0/1 | 2.2 | 2.7 | 45 | 50.6 | Low/ TNA < 20 MW < 160 |
| Cinnamon | ||||||||
| (E)-Trans-Cinnamaldehyde |  | 132.2 | 1/0/2 | 2.1 | 1.8 | 42 | 17.1 | Low/ TNA < 20 MW < 160 |
| (E)-Cinnamyl Acetate |  | 176.2 | 2/0/4 | 2.6 | 2.6 | 53 | 26.3 | High/0 |
| Eugenol |  | 164.2 | 2/1/3 | 2.2 | 2.5 | 49 | 29.5 | High/0 |
| Cuminaldehyde |  | 148.2 | 1/0/2 | 3.0 | 3.1 | 47 | 17.1 | Low/ MW < 160 |
| Protocatechuic Acid |  | 154.1 | 4/3/1 | 1.2 | −1.9 | 37 | 77.8 | Low/TNA < 20 MW < 160 A < 40 Negative LogD |
| Graviola | ||||||||
| Benzylisoquinoline |  | 219.3 | 1/0/2 | 4.0 | 4.3 | 72 | 12.9 | High/0 |
| Annonacin/Acetogenin |  | 596.9 | 7/4/26 | 6.4 | 7.3 | 169 | 116 | Low/ TNA > 70 MW > 500 RB > 10 LogP > 5.6 A > 130 High LogD |
| Cinnamic Acid |  | 148.2 | 2/1/2 | 2.4 | −0.7 | 44 | 37.3 | Low/ TNA < 20 MW < 160 Negative LogD |
| Coumaric Acid |  | 164.2 | 3/2/2 | 2.4 | −1.4 | 46 | 57.5 | High/0 Negative LogD |
| Caffeic Acid |  | 180.2 | 4/3/2 | 1.4 | −1.7 | 48 | 77.8 | High/0 Negative LogD |
| Rutin |  | 610.5 | 16/10/6 | 1.8 | −1.8 | 138 | 266 | Low/ TNA > 70 MW > 500 HBA > 10 HBD > 5 A > 130 PSA > 140 Negative LogD |
| Oregano | ||||||||
| Carvacrol |  | 150.2 | 1/1/1 | 3.3 | 3.1 | 47 | 20.2 | Low/ MW < 160 |
| Thymol |  | 150.2 | 1/1/1 | 3.3 | 3.1 | 47 | 20.2 | Low/ MW < 160 |
| O-Cymene |  | 134.2 | 0/0/1 | 4.0 | 4.1 | 45 | 0 | Low/ MW < 160 |
| Apigenin |  | 270.2 | 5/3/1 | 2.1 | 1.3 | 70 | 87 | High/0 |
| Luteolin |  | 286.2 | 6/4/1 | 2.4 | 1.1 | 72 | 107 | High/0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado, Y.; Cassé, C.; Ferrer-Acosta, Y.; Suárez-Arroyo, I.J.; Rodríguez-Zayas, J.; Torres, A.; Torres-Martínez, Z.; Pérez, D.; González, M.J.; Velázquez-Aponte, R.A.; et al. Biomedical Effects of the Phytonutrients Turmeric, Garlic, Cinnamon, Graviola, and Oregano: A Comprehensive Review. Appl. Sci. 2021, 11, 8477. https://doi.org/10.3390/app11188477
Delgado Y, Cassé C, Ferrer-Acosta Y, Suárez-Arroyo IJ, Rodríguez-Zayas J, Torres A, Torres-Martínez Z, Pérez D, González MJ, Velázquez-Aponte RA, et al. Biomedical Effects of the Phytonutrients Turmeric, Garlic, Cinnamon, Graviola, and Oregano: A Comprehensive Review. Applied Sciences. 2021; 11(18):8477. https://doi.org/10.3390/app11188477
Chicago/Turabian StyleDelgado, Yamixa, Céline Cassé, Yancy Ferrer-Acosta, Ivette J. Suárez-Arroyo, José Rodríguez-Zayas, Anamaris Torres, Zally Torres-Martínez, Daraishka Pérez, Michael J. González, Ricardo A. Velázquez-Aponte, and et al. 2021. "Biomedical Effects of the Phytonutrients Turmeric, Garlic, Cinnamon, Graviola, and Oregano: A Comprehensive Review" Applied Sciences 11, no. 18: 8477. https://doi.org/10.3390/app11188477
APA StyleDelgado, Y., Cassé, C., Ferrer-Acosta, Y., Suárez-Arroyo, I. J., Rodríguez-Zayas, J., Torres, A., Torres-Martínez, Z., Pérez, D., González, M. J., Velázquez-Aponte, R. A., Andino, J., Correa-Rodríguez, C., Franco, J. C., Milán, W., Rosario, G., Velázquez, E., Vega, J., Colón, J., & Batista, C. (2021). Biomedical Effects of the Phytonutrients Turmeric, Garlic, Cinnamon, Graviola, and Oregano: A Comprehensive Review. Applied Sciences, 11(18), 8477. https://doi.org/10.3390/app11188477