Investigation of Thermal Effects of Radiofrequency Ablation Mediated with Iron Oxide Nanoparticles Dispersed in Agarose and Chitosan Solvents
Abstract
:1. Introduction
2. Materials and Methods
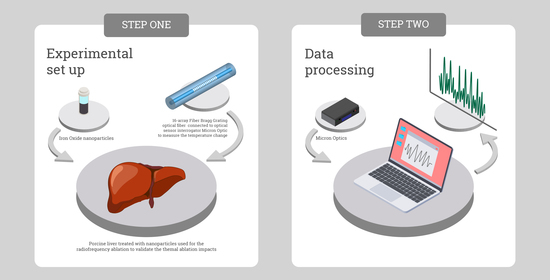
2.1. Experimental Setup
2.2. FBG Array Fiber Fabrication, Calibration, and Characterization
2.3. Iron Oxide Magnetic Nanoparticles Synthesis and Characterization
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- IARC. Latest Global Cancer Data; International Agency for Research on Cancer: Lyon, France, 2018. [Google Scholar]
- Dutz, S.; Hergt, R. Magnetic particle hyperthermia—A promising tumour therapy? Nanotechnology 2014, 25, 452001. [Google Scholar] [CrossRef]
- Schena, E.; Tosi, D.; Saccomandi, P.; Lewis, E.; Kim, T. Fiber Optic Sensors for Temperature Monitoring during Thermal Treatments: An Overview. Sensors 2016, 16, 1144. [Google Scholar] [CrossRef] [PubMed]
- Macchi, E.G.; Tosi, D.; Braschi, G.; Gallati, M.; Cigada, A.; Busca, G.; Lewis, E. Optical fiber sensors-based temperature distribution measurement inex vivoradiofrequency ablation with submillimeter resolution. J. Biomed. Opt. 2014, 19, 117004. [Google Scholar] [CrossRef]
- Goldberg, S. Radiofrequency tumor ablation: Principles and techniques. Eur. J. Ultrasound 2001, 13, 129–147. [Google Scholar] [CrossRef]
- Lencioni, R.; Crocetti, L.; Cioni, D.; Della Pina, C.; Bartolozzi, C. Percutaneous Radiofrequency Ablation of Hepatic Colorectal Metastases Technique, Indications, Results, and New Promises. Investig. Radiol. 2004, 39, 689–697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamuta, M.; Kohjima, M.; Morizono, S.; Yoshimoto, T.; Miyagi, Y.; Sakai, H.; Enjoji, M.; Kotoh, K. Comparison of tissue pressure and ablation time between the LeVeen and cool-tip needle methods. Comp. Hepatol. 2006, 5, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashikbayeva, Z.; Tosi, D.; Balmassov, D.; Schena, E.; Saccomandi, P.; Inglezakis, V. Application of Nanoparticles and Nanomaterials in Thermal Ablation Therapy of Cancer. Nanomaterials 2019, 9, 1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapareto, S.A.; Dewey, W.C. Thermal dose determination in cancer therapy. Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 787–800. [Google Scholar] [CrossRef]
- Palumbo, G.; Iadicicco, A.; Tosi, D.; Verze, P.; Carlomagno, N.; Tammaro, V.; Ippolito, J.; Campopiano, S. Temperature profile of ex-vivo organs during radio frequency thermal ablation by fiber Bragg gratings. J. Biomed. Opt. 2016, 21, 117003. [Google Scholar] [CrossRef] [Green Version]
- Beik, J.; Abed, Z.; Ghoreishi, F.S.; Hosseini-Nami, S.; Mehrzadi, S.; Shakeri-Zadeh, A.; Kamrava, S.K. Nanotechnology in hyperthermia cancer therapy: From fundamental principles to advanced applications. J. Control. Release 2016, 235, 205–221. [Google Scholar] [CrossRef]
- Suriyanto, N.; Ng, E.Y.K.; Kumar, S.D. Physical mechanism and modeling of heat generation and transfer in magnetic fluid hyperthermia through Néelian and Brownian relaxation: A review. Biomed. Eng. Online 2017, 16, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Jimeno, S.; Ortega-Palacios, R.; Cepeda-Rubio, M.; Vera, A.; Leija, L.; Estelrich, J. Improved thermal ablation efficacy using magnetic nanoparticles: A study in tumor phantoms. Prog. Electromagn. Res. 2012, 128, 229–248. [Google Scholar] [CrossRef] [Green Version]
- Hergt, R.; Andra, W.; D’Ambly, C.; Hilger, I.; Kaiser, W.; Richter, U.; Schmidt, H.-G. Physical limits of hyperthermia using magnetite fine particles. IEEE Trans. Magn. 1998, 34, 3745–3754. [Google Scholar] [CrossRef]
- Dennis, C.L.; Ivkov, R. Physics of heat generation using magnetic nanoparticles for hyperthermia. Int. J. Hyperth. 2013, 29, 715–729. [Google Scholar] [CrossRef]
- Amiri, S.; Mehrnia, M.R.; Sobhanifard, S.; Roudsari, F.P.; Hoseini, S.-N. Evaluation of agarose-entrapped magnetic nanoparticles influence on protein adsorption isotherm and kinetics using nickel-iminodiacetic acid ligand. Sep. Purif. Technol. 2017, 188, 423–430. [Google Scholar] [CrossRef]
- Ma, X.; Xia, Y.; Ni, L.; Song, L.; Wang, Z. Preparation of gold nanoparticles–agarose gel composite and its application in SERS detection. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2014, 121, 657–661. [Google Scholar] [CrossRef]
- Singh, M.K.; Prajapati, S.K.; Mahor, A.; Rajput, N.; Singh, R. Chitosan: A Novel Excipient in Pharmaceutical Formulation: A Review. Int. J. Pharm. Sci. Res. 2011, 2, 2266–2277. [Google Scholar]
- Singla, A.K.; Chawla, M. Chitosan: Some pharmaceutical and biological aspects—An update. J. Pharm. Pharmacol. 2001, 53, 1047–1067. [Google Scholar] [CrossRef] [PubMed]
- Tosi, D. Review and Analysis of Peak Tracking Techniques for Fiber Bragg Grating Sensors. Sensors 2017, 17, 2368. [Google Scholar] [CrossRef]
- Dostovalov, A.; Wolf, A.; Parygin, A.; Zyubin, V.; Babin, S. Femtosecond point-by-point inscription of Bragg gratings by drawing a coated fiber through ferrule. Opt. Express 2016, 24, 16232–16237. [Google Scholar] [CrossRef]
- Bernier, M.; Trépanier, F.; Carrier, J.; Vallée, R. High mechanical strength fiber Bragg gratings made with infrared femtosecond pulses and a phase mask. Opt. Lett. 2014, 39, 3646–3649. [Google Scholar] [CrossRef]
- Williams, R.J.; Voigtländer, C.; Marshall, G.D.; Tünnermann, A.; Nolte, S.; Steel, M.J.; Withford, M.J. Point-by-point inscription of apodized fiber Bragg gratings. Opt. Lett. 2011, 36, 2988–2990. [Google Scholar] [CrossRef] [Green Version]
- Wolf, A.; Dostovalov, A.; Bronnikov, K.; Babin, S. Arrays of fiber Bragg gratings selectively inscribed in different cores of 7-core spun optical fiber by IR femtosecond laser pulses. Opt. Express 2019, 27, 13978–13990. [Google Scholar] [CrossRef]
- Ozel, F.; Kockar, H.; Karaagac, O. Growth of Iron Oxide Nanoparticles by Hydrothermal Process: Effect of Reaction Parameters on the Nanoparticle Size. J. Supercond. Nov. Magn. 2015, 28, 823–829. [Google Scholar] [CrossRef]
- Wang, W.; Zheng, L.; Lu, F.; Hong, R.; Chen, M.Z.Q.; Zhuang, L. Facile synthesis and characterization of magnetochromatic Fe3O4 nanoparticles. AIP Adv. 2017, 7, 056317. [Google Scholar] [CrossRef] [Green Version]
- Rosensweig, R. Heating magnetic fluid with alternating magnetic field. J. Magn. Magn. Mater. 2002, 252, 370–374. [Google Scholar] [CrossRef]
- Day, E.S.; Morton, J.G.; West, J.L. Nanoparticles for Thermal Cancer Therapy. J. Biomech. Eng. 2009, 131, 074001. [Google Scholar] [CrossRef]












| Concentration of IONP (mg/mL) | Time to Reach 60 °C (s) | Time to Reach 100 °C (s) | Maximum Temperature (°C) | |||
|---|---|---|---|---|---|---|
| AGAROSE Solvent | CHITOSAN Solvent | AGAROSE Solvent | CHITOSAN Solvent | AGAROSE Solvent | CHITOSAN Solvent | |
| 0 | 5.58 | 6.48 | 14.94 | 16.53 | 133.03 | 136.11 |
| 1 | 6.09 | 7.11 | 17.15 | 18.21 | 130.60 | 143.27 |
| 2 | 4.53 | 7.77 | 13.14 | 19.44 | 139.36 | 137.67 |
| 3 | 7.08 | 6.48 | 16.53 | 19.17 | 124.98 | 130.94 |
| 4 | 7.44 | 7.38 | 22.14 | 18.90 | 122.33 | 129.80 |
| 5 | 5.37 | 6.96 | 15.75 | 18.96 | 141.88 | 135.27 |
| 6 | 5.52 | 6.75 | 15.18 | 19.08 | 138.60 | 137.99 |
| 7 | 5.61 | 6.66 | 14.73 | 21.78 | 136.50 | 133.02 |
| 8 | 5.76 | 7.50 | 16.38 | 19.80 | 130.16 | 149.56 |
| 9 | 5.61 | 7.11 | 16.05 | 18.24 | 126.62 | 135.89 |
| 10 | 5.19 | 7.26 | 13.56 | 19.86 | 134.99 | 137.19 |
| IONP Concentration mg/mL | TMAX, °C | t60, s | d60, cm | d42, cm | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Agarose | Chitosan | Agarose | Chitosan | Agarose | Chitosan | Agarose | Chitosan | |||||||||
| Mean Value | Standard Deviation | Mean Value | Standard Deviation | Mean Value | Standard Deviation | Mean Value | Standard Deviation | Mean Value | Standard Deviation | Mean Value | Standard Deviation | Mean Value | Standard Deviation | Mean Value | Standard Deviation | |
| 0 | 133.03 | 17.09 | 136.11 | 22.94 | 5.58 | 1.96 | 6.48 | 1.14 | 2.38 | 0.42 | 2.09 | 0.34 | 2.85 | 0.43 | 2.58 | 0.46 |
| 1 | 130.60 | 14.82 | 143.27 | 17.89 | 6.09 | 1.37 | 7.11 | 1.83 | 2.42 | 0.35 | 2.22 | 0.53 | 3.12 | 0.62 | 2.72 | 0.65 |
| 2 | 139.36 | 21.46 | 137.67 | 24.64 | 4.53 | 1.20 | 7.77 | 1.40 | 2.44 | 0.53 | 2.25 | 0.34 | 2.95 | 0.57 | 2.68 | 0.34 |
| 3 | 124.98 | 8.63 | 130.94 | 19.55 | 7.08 | 3.32 | 6.48 | 1.08 | 2.32 | 0.39 | 2.40 | 0.44 | 2.81 | 0.39 | 2.85 | 0.50 |
| 4 | 122.33 | 11.93 | 129.80 | 15.17 | 7.44 | 2.96 | 7.38 | 1.71 | 2.19 | 0.46 | 2.11 | 0.24 | 2.69 | 0.44 | 2.56 | 0.30 |
| 5 | 141.88 | 22.84 | 135.27 | 17.81 | 5.37 | 1.76 | 6.96 | 1.78 | 2.27 | 0.36 | 2.26 | 0.38 | 2.70 | 0.31 | 2.74 | 0.41 |
| 6 | 138.60 | 16.84 | 137.99 | 20.76 | 5.52 | 1.32 | 6.75 | 1.45 | 2.23 | 0.31 | 2.48 | 0.74 | 2.67 | 0.31 | 2.97 | 0.79 |
| 7 | 136.50 | 20.72 | 133.02 | 17.75 | 5.61 | 1.74 | 6.66 | 1.28 | 2.14 | 0.19 | 2.26 | 0.43 | 2.61 | 0.20 | 2.75 | 0.44 |
| 8 | 130.16 | 20.43 | 149.56 | 18.99 | 5.76 | 0.93 | 7.50 | 1.39 | 2.40 | 0.43 | 2.32 | 0.26 | 2.98 | 0.52 | 2.79 | 0.30 |
| 9 | 126.62 | 13.87 | 135.89 | 17.46 | 5.61 | 2.24 | 7.11 | 2.15 | 2.63 | 1.43 | 2.22 | 0.47 | 3.05 | 1.36 | 2.80 | 0.57 |
| 10 | 134.99 | 20.56 | 137.19 | 19.94 | 5.19 | 1.88 | 7.26 | 1.39 | 2.79 | 1.58 | 2.35 | 0.79 | 3.21 | 1.55 | 2.83 | 0.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashikbayeva, Z.; Aitkulov, A.; Wolf, A.; Dostovalov, A.; Amantayeva, A.; Kurbanova, A.; Inglezakis, V.J.; Tosi, D. Investigation of Thermal Effects of Radiofrequency Ablation Mediated with Iron Oxide Nanoparticles Dispersed in Agarose and Chitosan Solvents. Appl. Sci. 2021, 11, 2437. https://doi.org/10.3390/app11052437
Ashikbayeva Z, Aitkulov A, Wolf A, Dostovalov A, Amantayeva A, Kurbanova A, Inglezakis VJ, Tosi D. Investigation of Thermal Effects of Radiofrequency Ablation Mediated with Iron Oxide Nanoparticles Dispersed in Agarose and Chitosan Solvents. Applied Sciences. 2021; 11(5):2437. https://doi.org/10.3390/app11052437
Chicago/Turabian StyleAshikbayeva, Zhannat, Arman Aitkulov, Alexey Wolf, Alexander Dostovalov, Aida Amantayeva, Aliya Kurbanova, Vassilis J. Inglezakis, and Daniele Tosi. 2021. "Investigation of Thermal Effects of Radiofrequency Ablation Mediated with Iron Oxide Nanoparticles Dispersed in Agarose and Chitosan Solvents" Applied Sciences 11, no. 5: 2437. https://doi.org/10.3390/app11052437
APA StyleAshikbayeva, Z., Aitkulov, A., Wolf, A., Dostovalov, A., Amantayeva, A., Kurbanova, A., Inglezakis, V. J., & Tosi, D. (2021). Investigation of Thermal Effects of Radiofrequency Ablation Mediated with Iron Oxide Nanoparticles Dispersed in Agarose and Chitosan Solvents. Applied Sciences, 11(5), 2437. https://doi.org/10.3390/app11052437