Effect of Time and Temperature on Physicochemical and Microbiological Properties of Sous Vide Chicken Breast Fillets
Abstract
:1. Introduction
2. Materials and Methods
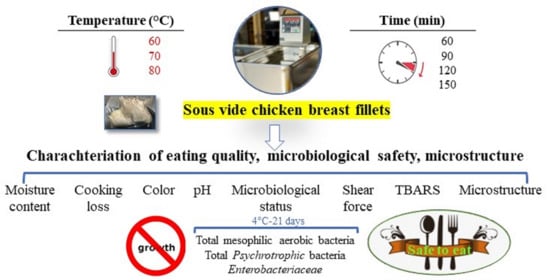
2.1. Experimental Design
2.2. Moisture Content and Cooking Loss
2.3. pH
2.4. Color
2.5. Warner-Bratzler Shear Force (WBSF)
2.6. Thiobarbituric Acid Reactive Substances (TBARS)
2.7. Microbiological Analyses
2.8. Statistical Analysis
3. Results and Discussion
3.1. Moisture Content, Cooking Loss, and pH
3.2. Color
3.3. Lipid Oxidation
3.4. Warner-Bratzler Shear Force (WBSF)
3.5. Principal Component Analysis (PCA) Analysis
3.6. Microbiology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Biesalski, H.-K. Meat as a component of a healthy diet—Are there any risks or benefits if meat is avoided in the diet? Meat Sci. 2005, 70, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Czarnowska-Kujawska, M.; Draszanowska, A.; Gujska, E. Effect of Different Cooking Methods on Folate Content in Chicken Liver. Foods 2020, 9, 1431. [Google Scholar] [CrossRef] [PubMed]
- Davey, C.L.; Gilbert, K.V. Temperature-dependent cooking toughness in beef. J. Sci. Food Agric. 1974, 25, 931–938. [Google Scholar] [CrossRef]
- Gök, V.; Uzun, T.; Tomar, O.; Çağlar, M.Y.; Çağlar, A. The effect of cooking methods on some quality characteristics of gluteus medius. Food Sci. Technol. 2019, 39, 999–1004. [Google Scholar] [CrossRef] [Green Version]
- Nithyalakshmi, V.; Preetha, R. Effect of cooking conditions on physico-chemical and textural properties of emu (Dromaius novaehollandiae) meat. Int. Food Res. J. 2015, 22, 1924–1930. [Google Scholar]
- Tornberg, E. Effects of heat on meat proteins—Implications on structure and quality of meat products. Meat Sci. 2005, 70, 493–508. [Google Scholar] [CrossRef]
- Roldán, M.; Antequera, T.; Martín, A.; Mayoral, A.I.; Ruiz, J. Effect of different temperature–time combinations on physicochemical, microbiological, textural and structural features of sous-vide cooked lamb loins. Meat Sci. 2013, 93, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Hernandez, E.; Salaseviciene, A.; Ertbjerg, P. Low-temperature long-time cooking of meat: Eating quality and underlying mechanisms. Meat Sci. 2018, 143, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, D.E. Sous vide cooking: A review. Int. J. Gastron. Food Sci. 2012, 1, 15–30. [Google Scholar] [CrossRef] [Green Version]
- Zavadlav, S.; Blažić, M.; Van De Velde, F.; Vignatti, C.; Fenoglio, C.; Piagentini, A.M.; Pirovani, M.E.; Perotti, C.M.; Kovačević, D.B.; Putnik, P. Sous-Vide as a Technique for Preparing Healthy and High-Quality Vegetable and Seafood Products. Foods 2020, 9, 1537. [Google Scholar] [CrossRef]
- Albistur, A.R.-; Gámbaro, A. Consumer perception of a non-traditional market on sous-vide dishes. Int. J. Gastron. Food Sci. 2018, 11, 20–24. [Google Scholar] [CrossRef]
- Christensen, L.; Gunvig, A.; Tørngren, M.A.; Aaslyng, M.D.; Knøchel, S.; Christensen, M. Sensory characteristics of meat cooked for prolonged times at low temperature. Meat Sci. 2012, 90, 485–489. [Google Scholar] [CrossRef]
- Cho, D.K.; Lee, B.; Oh, H.; Lee, J.S.; Kim, Y.S.; Choi, Y.M. Effect of Searing Process on Quality Characteristics and Storage Stability of Sous-Vide Cooked Pork Patties. Foods 2020, 9, 1011. [Google Scholar] [CrossRef]
- Rondanelli, M.; Daglia, M.; Meneghini, S.; Di Lorenzo, A.; Peroni, G.; Faliva, M.A.; Perna, S. Nutritional advantages of sous-vide cooking compared to boiling on cereals and legumes: Determination of ashes and metals content in ready-to-eat products. Food Sci. Nutr. 2017, 5, 827–833. [Google Scholar] [CrossRef] [Green Version]
- Schellekens, M. New research issues in sous-vide cooking. Trends Food Sci. Technol. 1996, 7, 256–262. [Google Scholar] [CrossRef]
- Jeong, S.-H.; Kim, E.-C.; Lee, D.-U. The Impact of a Consecutive Process of Pulsed Electric Field, Sous-Vide Cooking, and Reheating on the Properties of Beef Semitendinosus Muscle. Foods 2020, 9, 1674. [Google Scholar] [CrossRef]
- Hong, G.-E.; Kim, J.-H.; Ahn, S.-J.; Lee, C.-H. Changes in Meat Quality Characteristics of the Sous-vide Cooked Chicken Breast during Refrigerated Storage. Food Sci. Anim. Resour. 2015, 35, 757–764. [Google Scholar] [CrossRef] [Green Version]
- Rizzo, V.; Amoroso, L.; Licciardello, F.; Mazzaglia, A.; Muratore, G.; Restuccia, C.; Lombardo, S.; Pandino, G.; Strano, M.G.; Mauromicale, G. The effect of sous vide packaging with rosemary essential oil on storage quality of fresh-cut potato. LWT 2018, 94, 111–118. [Google Scholar] [CrossRef]
- Głuchowski, A.; Czarniecka-Skubina, E.; Wasiak-Zys, G.; Nowak, D.; Skubina, C.-; Zys, W. Effect of Various Cooking Methods on Technological and Sensory Quality of Atlantic Salmon (Salmo salar). Foods 2019, 8, 323. [Google Scholar] [CrossRef] [Green Version]
- Becker, A.; Boulaaba, A.; Pingen, S.; Krischek, C.; Klein, G. Low temperature cooking of pork meat—Physicochemical and sensory aspects. Meat Sci. 2016, 118, 82–88. [Google Scholar] [CrossRef]
- Christensen, L.; Ertbjerg, P.; Aaslyng, M.D.; Christensen, M. Effect of prolonged heat treatment from 48 °C to 63°C on toughness, cooking loss and color of pork. Meat Sci. 2011, 88, 280–285. [Google Scholar] [CrossRef]
- Jeong, K.; Hyeonbin, O.; Shin, S.Y.; Kim, Y.-S. Effects of sous-vide method at different temperatures, times and vacuum degrees on the quality, structural, and microbiological properties of pork ham. Meat Sci. 2018, 143, 1–7. [Google Scholar] [CrossRef] [PubMed]
- del Pulgar, J.S.; Gázquez, A.; Ruiz-Carrascal, J. Physico-chemical, textural and structural characteristics of sous-vide cooked pork cheeks as affected by vacuum, cooking temperature, and cooking time. Meat Sci. 2012, 90, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Botinestean, C.; Keenan, D.F.; Kerry, J.P.; Hamill, R.M. The effect of thermal treatments including sous-vide, blast freezing and their combinations on beef tenderness of M. semitendinosus steaks targeted at elderly consumers. LWT 2016, 74, 154–159. [Google Scholar] [CrossRef]
- Gómez, I.; Ibañez, F.C.; Beriain, M.J. Physicochemical and sensory properties of sous vide meat and meat analog products marinated and cooked at different temperature-time combinations. Int. J. Food Prop. 2019, 22, 1693–1708. [Google Scholar] [CrossRef] [Green Version]
- Bıyıklı, M.; Akoğlu, A.; Kurhan, Ş.; Akoğlu, İ.T. Effect of different Sous Vide cooking temperature-time combinations on the physicochemical, microbiological, and sensory properties of turkey cutlet. Int. J. Gastron. Food Sci. 2020, 20, 100204. [Google Scholar] [CrossRef]
- Akoğlu, I.; Bıyıklı, M.; Akoglu, A.; Kurhan, Ş.; Biyikli, M. Determination of the Quality and Shelf Life of Sous Vide Cooked Turkey Cutlet Stored at 4 and 12 °C. Braz. J. Poult. Sci. 2018, 20, 1–8. [Google Scholar] [CrossRef]
- Naveena, B.M.; Khansole, P.S.; Kumar, M.S.; Krishnaiah, N.; Kulkarni, V.V.; Deepak, S.J. Effect of sous vide processing on physicochemical, ultrastructural, microbial and sensory changes in vacuum packaged chicken sausages. Food Sci. Technol. Int. 2016, 23, 75–85. [Google Scholar] [CrossRef]
- Park, C.; Lee, B.; Oh, E.; Kim, Y.; Choi, Y. Combined effects of sous-vide cooking conditions on meat and sensory quality characteristics of chicken breast meat. Poult. Sci. 2020, 99, 3286–3291. [Google Scholar] [CrossRef]
- Karpińska-Tymoszczyk, M.; Draszanowska, A.; Danowska-Oziewicz, M.; Kurp, L. The effect of low-temperature thermal processing on the quality of chicken breast fillets. Food Sci. Technol. Int. 2020, 26, 563–573. [Google Scholar] [CrossRef]
- Can, Ö.; Harun, F. Shelf Life of Chicken Meat Balls Submitted to Sous Vide Treatment. Braz. J. Poult. Sci. 2015, 17, 137–144. [Google Scholar] [CrossRef] [Green Version]
- AOAC. AOAC Official Methods of Analysis, 18th ed.; AOAC International: Rockville, MD, USA, 2005; ISBN 0935584870. [Google Scholar]
- Da Silva-Buzanello, R.A.; Schuch, A.F.; Gasparin, A.W.; Torquato, A.S.; Scremin, F.R.; Canan, C.; Soares, A.L. Quality parameters of chicken breast meat affected by carcass scalding conditions. Asian-Australas. J. Anim. Sci. 2019, 32, 1186–1194. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, H.; Gullo, M.; La China, S.; Pfeifer, F.; Siesler, H.W.; Licciardello, F.; Pulvirenti, A. Characterization of bio-nanocomposite films based on gelatin/polyvinyl alcohol blend reinforced with bacterial cellulose nanowhiskers for food packaging applications. Food Hydrocoll. 2021, 113, 106454. [Google Scholar] [CrossRef]
- Honikel, K.O. Reference methods for the assessment of physical characteristics of meat. Meat Sci. 1998, 49, 447–457. [Google Scholar] [CrossRef]
- Siu, G.M.; Draper, H.H. A Survey of the Malonaldehyde Content of Retail Meats and Fish. J. Food Sci. 1978, 43, 1147–1149. [Google Scholar] [CrossRef]
- ISO. ISO 4833-1 Microbiology of the Food Chain—Horizontal Method for the Enumeration of Microorganisms, Part 1, Count at 30 °C by the Pour Plate Technique; ISO: Geneva, Switzerland, 2013; p. 9. [Google Scholar]
- ISO. ISO 17410 Microbiology of the Food Chain—Horizontal Method for the Enumeration of Psychrotrophic Microorganisms; ISO: Geneva, Switzerland, 2019; p. 10. [Google Scholar]
- ISO. ISO 21528-1 Microbiology of the Food Chain—Horizontal Method for the Detection and Enumeration of Enterobacteriaceae—Part 1: Detection of Enterobacteriaceae; ISO: Geneva, Switzerland, 2017; p. 17. [Google Scholar]
- Brambila, G.S.; Chatterjee, D.; Bowker, B.; Zhuang, H. Descriptive texture analyses of cooked patties made of chicken breast with the woody breast condition. Poult. Sci. 2017, 96, 3489–3494. [Google Scholar] [CrossRef] [PubMed]
- Murphy, R.Y.; Marks, B.P. Effect of meat temperature on proteins, texture, and cook loss for ground chicken breast patties. Poult. Sci. 2000, 79, 99–104. [Google Scholar] [CrossRef]
- Murphy, R.Y.; Johnson, E.R.; Duncan, L.K.; Clausen, E.C.; Davis, M.D.; March, J.A. Heat Transfer Properties, Moisture Loss, Product Yield, and Soluble Proteins in Chicken Breast Patties During Air Convection Cooking. Poult. Sci. 2001, 80, 508–514. [Google Scholar] [CrossRef]
- Ismail, I.; Hwang, Y.-H.; Bakhsh, A.; Joo, S.-T. The alternative approach of low temperature-long time cooking on bovine semitendinosus meat quality. Asian Australas. J. Anim. Sci. 2019, 32, 282–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aaslyng, M.D.; Bejerholm, C.; Ertbjerg, P.; Bertram, H.C.; Andersen, H.J. Cooking loss and juiciness of pork in relation to raw meat quality and cooking procedure. Food Qual. Prefer. 2003, 14, 277–288. [Google Scholar] [CrossRef]
- Roldán, M.; Antequera, T.; Hernández, A.; Ruiz, J.; Ruiz-Carrascal, J. Physicochemical and microbiological changes during the refrigerated storage of lamb loins sous-vide cooked at different combinations of time and temperature. Food Sci. Technol. Int. 2014, 21, 512–522. [Google Scholar] [CrossRef]
- Purslow, P.; Oiseth, S.; Hughes, J.; Warner, R. The structural basis of cooking loss in beef: Variations with temperature and ageing. Food Res. Int. 2016, 89, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, D.; Xu, W.; Gao, F.; Zhou, G. Effect of final cooked temperature on tenderness, protein solubility and microstructure of duck breast muscle. LWT 2013, 51, 266–274. [Google Scholar] [CrossRef]
- Wideman, N.; O’Bryan, C.; Crandall, P. Factors affecting poultry meat colour and consumer preferences—A review. World’s Poult. Sci. J. 2016, 72, 353–366. [Google Scholar] [CrossRef]
- Cobos, Á.; Diaz, O. Chemical Composition of Meat and Meat Products. In Handbook of Food Chemistry; Cheung, P.C.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–32. [Google Scholar]
- Khan, A.; Allen, K.; Wang, X. Effect of Type I and Type II Antioxidants on Oxidative Stability, Microbial Growth, pH, and Color in Raw Poultry Meat. Food Nutr. Sci. 2015, 6, 1541–1551. [Google Scholar] [CrossRef] [Green Version]
- García-Segovia, P.; Andrés-Bello, A.; Martínez-Monzó, J. Effect of cooking method on mechanical properties, color and structure of beef muscle (M. pectoralis). J. Food Eng. 2007, 80, 813–821. [Google Scholar] [CrossRef]
- Holownia, K.; Chinnan, M.S.; Reynolds, A.E.; Koehler, P.E. Evaluation of induced color changes in chicken breast meat during simulation of pink color defect. Poult. Sci. 2003, 82, 1049–1059. [Google Scholar] [CrossRef] [PubMed]
- Fabre, R.; Dalzotto, G.; Perlo, F.; Bonato, P.; Teira, G.; Tisocco, O. Cooking method effect on Warner-Bratzler shear force of different beef muscles. Meat Sci. 2018, 138, 10–14. [Google Scholar] [CrossRef]
- Turner, B.E.; Larick, D.K. Palatability of Sous Vide Processed Chicken Breast. Poult. Sci. 1996, 75, 1056–1063. [Google Scholar] [CrossRef]
- Barbanti, D.; Pasquini, M. Influence of cooking conditions on cooking loss and tenderness of raw and marinated chicken breast meat. LWT 2005, 38, 895–901. [Google Scholar] [CrossRef]
- Warner, R.; Ha, M.; Sikes, A.; Vaskoska, R. Cooking and Novel Postmortem Treatments to Improve Meat Texture. In New Aspects of Meat Quality; Elsevier BV: Amsterdam, The Netherlands, 2017; pp. 387–423. [Google Scholar]
- Laakkonen, E.; Wellington, G.H.; Sherbon, J.N. Low-Temperature, Long-Time Heating of Bovine Muscle 1. Changes in Tenderness, Water-Binding Capacity, pH and Amount of Water-Soluble Components. J. Food Sci. 1970, 35, 175–177. [Google Scholar] [CrossRef]
- Bigi, F.; Haghighi, H.; De Leo, R.; Ulrici, A.; Pulvirenti, A. Multivariate exploratory data analysis by PCA of the combined effect of film-forming composition, drying conditions, and UV-C irradiation on the functional properties of films based on chitosan and pectin. LWT 2021, 137, 110432. [Google Scholar] [CrossRef]


| Group | Temperature (°C) | Time (min) | Vacuum Degree (%) |
|---|---|---|---|
| Control | 100 | 60 | 0 |
| 1 | 60 | 60 | 98 |
| 2 | 60 | 90 | 98 |
| 3 | 60 | 120 | 98 |
| 4 | 60 | 150 | 98 |
| 5 | 70 | 60 | 98 |
| 6 | 70 | 90 | 98 |
| 7 | 70 | 120 | 98 |
| 8 | 70 | 150 | 98 |
| 9 | 80 | 60 | 98 |
| 10 | 80 | 90 | 98 |
| 11 | 80 | 120 | 98 |
| 12 | 80 | 150 | 98 |
| Parameters | Results |
|---|---|
| Moisture (%) | 72.4 ± 1.02 |
| TBARS (mg/Kg) | 0.08 ± 0.011 |
| Weight (g) | 125 ± 5 |
| L* | 58.4 ± 1.7 |
| a* | 0.8 ± 0.1 |
| b* | 9.1 ± 0.9 |
| pH | 5.8 ± 0.03 |
| Temp (°C) | 60 | 70 | 80 | 100 | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Time (min) | 60 | 90 | 120 | 150 | 60 | 90 | 120 | 150 | 60 | 90 | 120 | 150 | 60 | SEM | Temp | Time | Temp × Time |
| Moisture (%) | 71.41 f,g | 71.30 e,f,g | 71.72 f,g | 71.89 g | 71.71 f,g | 70.86 e,f,g | 69.97 c,d,e | 70.46 d,e,f | 70.43 d,e,f | 69.76 b,c,d | 69.47 a,b,c | 69.02 a,b | 68.25 a | 0.21 | * | * | * |
| Cooking loss (%) | 10.23 a | 11.02 a | 12.42 a,b | 12.47 a,b | 14.01 a,b,c | 16.88 b,c,d | 18.38 c,d,e | 18.69 c,d,e | 17.86 c,d,e | 21.77 d,e,f | 22.77 e,f | 24.23 f,g | 28.08 g | 3.11 | * | * | N.S |
| Shear force (kg) | 0.75 a | 0.83 a,b | 0.76 a | 0.62 a | 0.66 a | 0.73 a | 0.62 a | 0.63 a | 0.88 a,b | 0.97 b | 0.79 a | 0.88 a,b | 1.37 c | 0.02 | * | N.S | N.S |
| TBARS (mg/kg) | 0.29 a | 0.77 a,b | 0.92 a,b | 0.94 a,b | 1.50 b | 1.47 b | 1.63 b | 1.71 b | 2.31 c | 2.42 c | 2.54 c | 2.60 c | 2.91 d | 0.12 | * | N.S | N.S |
| L* | 80.94 a | 81.71 a | 80.11 a | 79.63 a | 80.82 a | 81.72 a | 82.27 a | 82.43 a | 81.39 a | 81.19 a | 80.85 a | 81.15 a | 80.75 a | 2.65 | N.S | N.S | N.S |
| a* | 1.95 a | 1.95 a | 1.81 a | 1.71 a | 1.73 a | 1.71 a | 1.74 a | 1.50 ab | 1.44 b | 1.39 b | 1.33 b | 1.29 b | 1.29 b | 0.05 | * | * | N.S |
| b* | 14.71 a | 14.65 a | 14.95 a | 15.15 a | 14.91 a | 14.83 a | 14.95 a | 15.40 a | 15.64 a | 15.55 a | 15.60 a | 15.36 a | 14.82 a | 0.41 | N.S | N.S | N.S |
| pH | 6.17 a,b,c | 6.14 a | 6.07 a | 6.08 a | 6.11 a | 6.14 a,b | 6.07 a | 6.13 a | 6.15 a,b | 6.25 b,c | 6.30 d | 6.27 c,d | 6.17 a,b,c | 0.93 | * | N.S | * |
| Table | Treatment | Total Mesophilic Aerobic Log (CFU/g) | Enterobacteriaceae Log (CFU/g) | Psychrotrophic Aerobic Log (CFU/g) |
|---|---|---|---|---|
| 0 | Raw meat | 2.8 ± 0.6 | 2.3 ± 0.4 | <1 |
| 0 | Sous vide | n.d | n.d | n.d |
| 5 | Sous vide | n.d | n.d | n.d |
| 10 | Sous vide | n.d | n.d | n.d |
| 15 | Sous vide | n.d | n.d | n.d |
| 21 | Sous vide | n.d | n.d | n.d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haghighi, H.; Belmonte, A.M.; Masino, F.; Minelli, G.; Lo Fiego, D.P.; Pulvirenti, A. Effect of Time and Temperature on Physicochemical and Microbiological Properties of Sous Vide Chicken Breast Fillets. Appl. Sci. 2021, 11, 3189. https://doi.org/10.3390/app11073189
Haghighi H, Belmonte AM, Masino F, Minelli G, Lo Fiego DP, Pulvirenti A. Effect of Time and Temperature on Physicochemical and Microbiological Properties of Sous Vide Chicken Breast Fillets. Applied Sciences. 2021; 11(7):3189. https://doi.org/10.3390/app11073189
Chicago/Turabian StyleHaghighi, Hossein, Anna Maria Belmonte, Francesca Masino, Giovanna Minelli, Domenico Pietro Lo Fiego, and Andrea Pulvirenti. 2021. "Effect of Time and Temperature on Physicochemical and Microbiological Properties of Sous Vide Chicken Breast Fillets" Applied Sciences 11, no. 7: 3189. https://doi.org/10.3390/app11073189
APA StyleHaghighi, H., Belmonte, A. M., Masino, F., Minelli, G., Lo Fiego, D. P., & Pulvirenti, A. (2021). Effect of Time and Temperature on Physicochemical and Microbiological Properties of Sous Vide Chicken Breast Fillets. Applied Sciences, 11(7), 3189. https://doi.org/10.3390/app11073189