Novel Luminescent Multilayer Films Containing π-Conjugated Anionic Polymer with Electronic Microenvironment
Abstract
:1. Introduction
2. Materials and Methods
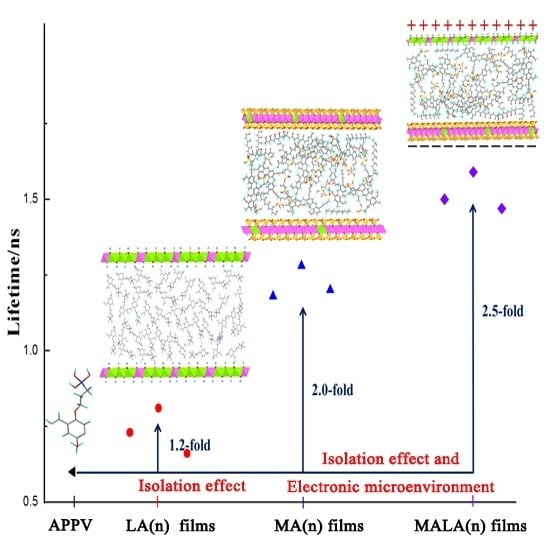
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Butler, S.Z.; Hollen, S.M.; Cao, L.Y.; Cui, Y.; Gupta, J.A.; Gutiérrez, H.R.; Heinz, T.F.; Hong, S.S.; Huang, J.X.; Ismach, A.F.; et al. Progress, challenges, and opportunities in two-dimensional materials beyond graphene. ACS Nano 2013, 7, 2898–2926. [Google Scholar] [CrossRef] [PubMed]
- Butt, F.K.; Tahir, M.; Cao, C.B.; Idrees, F.; Ahmed, R.; Khan, W.S.; Ali, Z.; Mahmood, N.; Tanveer, M.; Mahmood, A.; et al. Synthesis of novel ZnV2O4 hierarchical nanospheres and their applications as electrochemical supercapacitor and hydrogen storage material. ACS Appl. Mater. Interfaces 2014, 6, 13635–13641. [Google Scholar] [CrossRef] [PubMed]
- Gentili, D.; Valle, F.; Albonetti, C.; Liscio, F.; Cavallini, M. Self-organization of functional materials in confinement. Acc. Chem. Res. 2014, 47, 2692–2699. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, M.; D’Angelo, P.; Criado, V.V.; Gentili, D.; Shehu, A.; Leonardi, F.; Milita, S.; Liscio, F.; Biscarini, F. Ambipolar multi-stripe organic field-effect transistors. Adv. Mater. 2011, 23, 5091–5097. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, P.; Kaushik, A.K.; Arruda, E.M.; Waas, A.M.; Shim, B.S.; Xu, J.D.; Nandivada, H.; Pumplin, B.G.; Lahann, J.; Ramamoorthy, A.; et al. Ultrastrong and stiff layered polymer nanocomposites. Science 2007, 318, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Zhuk, A.; Mirza, R.; Sukhishvili, S. Multiresponsive clay-containing layer-by-layer films. ACS Nano 2011, 5, 8790–8799. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Cen, X.; Peng, H.D.; Guo, S.Z.; Wang, W.Z.; Liu, T.X. Heterogeneous ultrathin films of poly(vinyl alcohol)/layered double hydroxide and montmorillonite nanosheets via layer-by-layer assembly. J. Phys. Chem. B 2009, 113, 15225–15230. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.P.; Ma, R.Z.; Osada, M.; Iyi, N.; Ebina, Y.; Takada, K.K.; Sasaki, T. Synthesis, anion exchange, and delamination of Co-Al layered double hydroxide: Assembly of the exfoliated nanosheet/polyanion composite films and magneto-optical studies. J. Am. Chem. Soc. 2006, 128, 4872–4880. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; O’Hare, D. Recent advances in the synthesis and application of layered double hydroxide (LDH) nanosheets. Chem. Rev. 2011, 112, 4124–4155. [Google Scholar] [CrossRef] [PubMed]
- Gunjakar, J.L.; Kim, T.W.; Kim, H.N.; Kim, I.Y.; Hwang, S.J. Mesoporouslayer-by-layer ordered nanohybrids of layered double hydroxide and layered metal oxide: highly active visible light photocatalysts with improved chemical stability. J. Am. Chem. Soc. 2011, 133, 14998–15007. [Google Scholar] [CrossRef] [PubMed]
- Shu, Y.Q.; Yin, P.G.; Liang, B.L.; Wang, S.S.; Gao, L.; Wang, H.; Guo, L. Layer by layer assembly of heparin/layered double hydroxide completely renewable ultrathin films with enhanced strength and blood compatibility. J. Mater. Chem. 2012, 22, 21667–21672. [Google Scholar] [CrossRef]
- Tanaka, T.; Nishimoto, S.; Kameshima, Y.; Miyake, M. Fabrication of layered double hydroxide/photoresponsive dendron nanocomposite multilayer film by electrostatic layer-by-layer assembly. Mater. Lett. 2011, 65, 2315–2318. [Google Scholar] [CrossRef]
- Dou, Y.B.; Xu, S.M.; Liu, X.X.; Han, J.B.; Yan, H.; Wei, M.; Evans, D.G.; Duan, X. Transparent, flexible films based on layered double hydroxide/cellulose acetate with excellent oxygen barrier property. Adv. Funct. Mater. 2014, 24, 514–522. [Google Scholar] [CrossRef]
- Coiai, S.; Passaglia, E.; Pucci, A.; Ruggeri, G. Nanocomposites based on thermoplastic polymers and functional nanofiller for sensor applications. Materials 2015, 8, 3377–3427. [Google Scholar] [CrossRef]
- Yan, D.P.; Lu, J.; Ma, J.; Wei, M.; Wang, X.R.; Evans, D.G.; Duan, X. Anionic poly(p-phenylenevinylene)/layered double hydroxide ordered ultrathin films with multiple quantum well structure: A combined experimental and theoretical study. Langmuir 2009, 26, 7007–7014. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.L.; Liu, M.T.; Ma, H.W.; Liu, X.J.; Fu, Y.; Hu, K.R. Lifetime-ultra-prolonged luminescent multilayer thin films with electronic microenvironment. RSC Adv. 2014, 4, 40748–40752. [Google Scholar] [CrossRef]
- Liu, M.T.; Wang, T.L.; Ma, H.W.; Fu, Y.; Hu, K.R.; Guan, C. Assembly of luminescent ordered multilayer thin-films based on oppositely-charged MMT and magnetic NiFe-LDHs nanosheets with ultra-long lifetimes. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.T.; Wang, T.L.; Ma, H.W.; Fu, Y.; Hu, K.R.; Guan, C. Layer-by-layer assembly of luminescent multilayer thin films by MMT, anionic chromophores and magnetic CoAl-LDHs nanosheets. Mater. Lett. 2015, 153, 40–43. [Google Scholar] [CrossRef]
- Lee, W.E.; Jin, Y.J.; Park, L.S.; Kwak, G. Fluorescent actuator based on microporous conjugated polymer with intramolecular stack structure. Adv. Mater. 2012, 24, 5604–5609. [Google Scholar] [CrossRef] [PubMed]
- Bigall, N.C.; Parak, W.J.; Dorfs, D. Fluorescent, magnetic and plasmonic-hybrid multifunctional colloidal nano objects. Nano Today 2012, 7, 282–296. [Google Scholar] [CrossRef]
- Chung, S.J.; Kwon, K.Y.; Lee, S.W.; Jin, J.I.; Lee, C.H.; Lee, C.E.; Park, Y. Highly efficient light-emitting diodes based on an organic-soluble poly(p-phenylenevinylene) derivative carrying the electron-transporting PIED moiety. Adv. Mater. 1998, 10, 1112–1116. [Google Scholar] [CrossRef]
- Chen, Z.K.; Meng, H.; Lai, Y.H.; Huang, W. Photoluminescent poly(p-phenylenevinylene) with an aromatic oxadiazole moiety as the side chain: Synthesis, electrochemistry, and spectroscopy study. Macromolecules 1999, 32, 4351–4358. [Google Scholar] [CrossRef]
- Becker, H.; Spreitzer, H.; Kreuder, W.; Kluge, E.; Schenk, H.; Parker, I.; Cao, Y. Soluble PPVs with enhanced performance-A mechanistic approach. Adv. Mater. 2000, 12, 42–48. [Google Scholar] [CrossRef]
- Chen, Z.K.; Lee, N.H.S.; Huang, W. New phenyl-substituted PPV derivatives for polymer light-emitting diodes-synthesis, characterization and structure-property relationship study. Macromolecules 2003, 36, 1009–1020. [Google Scholar] [CrossRef]
- Jenekhe, S.A. Excited-state complexes of conjugated polymers. Adv. Mater. 1995, 7, 309–311. [Google Scholar] [CrossRef]
- Nguyen, T.Q.; Martini, I.B.; Liu, J.; Schwartz, B.J. Controlling interchain interactions in conjugated polymers: The effects of chain morphology on exciton-exciton annihilation and aggregation in MEH-PPV films. J. Phys. Chem. B 2000, 104, 237–255. [Google Scholar] [CrossRef]




© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, T.; Liu, M.; Ma, H. Novel Luminescent Multilayer Films Containing π-Conjugated Anionic Polymer with Electronic Microenvironment. Appl. Sci. 2016, 6, 272. https://doi.org/10.3390/app6100272
Wang T, Liu M, Ma H. Novel Luminescent Multilayer Films Containing π-Conjugated Anionic Polymer with Electronic Microenvironment. Applied Sciences. 2016; 6(10):272. https://doi.org/10.3390/app6100272
Chicago/Turabian StyleWang, Tianlei, Meitang Liu, and Hongwen Ma. 2016. "Novel Luminescent Multilayer Films Containing π-Conjugated Anionic Polymer with Electronic Microenvironment" Applied Sciences 6, no. 10: 272. https://doi.org/10.3390/app6100272
APA StyleWang, T., Liu, M., & Ma, H. (2016). Novel Luminescent Multilayer Films Containing π-Conjugated Anionic Polymer with Electronic Microenvironment. Applied Sciences, 6(10), 272. https://doi.org/10.3390/app6100272