Chip-Based Cytometry Illuminated by a Blade-Shape Continuous Light for Multispectral Detection
Abstract
:1. Introduction
2. Materials and Methods
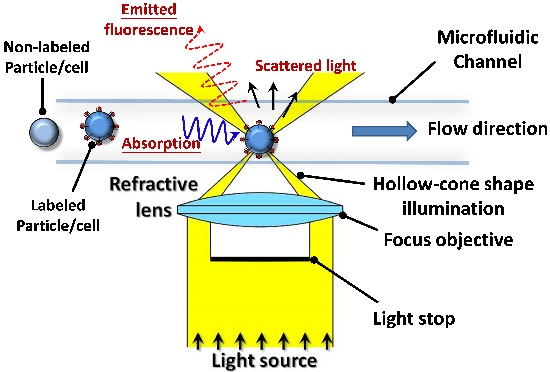
2.1. Working Principle
2.2. Instrumental Setup and Flow Cytometry
2.3. Reagents and Bio-Sample Preparation
3. Results and Discussion
3.1. Spectral Analysis and Performance Measurement
3.2. Discrimination for Particles and Bio-Cells
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cheng, M.Y.; Lin, C.L.; Lai, Y.T.; Yang, Y.J. A polymer-based capacitive sensing array for normal and shear force measurement. Sensors 2010, 10, 10211–10225. [Google Scholar] [CrossRef] [PubMed]
- Roederer, M.; Brenchley, J.M.; Betts, M.R.; De Rosa, S.C. Flow cytometric analysis of vaccine responses: How many colors are enough? Clin. Immunol. 2004, 110, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Sun, H. Up-regulation of foxp3 inhibits cell proliferation, migration and invasion in epithelial ovarian cancer. Cancer Lett. 2010, 287, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, I.S.; Yu, H.W. Flow cytometric detection of bacillus spoOA gene in biofilm using quantum dot labeling. Anal. Chem. 2010, 82, 2836–2843. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Anderson, G.P.; Erickson, J.S.; Golden, J.P.; Nasir, M.; Ligler, F.S. Multiplexed detection of bacteria and toxins using a microflow cytometer. Anal. Chem. 2009, 81, 5426–5432. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.Y.; Nandra, M.S.; Shih, C.Y.; Li, W.; Tai, Y.C. Resonance impedance sensing of human blood cells. Sens. Actuators A 2008, 145, 29–36. [Google Scholar] [CrossRef]
- Zheng, S.Y.; Lin, J.C.H.; Kasdan, H.L.; Tai, Y.C. Fluorescent labeling, sensing, and differentiation of leukocytes from undiluted whole blood samples. Sens. Actuators B 2008, 132, 558–567. [Google Scholar] [CrossRef]
- Orlova, D.Y.; Yurkin, M.A.; Hoekstra, A.G.; Maltsev, V.P. Light scattering by neutrophils: Model, simulation, and experiment. J. Biomed. Opt. 2008. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Cordero, J.L.; Barrett, L.M.; O'Kennedy, R.; Ricco, A.J. Microfluidic sedimentation cytometer for milk quality and bovine mastitis monitoring. Biomed. Microdevices 2010, 12, 1051–1059. [Google Scholar] [CrossRef] [PubMed]
- Darzynkiewicz, Z.; Bedner, E.; Li, X.; Gorczyca, W.; Melamed, M.R. Laser-scanning cytometry: A new instrumentation with many applications. Exp. Cell Res. 1999, 249, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.; Kelso, D.M. Multispectral image analysis of binary encoded microspheres for highly multiplexed suspension arrays. Cytom. Part A 2010, 77A, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Barat, D.; Spencer, D.; Benazzi, G.; Mowlem, M.C.; Morgan, H. Simultaneous high speed optical and impedance analysis of single particles with a microfluidic cytometer. Lab Chip 2012, 12, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Bigos, A.; Baumgarth, N.; Jager, G.C.; Herman, O.C.; Nozaki, T.; Stovel, R.T.; Parks, D.R.; Herzenberg, L.A. Nine color eleven parameter immunophenotyping using three laser flow cytometry. Cytom. Part A 1999, 36, 36–45. [Google Scholar] [CrossRef]
- Perfetto, S.P.; Chattopadhyay, P.K.; Roederer, M. Innovation-seventeen-colour flow cytometry: Unravelling the immune system. Nat. Rev. Immunol. 2004, 4, 648–655. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Lee, G.B.; Chen, S.H.; Chang, G.L. Micro capillary electrophoresis chips integrated with buried su-8/sog optical waveguides for bio-analytical applications. Sens. Actuators A 2003, 107, 125–131. [Google Scholar] [CrossRef]
- Guo, J.H.; Huang, X.W.; Shi, D.Y.; Yu, H.; Ai, Y.; Li, C.M.; Kang, Y.J. Portable resistive pulse-activated lens-free cell imaging system. Rsc Adv. 2014, 4, 56342–56345. [Google Scholar] [CrossRef]
- Golden, J.P.; Kim, J.S.; Erickson, J.S.; Hilliard, L.R.; Howell, P.B.; Anderson, G.P.; Nasir, M.; Ligler, F.S. Multi-wavelength microflow cytometer using groove-generated sheath flow. Lab Chip 2009, 9, 1942–1950. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Lee, G.B. Micromachined flow cytometers with embedded etched optic fibers for optical detection. J. Micromech. Microeng. 2003, 13, 447–453. [Google Scholar] [CrossRef]
- Tung, Y.C.; Zhang, M.; Lin, C.T.; Kurabayashi, K.; Skerlos, S.J. Pdms-based opto-fluidic micro flow cytometer with two-color, multi-angle fluorescence detection capability using pin photodiodes. Sens. Actuators B 2004, 98, 356–367. [Google Scholar] [CrossRef]
- Kennedy, M.J.; Stelick, S.J.; Sayam, L.G.; Yen, A.; Erickson, D.; Batt, C.A. Hydrodynamic optical alignment for microflow cytometry. Lab Chip 2011, 11, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Schonbrun, E.; Gorthi, S.S.; Schaak, D. Microfabricated multiple field of view imaging flow cytometry. Lab Chip 2012, 12, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Isailovic, D.; Li, H.W.; Phillips, G.J.; Yeung, E.S. High-throughput single-cell fluorescence spectroscopy. Appl. Spectrosc. 2005, 59, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.X.; Glidle, A.; Ironside, C.; Cooper, J.M.; Yin, H.B. An integrated microspectrometer for localised multiplexing measurements. Lab Chip 2015, 15, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, A.; Packirisamy, M. Integrated microfluidic biophotonic chip for laser induced fluorescence detection. Biomed. Microdevices 2010, 12, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Sunamura, M.; Maruyama, A.; Tsuji, T.; Kurane, R. Spectral imaging detection and counting of microbial cells in marine sediment. J. Microbiol. Methods 2003, 53, 57–65. [Google Scholar] [CrossRef]
- Goddard, G.; Martin, J.C.; Naivar, M.; Goodwin, P.M.; Graves, S.W.; Habbersett, R.; Nolan, J.P.; Jett, J.H. Single particle high resolution spectral analysis flow cytometry. Cytom. Part A 2006, 69A, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, B.C.; Bergquist, P.L. Quantum dots as alternatives to organic fluorophores for cryptosporidium detection using conventional flow cytometry and specific monoclonal antibodies: Lessons learned. Cytom. Part A 2007, 71A, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, P.K.; Price, D.A.; Harper, T.F.; Betts, M.R.; Yu, J.; Gostick, E.; Perfetto, S.P.; Goepfert, P.; Koup, R.A.; De Rosa, S.C.; et al. Quantum dot semiconductor nanocrystals for immunophenotyping by polychromatic flow cytometry. Nat. Med. 2006, 12, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.; Bang, H.; Min, J.; Chung, C.; Chang, J.K.; Han, D.C. Simultaneous counting of two subsets of leukocytes using fluorescent silica nanoparticles in a sheathless microchip flow cytometer. Lab Chip 2010, 10, 3243–3254. [Google Scholar] [CrossRef] [PubMed]
- Watson, D.A.; Brown, L.O.; Gaskill, D.R.; Naivar, M.; Graves, S.W.; Doorn, S.K.; Nolan, J.P. A flow cytometer for the measurement of raman spectra. Cytom. Part A 2008, 73A, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Braslavsky, I.; Amit, R.; Ali, B.M.J.; Gileadi, O.; Oppenheim, A.; Stavans, J. Objective-type dark-field illumination for scattering from microbeads. Appl. Opt. 2001, 40, 5650–5657. [Google Scholar] [CrossRef] [PubMed]
- Piper, T.; Piper, J. Variable phase dark-field contrast—A variant illumination technique for improved visualizations of transparent specimens. Microsc. Microanal. 2012, 18, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.W.; Hsu, J.H.; Chang, C.H.; Lin, C.H. Objective-type dark-field system applied to multi-wavelength capillary electrophoresis for fluorescent detection and analysis. Biosens. Bioelectron. 2009, 25, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.W.; Chang, G.L.; Lin, C.H. Novel wave length-resolved fluorescence detection for a high-throughput capillary electrophoresis system under a diascopic configuration. J Chromatogr. A 2008, 1192, 198–201. [Google Scholar] [CrossRef] [PubMed]
- Rebner, K.; Schmitz, M.; Boldrini, B.; Kienle, A.; Oelkrug, D.; Kessler, R.W. Dark-field scattering microscopy for spectral characterization of polystyrene aggregates. Opt. Express 2010, 18, 3116–3127. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.W.; Wang, P.Y.; Chen, A.; Chang, C.H.; Lin, C.H. Wavelength-resolved flow cytometer under a dark-field illumination configuration. IEEE Sens. J. 2011, 11, 2845–2851. [Google Scholar] [CrossRef]
- Lin, C.H.; Lee, G.B.; Lin, Y.H.; Chang, G.L. A fast prototyping process for fabrication of microfluidic systems on soda-lime glass. J. Micromech. Microeng. 2001, 11, 726–732. [Google Scholar] [CrossRef]
- Lee, G.B.; Lin, C.H.; Chang, G.L. Micro flow cytometers with buried su-8/sog optical waveguides. Sens. Actuators A 2003, 103, 165–170. [Google Scholar] [CrossRef]







© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.-W.; Lin, C.-H. Chip-Based Cytometry Illuminated by a Blade-Shape Continuous Light for Multispectral Detection. Appl. Sci. 2016, 6, 229. https://doi.org/10.3390/app6080229
Lin S-W, Lin C-H. Chip-Based Cytometry Illuminated by a Blade-Shape Continuous Light for Multispectral Detection. Applied Sciences. 2016; 6(8):229. https://doi.org/10.3390/app6080229
Chicago/Turabian StyleLin, Shi-Wei, and Che-Hsin Lin. 2016. "Chip-Based Cytometry Illuminated by a Blade-Shape Continuous Light for Multispectral Detection" Applied Sciences 6, no. 8: 229. https://doi.org/10.3390/app6080229
APA StyleLin, S. -W., & Lin, C. -H. (2016). Chip-Based Cytometry Illuminated by a Blade-Shape Continuous Light for Multispectral Detection. Applied Sciences, 6(8), 229. https://doi.org/10.3390/app6080229