Oxidation Products of Ester-Based Oils with and without Antioxidants Identified by Stable Isotope Labelling and Mass Spectrometry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
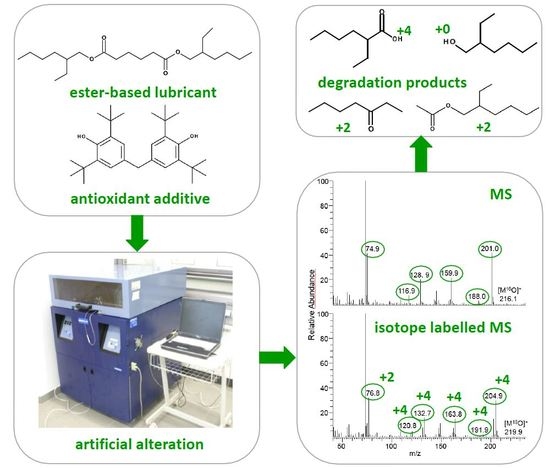
2.2. Approach of Isotope Labelling
2.3. Artificial Alteration
2.4. Analytical Approach
2.4.1. Conventional Analytical Methods
Neutralisation Number (NN)
Water Content
2.4.2. Advanced Analytical Methods
3. Results and Discussion
3.1. General Degradation Behaviour Determined by Conventional Analytical Methods
3.2. Degradation Behaviour Identified by Advanced Analytical Methods
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- American Petroleum Institute (API), API 1509 2016, Appendix E. Available online: www.api.org (accessed on 5 December 2016).
- Rudnick, L.E. Synthetics, Mineral Oils, and Bio-Based Lubricants, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014; pp. 47–74. [Google Scholar]
- Global Market Insights Inc. Synthetic Lubricants Market Size to Exceed USD 5 Billion by 2023. Available online: www.gminsights.com (accessed on 5 December 2016).
- Eastwood, J. Esters—The most versatile of base stock technologies. Lube Mag. 2015, 129, 32–37. [Google Scholar]
- Nagendramma, P.; Kaul, S. Development of ecofriendly/biodegradable lubricants: An overview. Renew. Sustain. Energy Rev. 2012, 16, 764–774. [Google Scholar] [CrossRef]
- Erhan, S.Z.; Sharma, B.K.; Perez, J.M. Oxidation and low temperature stability of vegetable oil-based lubricants. Ind. Crop. Prod. 2006, 24, 292–299. [Google Scholar] [CrossRef]
- Salimon, J.; Salih, N.; Yousif, E. Improvement of pour point and oxidative stability of synthetic ester basestocks for biolubricant applications. Arab. J. Chem. 2012, 5, 193–200. [Google Scholar] [CrossRef]
- Wu, Y.; Li, W.; Zhang, M.; Wang, X. Improvement of oxidative stability of trimethylolpropane trioleatelubricant. Thermochim. Acta 2013, 569, 112–118. [Google Scholar] [CrossRef]
- Cavalcante, I.M.; de Rocha, N.R.C.; Maier, M.E.; Lima, A.P.D.; Neto, D.M.A.; Brito, D.H.A.; Petzhold, C.L.; Schanz, M.T.G.F.; Ricardo, N.M.P.S. Synthesis and characterization of new esters of oleic acid and glycerol analogues as potential lubricants. Ind. Crop. Prod. 2014, 62, 453–459. [Google Scholar] [CrossRef]
- Housel, T. Synthetic esters: Engineered to perform. Available online: http://machinerylubrication.com/Read/29703/synthetic-esters-perform (accessed on 14 April 2017).
- Gryglewicz, S.; Piechicki, W.; Gryglewicz, G. Preparation of polyol esters based on vegetable and animal fats. Bioresour. Technol. 2003, 87, 35–39. [Google Scholar] [CrossRef]
- Bünemann, T.F.; Vank Aken, R.P. Synthetische Ester––Wie kann man die Lebensdauer von biologisch schnell abbauenden Hydraulikflüssigkeiten verlängern. In Tribology 2000—Plus, Proceedings of the 12th International Colloquium, Ostfildern, Germany, 11–13 January 2000; Emerald Group Publishing Limited: Bingley, UK, 2000; pp. 109–110. [Google Scholar]
- Moser, B.R.; Sharma, B.K.; Doll, K.M.; Erhan, S.Z. Diesters from Oleic Acid: Synthesis, Low Temperature Properties, and Oxidation Stability. J. Am. Oil Chem. Soc. 2007, 84, 675–680. [Google Scholar] [CrossRef]
- Alias, N.H.; Yunus, R.; Idris, A.; Omar, R. Effects of additives on oxidation characteristics of palm oilbased trimethylolpropane ester in hydraulics applications. Eur. J. Lipid Sci. Technol. 2009, 111, 368–375. [Google Scholar] [CrossRef]
- Chao, M.; Li, W.; Wang, X. Influence of antioxidant on the thermal–oxidative degradation behavior and oxidation stability of synthetic ester. Thermochim. Acta 2014, 561, 16–21. [Google Scholar] [CrossRef]
- Deutsches Institut für Normung. 4263 Petroleum and Related Products—Determination of the Ageing Behaviour of Inhibited Oils and Fluids Using the TOST Test; Deutsches Institut für Normung: Berlin, Germany, 2016. [Google Scholar]
- Deutsches Institut für Normung. 51808 Testing of Lubricants—Determination of Oxidation Stability of Greases—Oxygen Method, Deutsches Institut für Normung (Draft); Deutsches Institut für Normung: Berlin, Germany, 2015. [Google Scholar]
- ASTM International. D 2272 Standard Test Method for Oxidation Stability of Steam Turbine Oils by Rotating Pressure Vessel; ASTM International: West Conshohocken, PA, USA, 1998. [Google Scholar]
- Santos, J.C.O.; Santos, I.M.G.; Souza, A.G. Thermal degradation process of synthetic lubricating oils: Part I—Spectroscopic study. Petrol. Sci. Technol. 2015, 33, 1238–1245. [Google Scholar] [CrossRef]
- Keller, M.A.; Saba, C.S. Gas chromatographic monitoring of hydroxyl components in oxidized turbine engine lubricants. Tribol. Trans. 2003, 46, 576–579. [Google Scholar] [CrossRef]
- Tripathi, A.K.; Vinu, R. Characterization of thermal stability of synthetic and semi-synthetic engine oils. Lubricants 2015, 3, 54–79. [Google Scholar] [CrossRef]
- Frauscher, M.; Besser, C.; Dörr, N.; Allmaier, G. Unambiguous identification of degradation products related to elucidation of the degradation mechanism of oxygen containing fuel components by shared use of isotope labelling and mass spectrometry. Anal. Chem. 2017. submitted. [Google Scholar]
- Besser, C.; Pisarova, L.; Frauscher, M.; Hunger, H.; Dörr, N.; Litzow, U.; Orfaniotis, A. Oxidation products of biodiesel in diesel fuel generated by artificial alteration and identified by mass spectrometry. Fuel 2017. submitted. [Google Scholar]
- Deutsches Institut für Normung. DIN 51558 Testing of Mineral Oils, Determination of Neutralization Number by Color-Indicator Titration; Deutsches Institut für Normung: Berlin, Germany, 1979. [Google Scholar]
- Deutsches Institut für Normung. DIN 51777-2 Testing of Mineral Oil Hydrocarbons and Solvents, Determination of Water Content According to Karl Fischer; Indirect Method; Deutsches Institut für Normung: Berlin, Germany, 1974. [Google Scholar]











| 16O2 | Analyte | Retention Time (min) | Calculated Monoisotopic m/z Value of Molecular Ion | Detected Monoisotopic Molecular ion (RI %) | Highest Detected m/z Value in the Mass Spectrum |
|---|---|---|---|---|---|
| a | Propionic acid TMS ester | 4.04 | 146.1 | 145.9 (0.6) | 130.9 |
| b | Butanoic acid TMS ester | 5.37 | 160.1 | 159.9 (1.0) | 144.9 |
| c | 3-heptanone | 6.34 | 114.2 | 114.0 (13.5) | 114.0 |
| d | (2-ethylhexyl)oxy TMS silane | 8.55 | 202.4 | 202.1 (0.8) | 187.0 |
| e | 2-ethylhexyl formic acid ester | 9.77 | 158.2 | - (<0.5) | 112.0 |
| f | 2-ethylhexanoic acid TMS ester | 10.63 | 216.2 | - (<0.5) | 201.0 |
| g | 2-ethylhexyl acetic acid ester | 11.28 | 172.3 | - (<0.5) | 124.8 |
| h | 2-ethylhexyl propionic acid ester | 12.97 | 186.3 | - (<0.5) | 156.9 |
| i | 2-ethylhexyl butanoic acid ester | 14.49 | 200.3 | - (<0.5) | 156.9 |
| j | 2-ethylhexyl pentanoic acid ester | 16.16 | 214.3 | - (<0.5) | 146.9 |
| k | Adipic acid TMS ester | 17.88 | 290.5 | - (<0.5) | 275.0 |
| l | 2-ethylhexyl 6-oxohexanoate | 20.51 | 242.4 | - (<0.5) | 139.8 |
| m | 2-ethylhexyl 2-oxobutyl adipate | 24.74 | 328.4 | - (<0.5) | 271.0 |
| n | Bis(2-ethylhexyl) adipate | 30.82 | 370.6 | 371.1 (1.7) | 371.1 |
| 18O2 | Analyte | No. of 18O2 Atoms | Retention Time (min) | Calculated Monoisotopic m/z Value of Molecular Ion | Detected Monoisotopic Molecular Ion (RI %) | Highest Detected m/z Value in the Mass Spectrum |
|---|---|---|---|---|---|---|
| a | Propionic acid TMS ester | 2 | 4.04 | 150.1 | 149.8 (<0.5) | 134.7 |
| b | Butanoic acid TMS ester | 2 | 5.37 | 164.1 | 163.8 (1.0) | 148.8 |
| c | 3-heptanone | 2 | 6.34 | 116.2 | 115.8 (12.9) | 115.8 |
| d | (2-ethylhexyl)oxy TMS silane | - | 8.55 | 202.4 | 201.9 (0.7) | 186.9 |
| e | 2-ethylhexyl formic acid ester | - | 9.77 | 158.2 | - (<0.5) | 111.9 |
| f | 2-ethylhexanoic acid TMS ester | 2 | 10.63 | 220.2 | - (<0.5) | 204.9 |
| g | 2-ethylhexyl acetic acid ester | 1 | 11.28 | 174.3 | - (<0.5) | 126.8 |
| h | 2-ethylhexyl propionic acid ester | 1 | 12.97 | 188.3 | - (<0.5) | 158.8 |
| i | 2-ethylhexyl butanoic acid ester | 1 | 14.49 | 202.3 | - (<0.5) | 158.8 |
| j | 2-ethylhexyl pentanoic acid ester | - | 16.16 | 214.3 | - (<0.5) | 148.8 |
| k | Adipic acid TMS ester | 2 | 17.88 | 294.5 | - (<0.5) | 278.9 |
| l | 2-ethylhexyl 6-oxohexanoate | 1 | 20.51 | 244.4 | - (<0.5) | 141.8 |
| m | 2-ethylhexyl 2-oxobutyl adipate | 1 | 24.74 | 330.4 | - (<0.5) | 271.0 |
| n | Bis(2-ethylhexyl) adipate | - | 30.82 | 370.6 | 371.0 (1.8) | 371.0 |
| 16O2 | Analyte | Retention Time (min) | Calculated Monoisotopic m/z Value of Molecular Ion | Detected Monoisotopic Molecular Ion (RI %) | Highest Detected m/z Value in the Mass Spectrum |
| o | 2,6-di-tert-butyl-p-benzoquinone | 18.11 | 220.3 | 220.0 (40.2) | 220.0 |
| p | 3,5-di-tert-butyl-4-hydroxybenzoic acid TMS ester | 25.50 | 322.20 | 322.0 (14.5) | 322.0 |
| 18O2 | Analyte | Retention Time (min) | Calculated Monoisotopic m/z Value of Molecular Ion | Detected Monoisotopic Molecular Ion (RI %) | Highest Detected m/z Value in the Mass Spectrum |
| o | 2,6-di-tert-butyl-p-benzoquinone | 18.11 | 220.3 | 220.0 (38.3) | 220.0 |
| p | 3,5-di-tert-butyl-4-hydroxybenzoic acid TMS ester | 25.52 | 326.2 | 326.0 (12.8) | 326.0 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frauscher, M.; Besser, C.; Allmaier, G.; Dörr, N. Oxidation Products of Ester-Based Oils with and without Antioxidants Identified by Stable Isotope Labelling and Mass Spectrometry. Appl. Sci. 2017, 7, 396. https://doi.org/10.3390/app7040396
Frauscher M, Besser C, Allmaier G, Dörr N. Oxidation Products of Ester-Based Oils with and without Antioxidants Identified by Stable Isotope Labelling and Mass Spectrometry. Applied Sciences. 2017; 7(4):396. https://doi.org/10.3390/app7040396
Chicago/Turabian StyleFrauscher, Marcella, Charlotte Besser, Günter Allmaier, and Nicole Dörr. 2017. "Oxidation Products of Ester-Based Oils with and without Antioxidants Identified by Stable Isotope Labelling and Mass Spectrometry" Applied Sciences 7, no. 4: 396. https://doi.org/10.3390/app7040396
APA StyleFrauscher, M., Besser, C., Allmaier, G., & Dörr, N. (2017). Oxidation Products of Ester-Based Oils with and without Antioxidants Identified by Stable Isotope Labelling and Mass Spectrometry. Applied Sciences, 7(4), 396. https://doi.org/10.3390/app7040396