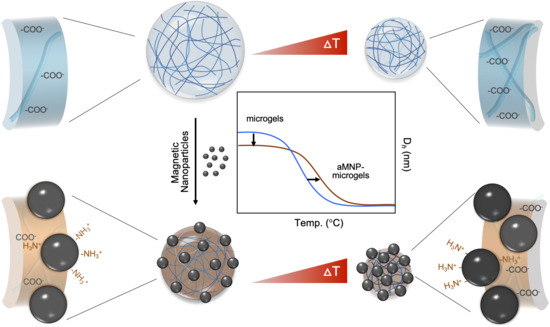
Thermoresponsive Behavior of Magnetic Nanoparticle Complexed pNIPAm-co-AAc Microgels
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Microgel Synthesis
2.3. MNP Synthesis
2.4. Dynamic Light Scattering (DLS) Analysis
2.5. Transmission Electron Microscopy (TEM) Analysis
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Miyata, T.; Asami, N.; Uragami, T. A reversibly antigen-responsive hydrogel. Nature 1999, 399, 766–769. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, Y.; Brown, A.C.; Lyon, L.A. Direct observation of ligand-induced receptor dimerization with a bioresponsive hydrogel. RSC Adv. 2014, 4, 65173–65175. [Google Scholar] [CrossRef]
- Kim, J.; Singh, N.; Lyon, L.A. Label-free biosensing with hydrogel microlenses. Angew. Chem. Int. Ed. Engl. 2006, 45, 1446–1449. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Nayak, S.; Lyon, L.A. Bioresponsive hydrogel microlenses. J. Am. Chem. Soc. 2005, 127, 9588–9592. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.S.; Kim, J.; Sung, H.K. Characterization of a functional hydrogel layer on a silicon-based grating waveguide for a biochemical sensor. Sensors 2016, 16, 914. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Tanaka, M. Designing smart biomaterials for tissue engineering. Int. J. Mol. Sci. 2018, 19, 17. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Jang, H.; Lee, J.; Kim, J.H.; Kim, S.H.; Sun, K.; Park, Y. Regeneration of chronic myocardial infarction by injectable hydrogels containing stem cell homing factor sdf-1 and angiogenic peptide ac-sdkp. Biomaterials 2014, 35, 2436–2445. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Serpe, M.J.; Lyon, L.A. Hydrogel microparticles as dynamically tunable microlenses. J. Am. Chem. Soc. 2004, 126, 9512–9513. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Fillmore, D.J. Kinetics of swelling of gels. J. Chem. Phys. 1979, 70, 1214–1218. [Google Scholar] [CrossRef]
- Dusek, K.; Patterson, K. Transition on swollen polymer networks induced by intramolecular condensation. J. Polym. Sci. Polym. Phys. Ed. 1968, 6, 1209–1216. [Google Scholar]
- Pelton, R. Temperature-sensitive aqueous microgels. Adv. Colloid Interface Sci. 2000, 85, 1–33. [Google Scholar] [CrossRef]
- Serpe, M.J.; Jones, C.D.; Lyon, L.A. Layer-by-layer deposition of thermoresponsive microgel thin films. Langmuir 2003, 19, 8759–8764. [Google Scholar] [CrossRef]
- Jones, C.D.; Lyon, L.A. Synthesis and characterization of multiresponsive core-shell microgels. Macromolecules 2000, 33, 8301–8306. [Google Scholar] [CrossRef]
- Yin, X.; Hoffman, A.S.; Stayton, P.S. Poly(N-isopropylacrylamide-co-propylacrylic acid) copolymers that respond sharply to temperature and ph. Biomacromolecules 2006, 7, 1381–1385. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qiu, X.; Wu, C. Comparison of the coil-to-globule and the globule-to-coil transitions of a single poly(N-isopropylacrylamide) homopolymer chain in water. Macromolecules 1998, 31, 2972–2976. [Google Scholar] [CrossRef]
- Yoo, M.K.; Sung, Y.K.; Lee, Y.M.; Cho, C.S. Effect of polyelectrolyte on the lower critical solution temperature of poly(N-isopropyl acrylamide) in the poly(nipaam-co-acrylic acid) hydrogel. Polymer 2000, 41, 5713–5719. [Google Scholar] [CrossRef]
- Gan, D.; Lyon, L.A. Tunable swelling kinetics in core-shell hydrogel nanoparticles. J. Am. Chem. Soc. 2001, 123, 7511–7517. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Lyon, L.A. Soft nanotechnology with soft nanoparticles. Angew. Chem. Int. Ed. 2005, 44, 7686–7708. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Serpe, M.J.; Lyon, L.A. Photoswitchable microlens arrays. Angew. Chem. Int. Ed. 2005, 44, 1333–1336. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in regenerative medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [PubMed]
- Boffito, M.; Sirianni, P.; Di Rienzo, A.M.; Chiono, V. Thermosensitive block copolymer hydrogels based on poly(epsilon-caprolactone) and polyethylene glycol for biomedical applications: State of the art and future perspectives. J. Biomed. Mater. Res. A 2015, 103, 1276–1290. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zu, S.; Zhou, J.; Jiang, Q.; Du, B.; Shan, H.; Luo, Y.; Liu, Z.; Zhu, X.; Fang, Z. Single-nanoparticle plasmonic electro-optic modulator based on MoS2 monolayers. ACS Nano 2017, 11, 9720–9727. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.T.; Chuang, Y.J.; Wu, Y.C.; Yang, C.S.; Wang, M.C.; Tseng, F.G. A gold-nanoparticle-enhanced immune sensor based on fiber optic interferometry. Nanotechnology 2008, 19, 345501. [Google Scholar] [CrossRef] [PubMed]
- Shu, T.; Shen, Q.M.; Wan, Y.; Zhang, W.; Su, L.; Zhang, X.J.; Serpe, M.J. Silver nanoparticle-loaded microgel-based etalons for H2O2 sensing. RSC Adv. 2018, 8, 15567–15574. [Google Scholar] [CrossRef]
- Kim, J.; Park, J.E.; Nahrendorf, M.; Kim, D.-E. Direct thrombus imaging in stroke. J. Stroke 2016, 18, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Alkilany, A.M.; Lohse, S.E.; Murphy, C.J. The gold standard: Gold nanoparticle libraries to understand the nano-bio interface. Acc. Chem. Res. 2013, 46, 650–661. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yang, Z.; Wang, Y.; Zhang, G.; Shao, Y.; Jia, H.; Cao, T.; Wang, R.; Liu, D. Remote controlling DNA hydrogel by magnetic field. ACS Appl. Mater. Interfaces 2017, 9, 1995–2000. [Google Scholar] [CrossRef] [PubMed]
- Yata, T.; Takahashi, Y.; Tan, M.; Nakatsuji, H.; Ohtsuki, S.; Murakami, T.; Imahori, H.; Umeki, Y.; Shiomi, T.; Takakura, Y.; et al. DNA nanotechnology-based composite-type gold nanoparticle-immunostimulatory DNA hydrogel for tumor photothermal immunotherapy. Biomaterials 2017, 146, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Zhou, Q.; Mu, K.; Xie, H.; Zhu, Y.; Zhu, W.; Zhao, Y.; Xu, H.; Yang, X. Ph/temperature sensitive magnetic nanogels conjugated with cy5.5-labled lactoferrin for mr and fluorescence imaging of glioma in rats. Biomaterials 2013, 34, 7418–7428. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.-Q.; Wang, T.-X.; Liu, W.; Wang, C.-D.; Wang, D.; Shang, T.; Shen, L.-H.; Ren, L. Multifunctional au@ipn-pnipaam nanogels for cancer cell imaging and combined chemo-photothermal treatment. J. Mater. Chem. 2011, 21, 7240–7247. [Google Scholar] [CrossRef]
- Rittikulsittichai, S.; Kolhatkar, A.G.; Sarangi, S.; Vorontsova, M.A.; Vekilov, P.G.; Brazdeikis, A.; Randall Lee, T. Multi-responsive hybrid particles: Thermo-, ph-, photo-, and magneto-responsive magnetic hydrogel cores with gold nanorod optical triggers. Nanoscale 2016, 8, 11851–11861. [Google Scholar] [CrossRef] [PubMed]
- Laurenti, M.; Guardia, P.; Contreras-Caceres, R.; Perez-Juste, J.; Fernandez-Barbero, A.; Lopez-Cabarcos, E.; Rubio-Retama, J. Synthesis of thermosensitive microgels with a tunable magnetic core. Langmuir 2011, 27, 10484–10491. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.H.; Kim, S.; Lee, J.H.; Shin, T.H.; Yoo, D.; Cheon, J. Magnetic tandem apoptosis for overcoming multidrug-resistant cancer. Nano Lett. 2016, 16, 7455–7460. [Google Scholar] [CrossRef] [PubMed]
- Gan, D.; Lyon, L.A. Interfacial nonradiative energy transfer in responsive core-shell hydrogel nanoparticles. J. Am. Chem. Soc. 2001, 123, 8203–8209. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.T.; Nah, H.; Lee, J.H.; Moon, S.H.; Kim, M.G.; Cheon, J. Critical enhancements of mri contrast and hyperthermic effects by dopant-controlled magnetic nanoparticles. Angew. Chem. Int. Ed. Engl. 2009, 48, 1234–1238. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Seo, D.; Lee, J.U.; Southard, K.M.; Lim, Y.; Kim, D.; Gartner, Z.J.; Jun, Y.W.; Cheon, J. Single-cell mechanogenetics using monovalent magnetoplasmonic nanoparticles. Nat. Protoc. 2017, 12, 1871–1889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.K.; Hwang, G.; Woo, J.; Park, J.; Kim, J. Characterization of responsive hydrogel nanoparticles upon polyelectrolyte complexation. Polymers 2017, 9, 66. [Google Scholar] [CrossRef]
- Brown, A.C.; Stabenfeldt, S.E.; Ahn, B.; Hannan, R.T.; Dhada, K.S.; Herman, E.S.; Stefanelli, V.; Guzzetta, N.; Alexeev, A.; Lam, W.A.; et al. Ultrasoft microgels displaying emergent platelet-like behaviours. Nat. Mater. 2014, 13, 1108–1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burmistrova, A.; Richter, M.; Eisele, M.; Uzum, C.; von Klitzing, R. The effect of co-monomer content on the swelling/shrinking and mechanical behaviour of individually adsorbed pnipam microgel particles. Polymers 2011, 3, 1575–1590. [Google Scholar] [CrossRef]







| Temp (°C) | Dh (nm) | ||||||
|---|---|---|---|---|---|---|---|
| Transition Start (TS) | Transition End (TE) | VPTT | Transition Start (Ts) | Transition End (TE) | TE/TS | ||
| microgels | 5% AAc | 31 | 41 | 36 | 400 | 130 | 0.33 |
| 10% AAc | 32 | 42 | 37 | 480 | 150 | 0.31 | |
| 20% AAc | 32 | 50 | 41 | 560 | 190 | 0.34 | |
| aMNP-microgels | 5% AAc | 34 | 41 | 38 | 330 | 140 | 0.42 |
| 10% AAc | 34 | 45 | 40 | 440 | 150 | 0.34 | |
| 20% AAc | 35 | 53 | 44 | 610 | 190 | 0.31 | |
| microgels | 0% BIS | 32 | 41 | 37 | 670 | 140 | 0.21 |
| 2% BIS | 32 | 42 | 37 | 480 | 150 | 0.31 | |
| 5% BIS | 33 | 43 | 38 | 390 | 170 | 0.44 | |
| aMNP-microgels | 0% BIS | 34 | 42 | 38 | 600 | 140 | 0.23 |
| 2% BIS | 34 | 45 | 40 | 440 | 140 | 0.32 | |
| 5% BIS | 35 | 51 | 43 | 360 | 170 | 0.47 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.-K.; Park, Y.; Kim, J. Thermoresponsive Behavior of Magnetic Nanoparticle Complexed pNIPAm-co-AAc Microgels. Appl. Sci. 2018, 8, 1984. https://doi.org/10.3390/app8101984
Lee S-K, Park Y, Kim J. Thermoresponsive Behavior of Magnetic Nanoparticle Complexed pNIPAm-co-AAc Microgels. Applied Sciences. 2018; 8(10):1984. https://doi.org/10.3390/app8101984
Chicago/Turabian StyleLee, Su-Kyoung, Yongdoo Park, and Jongseong Kim. 2018. "Thermoresponsive Behavior of Magnetic Nanoparticle Complexed pNIPAm-co-AAc Microgels" Applied Sciences 8, no. 10: 1984. https://doi.org/10.3390/app8101984
APA StyleLee, S. -K., Park, Y., & Kim, J. (2018). Thermoresponsive Behavior of Magnetic Nanoparticle Complexed pNIPAm-co-AAc Microgels. Applied Sciences, 8(10), 1984. https://doi.org/10.3390/app8101984