Cu-Doped TiO2: Visible Light Assisted Photocatalytic Antimicrobial Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of Cu-TiO2
2.3. Density Functional Theory (DFT) Computations
2.4. Characterization
2.5. Measurement of Antimicrobial Activity
3. Results
3.1. Density Functional Theory Simulations
3.2. X-ray Diffraction (XRD)
3.3. Raman Spectroscopy
3.4. X-ray Photoelectron Spectroscopy (XPS)
3.5. UV–Vis Absorption and Bandgap Estimation
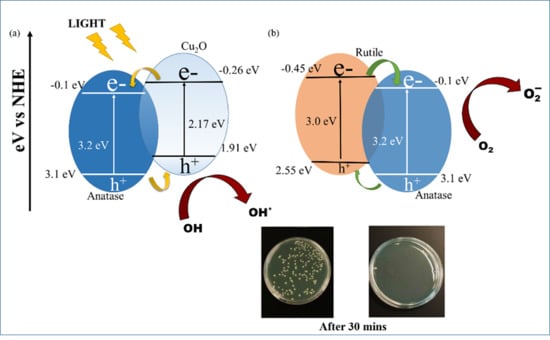
3.6. Photocatalytic Antibacterial Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Heseltine, E.; Rosen, J. WHO Guidelines for Indoor Air Quality: Dampness and Mould; WHO Regional Office Europe: København, Denmark, 2009. [Google Scholar]
- Jones, A.P. Indoor air quality and health. Atmos. Environ. 1999, 33, 4535–4564. [Google Scholar] [CrossRef]
- Burger, H. Bioaerosols: Prevalence and health effects in the indoor environment. J. Allergy Clin. Immunol. 1990, 86, 687–701. [Google Scholar] [CrossRef]
- Robbins, C.A.; Swenson, L.J.; Nealley, M.L.; Kelman, B.J.; Gots, R.E. Health effects of mycotoxins in indoor air: A critical review. Appl. Occup. Environ. Hyg. 2000, 15, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Verdier, T.; Coutand, M.; Bertron, A.; Roques, C. A review of indoor microbial growth across building materials and sampling and analysis methods. Build. Environ. 2014, 80, 136–149. [Google Scholar] [CrossRef]
- Dillon, H.K.; Miller, J.D.; Sorenson, W.; Douwes, J.; Jacobs, R.R. Review of methods applicable to the assessment of mold exposure to children. Environ. Health Perspect. 1999, 107 (Suppl. 3), 473. [Google Scholar] [CrossRef] [PubMed]
- Redlich, C.A.; Sparer, J.; Cullen, M.R. Sick-building syndrome. Lancet 1997, 349, 1013–1016. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Jalali, S. Nanotechnology: Advantages and drawbacks in the field of construction and building materials. Constr. Build. Mater. 2011, 25, 582–590. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Ho, S.S.H.; Lu, Y.; Niu, R.; Xu, L.; Cao, J.; Lee, S. Removal of indoor volatile organic compounds via photocatalytic oxidation: A short review and prospect. Molecules 2016, 21, 56. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.; Subramanian, G.; Pillai, S.C. Recent advances in photocatalysis for environmental applications. J. Environ. Chem. Eng. 2018, 6, 3531–3555. [Google Scholar] [CrossRef]
- Verdier, T.; Coutand, M.; Bertron, A.; Roques, C. Antibacterial activity of TiO2 photocatalyst alone or in coatings on E. coli: The influence of methodological aspects. Coatings 2014, 4, 670–686. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C 2015, 25, 1–29. [Google Scholar] [CrossRef]
- Pillai, S.C.; Periyat, P.; George, R.; McCormack, D.E.; Seery, M.K.; Hayden, H.; Colreavy, J.; Corr, D.; Hinder, S.J. Synthesis of high-temperature stable anatase TiO2 photocatalyst. J. Phys. Chem. C 2007, 111, 1605–1611. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Pillai, S.C.; Falaras, P.; O’shea, K.E.; Byrne, J.A.; Dionysiou, D.D. New insights into the mechanism of visible light photocatalysis. J. Phys. Chem. Lett. 2014, 5, 2543–2554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrne, C.; Fagan, R.; Hinder, S.; McCormack, D.E.; Pillai, S.C. New approach of modifying the anatase to rutile transition temperature in TiO2 photocatalysts. RSC Adv. 2016, 6, 95232–95238. [Google Scholar] [CrossRef]
- Periyat, P.; Baiju, K.; Mukundan, P.; Pillai, P.; Warrier, K. High temperature stable mesoporous anatase TiO2 photocatalyst achieved by silica addition. Appl. Catal. A 2008, 349, 13–19. [Google Scholar] [CrossRef]
- Panneri, S.; Ganguly, P.; Nair, B.N.; Mohamed, A.A.P.; Warrier, K.G.K.; Hareesh, U.N.S. Role of precursors on the photophysical properties of carbon nitride and its application for antibiotic degradation. Environ. Sci. Pollut. Res. 2017, 24, 8609–8618. [Google Scholar] [CrossRef] [PubMed]
- Vignesh, K.; Suganthi, A.; Min, B.-K.; Kang, M. Photocatalytic activity of magnetically recoverable MnFe2O4/g-C3N4/TiO2 nanocomposite under simulated solar light irradiation. J. Mol. Catal. A 2014, 395, 373–383. [Google Scholar] [CrossRef]
- Vignesh, K.; Priyanka, R.; Hariharan, R.; Rajarajan, M.; Suganthi, A. Fabrication of CdS and CuWO4 modified TiO2 nanoparticles and its photocatalytic activity under visible light irradiation. J. Ind. Eng. Chem. 2014, 20, 435–443. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Sorrell, C.C. Review of the anatase to rutile phase transformation. J. Mater. Sci. 2011, 46, 855–874. [Google Scholar] [CrossRef]
- El-Sheikh, S.M.; Zhang, G.; El-Hosainy, H.M.; Ismail, A.A.; O’Shea, K.E.; Falaras, P.; Kontos, A.G.; Dionysiou, D.D. High performance sulfur, nitrogen and carbon doped mesoporous anatase–brookite TiO2 photocatalyst for the removal of microcystin-LR under visible light irradiation. J. Hazard. Mater. 2014, 280, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Etacheri, V.; Seery, M.K.; Hinder, S.J.; Pillai, S.C. Nanostructured Ti1−x SxO2−yNy Heterojunctions for Efficient Visible-Light-Induced Photocatalysis. Inorg. Chem. 2012, 51, 7164–7173. [Google Scholar] [CrossRef] [PubMed]
- Etacheri, V.; Seery, M.K.; Hinder, S.J.; Pillai, S.C. Oxygen rich titania: A dopant free, high temperature stable, and visible-light active anatase photocatalyst. Adv. Funct. Mater. 2011, 21, 3744–3752. [Google Scholar] [CrossRef] [Green Version]
- Seery, M.K.; George, R.; Floris, P.; Pillai, S.C. Silver doped titanium dioxide nanomaterials for enhanced visible light photocatalysis. J. Photochem. Photobiol. A 2007, 189, 258–263. [Google Scholar] [CrossRef] [Green Version]
- Yoong, L.; Chong, F.K.; Dutta, B.K. Development of copper-doped TiO2 photocatalyst for hydrogen production under visible light. Energy 2009, 34, 1652–1661. [Google Scholar] [CrossRef]
- Xu, S.; Du, A.J.; Liu, J.; Ng, J.; Sun, D.D. Highly efficient CuO incorporated TiO2 nanotube photocatalyst for hydrogen production from water. Int. J. Hydrogen Energy 2011, 36, 6560–6568. [Google Scholar] [CrossRef]
- Lalitha, K.; Sadanandam, G.; Kumari, V.D.; Subrahmanyam, M.; Sreedhar, B.; Hebalkar, N.Y. Highly stabilized and finely dispersed Cu2O/TiO2: A promising visible sensitive photocatalyst for continuous production of hydrogen from glycerol: Water mixtures. J. Phys. Chem. C 2010, 114, 22181–22189. [Google Scholar] [CrossRef]
- Wu, F.; Hu, X.; Fan, J.; Liu, E.; Sun, T.; Kang, L.; Hou, W.; Zhu, C.; Liu, H. Photocatalytic activity of Ag/TiO2 nanotube arrays enhanced by surface plasmon resonance and application in hydrogen evolution by water splitting. Plasmonics 2013, 8, 501–508. [Google Scholar] [CrossRef]
- Nasution, H.W.; Purnama, E.; Kosela, S.; Gunlazuardi, J. Photocatalytic reduction of CO2 on copper-doped Titania catalysts prepared by improved-impregnation method. Catal. Commun. 2005, 6, 313–319. [Google Scholar] [CrossRef]
- Kim, T.W.; Ha, H.-W.; Paek, M.-J.; Hyun, S.-H.; Choy, J.-H.; Hwang, S.-J. Unique phase transformation behavior and visible light photocatalytic activity of titanium oxide hybridized with copper oxide. J. Mater. Chem. 2010, 20, 3238–3245. [Google Scholar] [CrossRef]
- Varghese, O.K.; Paulose, M.; LaTempa, T.J.; Grimes, C.A. High-rate solar photocatalytic conversion of CO2 and water vapor to hydrocarbon fuels. Nano Lett. 2009, 9, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Colon, G.; Maicu, M.; Hidalgo, M.S.; Navio, J. Cu-doped TiO2 systems with improved photocatalytic activity. Appl. Catal. B 2006, 67, 41–51. [Google Scholar] [CrossRef]
- Hassan, M.S.; Amna, T.; Kim, H.Y.; Khil, M.-S. Enhanced bactericidal effect of novel CuO/TiO2 composite nanorods and a mechanism thereof. Compos. Part B 2013, 45, 904–910. [Google Scholar] [CrossRef]
- Sunada, K.; Watanabe, T.; Hashimoto, K. Bactericidal activity of copper-deposited TiO2 thin film under weak UV light illumination. Environ. Sci. Technol. 2003, 37, 4785–4789. [Google Scholar] [CrossRef] [PubMed]
- Yousef, A.; El-Halwany, M.; Barakat, N.A.; Al-Maghrabi, M.N.; Kim, H.Y. Cu0-doped TiO2 nanofibers as potential photocatalyst and antimicrobial agent. J. Ind. Eng. Chem. 2015, 26, 251–258. [Google Scholar] [CrossRef]
- Karunakaran, C.; Abiramasundari, G.; Gomathisankar, P.; Manikandan, G.; Anandi, V. Cu-doped TiO2 nanoparticles for photocatalytic disinfection of bacteria under visible light. J. Colloid Interface Sci. 2010, 352, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Leyland, N.S.; Podporska-Carroll, J.; Browne, J.; Hinder, S.J.; Quilty, B.; Pillai, S.C. Highly Efficient F, Cu doped TiO2 anti-bacterial visible light active photocatalytic coatings to combat hospital-acquired infections. Sci. Rep. 2016, 6, 24770. [Google Scholar] [CrossRef] [PubMed]
- Yadav, H.M.; Otari, S.V.; Koli, V.B.; Mali, S.S.; Hong, C.K.; Pawar, S.H.; Delekar, S.D. Preparation and characterization of copper-doped anatase TiO2 nanoparticles with visible light photocatalytic antibacterial activity. J. Photochem. Photobiol. A 2014, 280, 32–38. [Google Scholar] [CrossRef]
- Miao, Y.; Xu, X.; Liu, K.; Wang, N. Preparation of novel Cu/TiO2 mischcrystal composites and antibacterial activities for Escherichia coli under visible light. Ceram. Int. 2017, 43, 9658–9663. [Google Scholar] [CrossRef]
- Ohwaki, T.; Saeki, S.; Aoki, K.; Morikawa, T. Evaluation of photocatalytic activities and characteristics of Cu-or Fe-modified nitrogen-doped titanium dioxides for applications in environmental purification. Jpn. J. Appl. Phys. 2015, 55, 01AA05. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [Green Version]
- Chand, R.; Obuchi, E.; Katoh, K.; Luitel, H.N.; Nakano, K. Enhanced photocatalytic activity of TiO2/SiO2 by the influence of Cu-doping under reducing calcination atmosphere. Catal. Commun. 2011, 13, 49–53. [Google Scholar] [CrossRef]
- Nolan, M.; Watson, G.W. Hole localization in Al doped silica: A DFT + U description. J. Chem. Phys. 2006, 125, 144701. [Google Scholar] [CrossRef] [PubMed]
- Nolan, M.; Watson, G.W. The electronic structure of alkali doped alkaline earth metal oxides: Li doping of MgO studied with DFT-GGA and GGA + U. Surf. Sci. 2005, 586, 25–37. [Google Scholar] [CrossRef]
- Carey, J.J.; Nolan, M. Dissociative adsorption of methane on the Cu and Zn doped surface of CeO2. Appl. Catal. B 2016, 197, 324–336. [Google Scholar] [CrossRef]
- Nolan, M.; Elliott, S.D. The p-type conduction mechanism in Cu2O: A first principles study. Phys. Chem. Chem. Phys. 2006, 8, 5350–5358. [Google Scholar] [CrossRef] [PubMed]
- Morgan, B.J.; Watson, G.W. A DFT + U description of oxygen vacancies at the TiO2 rutile (1 1 0) surface. Surf. Sci. 2007, 601, 5034–5041. [Google Scholar] [CrossRef]
- Nolan, M.; Elliott, S.D.; Mulley, J.S.; Bennett, R.A.; Basham, M.; Mulheran, P. Electronic structure of point defects in controlled self-doping of the TiO2 surface: Combined photoemission spectroscopy and density functional theory study. Phys. Rev. B 2008, 77, 235424. [Google Scholar] [CrossRef]
- Kowalski, P.M.; Camellone, M.F.; Nair, N.N.; Meyer, B.; Marx, D. Charge localization dynamics induced by oxygen vacancies on the TiO2 surface. Phys. Rev. Lett. 2010, 105, 146405. [Google Scholar] [CrossRef] [PubMed]
- Morgan, B.J.; Watson, G.W. Polaronic trapping of electrons and holes by native defects in anatase TiO2. Phys. Rev. B 2009, 80, 233102. [Google Scholar] [CrossRef]
- Henkelman, G.; Arnaldsson, A.; Jónsson, H. A fast and robust algorithm for Bader decomposition of charge density. Comput. Mater. Sci. 2006, 36, 354–360. [Google Scholar] [CrossRef]
- Scherrer, P. Estimation of the size and internal structure of colloidal particles by means of röntgen. Nachr. Ges. Wiss. Göttingen 1918, 2, 96–100. [Google Scholar]
- Panneri, S.; Ganguly, P.; Mohan, M.; Nair, B.N.; Mohamed, A.A.P.; Warrier, K.G.; Hareesh, U.S. Photoregenerable, Bifunctional Granules of Carbon-Doped g-C3N4 as Adsorptive Photocatalyst for the Efficient Removal of Tetracycline Antibiotic. ACS Sustain. Chem. Eng. 2017, 5, 1610–1618. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, R.; Li, J.; Li, L.; Lin, S. First-principles study on transition metal-doped anatase TiO2. Nanoscale Res. Lett. 2014, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Navas, J.; Sánchez-Coronilla, A.; Aguilar, T.; Hernández, N.C.; Desireé, M.; Sánchez-Márquez, J.; Zorrilla, D.; Fernández-Lorenzo, C.; Alcántara, R.; Martín-Calleja, J. Experimental and theoretical study of the electronic properties of Cu-doped anatase TiO2. Phys. Chem. Chem. Phys. 2014, 16, 3835–3845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yu, X.; McLeod, J.A.; Sun, X. First-principles study of Cu-doping and oxygen vacancy effects on TiO2 for water splitting. Chem. Phys. Lett. 2014, 612, 106–110. [Google Scholar] [CrossRef]
- Guo, M.; Du, J. First-principles study of electronic structures and optical properties of Cu, Ag, and Au-doped anatase TiO2. Physica B 2012, 407, 1003–1007. [Google Scholar] [CrossRef]
- Maimaiti, Y.; Nolan, M.; Elliott, S.D. Reduction mechanisms of the CuO (111) surface through surface oxygen vacancy formation and hydrogen adsorption. Phys. Chem. Chem. Phys. 2014, 16, 3036–3046. [Google Scholar] [CrossRef] [PubMed]
- Assadi, M.H.N.; Hanaor, D.A. The effects of copper doping on photocatalytic activity at planes of anatase TiO2: A theoretical study. Appl. Surf. Sci. 2016, 387, 682–689. [Google Scholar] [CrossRef]
- Zhang, W.; Yin, J.-R.; Tang, X.-Q.; Zhang, P.; Ding, Y.-H. Density functional theory studies on the structural and physical properties of Cu-doped anatase TiO2 surface. Physica E 2017, 85, 259–263. [Google Scholar] [CrossRef]
- Duhalde, S.; Vignolo, M.F.; Golmar, F.; Chiliotte, C.; Torres, C.E.R.; Errico, L.A.; Cabrera, A.F.; Rentería, M.; Sánchez, F.H.; Weissmann, M. Appearance of room-temperature ferromagnetism in Cu-doped TiO2−δ films. Phys. Rev. B 2005, 72, 161313. [Google Scholar] [CrossRef]
- Iwaszuk, A.; Nolan, M. Charge compensation in trivalent cation doped bulk rutile TiO2. J. Phys. 2011, 23, 334207. [Google Scholar] [CrossRef] [PubMed]
- Du, M.-H.; Zhang, S.B. Impurity-bound small polarons in ZnO: Hybrid density functional calculations. Phys. Rev. B 2009, 80, 115217. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Walsh, A.; Morgan, B.J.; Nolan, M.; Fearon, J.; Watson, G.W. Surface sensitivity in lithium-doping of MgO: A density functional theory study with correction for on-site Coulomb interactions. J. Phys. Chem. C 2007, 111, 7971–7979. [Google Scholar] [CrossRef]
- Schirmer, O.F. O–bound small polarons in oxide materials. J. Phys. 2006, 18, R667. [Google Scholar] [CrossRef]
- Stoneham, A.M.; Gavartin, J.; Shluger, A.L.; Kimmel, A.V.; Ramo, D.M.; Rønnow, H.M.; Aeppli, G.; Renner, C. Trapping, self-trapping and the polaron family. J. Phys. 2007, 19, 255208. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-F.; Lee, C.-Y.; Yeng, M.-Y.; Chiu, H.-T. The effect of calcination temperature on the crystallinity of TiO2 nanopowders. J. Cryst. Growth 2003, 247, 363–370. [Google Scholar] [CrossRef]
- Ako, R.T.; Ekanayake, P.; Young, D.J.; Hobley, J.; Chellappan, V.; Tan, A.L.; Gorelik, S.; Subramanian, G.S.; Lim, C.M. Evaluation of surface energy state distribution and bulk defect concentration in DSSC photoanodes based on Sn, Fe, and Cu doped TiO2. Appl. Surf. Sci. 2015, 351, 950–961. [Google Scholar] [CrossRef]
- Jaiswal, R.; Bharambe, J.; Patel, N.; Dashora, A.; Kothari, D.; Miotello, A. Copper and Nitrogen co-doped TiO2 photocatalyst with enhanced optical absorption and catalytic activity. Appl. Catal. B 2015, 168, 333–341. [Google Scholar] [CrossRef]
- Sinatra, L.; LaGrow, A.P.; Peng, W.; Kirmani, A.R.; Amassian, A.; Idriss, H.; Bakr, O.M. A Au/Cu2O–TiO2 system for photo-catalytic hydrogen production. A pn-junction effect or a simple case of in situ reduction? J. Catal. 2015, 322, 109–117. [Google Scholar] [CrossRef]
- Ganesh, I.; Kumar, P.P.; Annapoorna, I.; Sumliner, J.M.; Ramakrishna, M.; Hebalkar, N.Y.; Padmanabham, G.; Sundararajan, G. Preparation and characterization of Cu-doped TiO2 materials for electrochemical, photoelectrochemical, and photocatalytic applications. Appl. Surf. Sci. 2014, 293, 229–247. [Google Scholar] [CrossRef]
- Dashora, A.; Patel, N.; Kothari, D.; Ahuja, B.; Miotello, A. Formation of an intermediate band in the energy gap of TiO2 by Cu–N-codoping: First principles study and experimental evidence. Sol. Energy Mater. Sol. Cells 2014, 125, 120–126. [Google Scholar] [CrossRef]
- Jung, M.; Hart, J.N.; Scott, J.; Ng, Y.H.; Jiang, Y.; Amal, R. Exploring Cu oxidation state on TiO2 and its transformation during photocatalytic hydrogen evolution. Appl. Catal. A 2016, 521, 190–201. [Google Scholar] [CrossRef]
- Hu, Q.; Huang, J.; Li, G.; Jiang, Y.; Lan, H.; Guo, W.; Cao, Y. Origin of the improved photocatalytic activity of Cu incorporated TiO2 for hydrogen generation from water. Appl. Surf. Sci. 2016, 382, 170–177. [Google Scholar] [CrossRef]
- Parmigiani, F.; Pacchioni, G.; Illas, F.; Bagus, P. Studies of the CuO bond in cupric oxide by X-ray photoelectron spectroscopy and ab initio electronic structure models. J. Electron Spectrosc. Relat. Phenom. 1992, 59, 255–269. [Google Scholar] [CrossRef]
- Hu, Q.; Huang, J.; Li, G.; Chen, J.; Zhang, Z.; Deng, Z.; Jiang, Y.; Guo, W.; Cao, Y. Effective water splitting using CuOx/TiO2 composite films: Role of Cu species and content in hydrogen generation. Appl. Surf. Sci. 2016, 369, 201–206. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. Sect. A 1976, 32, 751–767. [Google Scholar] [CrossRef] [Green Version]
- Kubacka, A.; Munoz-Batista, M.; Fernández-García, M.; Obregón, S.; Colón, G. Evolution of H2 photoproduction with Cu content on CuOx-TiO2 composite catalysts prepared by a microemulsion method. Appl. Catal. B 2015, 163, 214–222. [Google Scholar] [CrossRef]
- Aguilera-Ruiz, E.; García-Pérez, U.M.; de la Garza-Galván, M.; Zambrano-Robledo, P.; Bermúdez-Reyes, B.; Peral, J. Efficiency of Cu2O/BiVO4 particles prepared with a new soft procedure on the degradation of dyes under visible-light irradiation. Appl. Surf. Sci. 2015, 328, 361–367. [Google Scholar] [CrossRef]
- Siripala, W.; Ivanovskaya, A.; Jaramillo, T.F.; Baeck, S.-H.; McFarland, E.W. A Cu2O/TiO2 heterojunction thin film cathode for photoelectrocatalysis. Sol. Energy Mater. Sol. Cells 2003, 77, 229–237. [Google Scholar] [CrossRef]
- Scanlon, D.O.; Dunnill, C.W.; Buckeridge, J.; Shevlin, S.A.; Logsdail, A.J.; Woodley, S.M.; Catlow, C.R.A.; Powell, M.J.; Palgrave, R.G.; Parkin, I.P.; et al. Band alignment of rutile and anatase TiO2. Nat. Mater. 2013, 12, 798. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Tang, M.; Chen, C.; Chen, M.; Luo, K.; Xu, J.; Zhou, D.; Wu, F.J.E.S. Technology, Efficient bacterial inactivation by transition metal catalyzed auto-oxidation of sulfite. Environ. Sci. Technol. 2017, 51, 12663–12671. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, P.; Byrne, C.; Breen, A.; Pillai, S.C. Antimicrobial activity of photocatalysts: Fundamentals, mechanisms, kinetics and recent advances. Appl. Catal. B 2018, 225, 51–75. [Google Scholar] [CrossRef]












| Sample | Calcined Temperature (in °C) | Particle Size (in nm) |
|---|---|---|
| Control● | 500 | 8.83 |
| 0.5% Cu-TiO2 | 500 | 11.34 |
| 0.5% Cu-TiO2 | 600 | 22.35 |
| 0.5% Cu-TiO2 | 650 | 28.84 |
| 0.5% Cu-TiO2 | 700 | 32.93 |
| Control● | 700 | 33.80 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mathew, S.; Ganguly, P.; Rhatigan, S.; Kumaravel, V.; Byrne, C.; Hinder, S.J.; Bartlett, J.; Nolan, M.; Pillai, S.C. Cu-Doped TiO2: Visible Light Assisted Photocatalytic Antimicrobial Activity. Appl. Sci. 2018, 8, 2067. https://doi.org/10.3390/app8112067
Mathew S, Ganguly P, Rhatigan S, Kumaravel V, Byrne C, Hinder SJ, Bartlett J, Nolan M, Pillai SC. Cu-Doped TiO2: Visible Light Assisted Photocatalytic Antimicrobial Activity. Applied Sciences. 2018; 8(11):2067. https://doi.org/10.3390/app8112067
Chicago/Turabian StyleMathew, Snehamol, Priyanka Ganguly, Stephen Rhatigan, Vignesh Kumaravel, Ciara Byrne, Steven J. Hinder, John Bartlett, Michael Nolan, and Suresh C. Pillai. 2018. "Cu-Doped TiO2: Visible Light Assisted Photocatalytic Antimicrobial Activity" Applied Sciences 8, no. 11: 2067. https://doi.org/10.3390/app8112067
APA StyleMathew, S., Ganguly, P., Rhatigan, S., Kumaravel, V., Byrne, C., Hinder, S. J., Bartlett, J., Nolan, M., & Pillai, S. C. (2018). Cu-Doped TiO2: Visible Light Assisted Photocatalytic Antimicrobial Activity. Applied Sciences, 8(11), 2067. https://doi.org/10.3390/app8112067