A Comparison of Palladium Sorption Using Polyethylenimine Impregnated Alginate-Based and Carrageenan-Based Algal Beads
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of LD/PEI and CC/PEI Beads
2.3. Characterization
2.4. Sorption in Mono-Metal System
2.5. Sorption in Complex System
2.6. Modeling
- (a)
- the determination of the sorption capacities in function of the residual metal concentration (principle of the sorption isotherms); this may be helpful for predicting the sorption capacity under selected experimental conditions, where a good fit is of great importance;
- (b)
- an approach of binding mechanism (the hypotheses attached to the model are indicative of mechanisms possibly involved in metal binding; however, the mathematical fit does not necessarily mean that the hypothesized mechanisms are fulfilled; this should be confirmed by physico-chemical analyses);
- (c)
- the determination of maximum sorption capacities (saturation level) and the affinity (correlated to the initial slope of the curve) between the sorbent and the solute under selected experimental conditions (for the comparison of sorbents).
2.7. Statistical Analysis
3. Results and Discussion
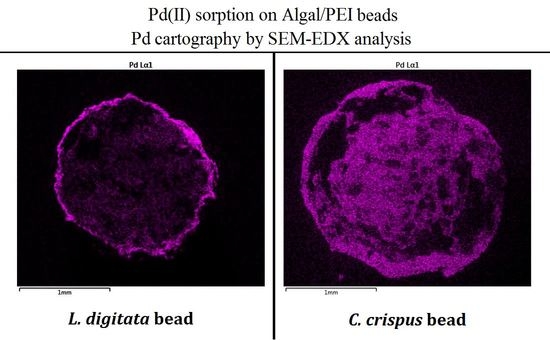
3.1. Characterization
3.2. Pd(II) Sorption in Mono Metal System
3.2.1. pH Effect
3.2.2. Uptake Kinetics
3.2.3. Sorption Isotherms
3.3. Pd(II) Sorption in Complex Systems
3.3.1. Effect of Anions and Cations
3.3.2. Application to the Treatment of “Synthesized” Wastewaters
4. Perspectives
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Lim, A.; Song, M.-H.; Cho, C.-W.; Yun, Y.-S. Development of Surface-Modified Polyacrylonitrile Fibers and Their Selective Sorption Behavior of Precious Metals. Appl. Sci. 2016, 6, 378. [Google Scholar] [CrossRef]
- Hirano, Y.; Kasai, Y.; Sagata, K.; Kita, Y. Unique approach for transforming glucose to C3 platform chemicals using metallic iron and a Pd/C catalyst in water. Bull. Chem. Soc. Jpn. 2016, 89, 1026–1033. [Google Scholar] [CrossRef]
- Zhou, C.; Ontiveros-Valencia, A.; Wang, Z.; Maldonado, J.; Zhao, H.-P.; Krajmalnik-Brown, R.; Rittmann, B.E. Palladium recovery in a H2-based membrane biofilm reactor: Formation of Pd (0) nanoparticles through enzymatic and autocatalytic reductions. Environ. Sci. Technol. 2016, 50, 2546–2555. [Google Scholar] [CrossRef] [PubMed]
- Shams, K.; Beiggy, M.; Shirazi, A.G. Platinum recovery from a spent industrial dehydrogenation catalyst using cyanide leaching followed by ion exchange. Appl. Catal. A Gen. 2004, 258, 227–234. [Google Scholar] [CrossRef]
- Di Natale, F.; Orefice, M.; La Motta, F.; Erto, A.; Lancia, A. Unveiling the potentialities of activated carbon in recovering palladium from model leaching solutions. Sep. Purif. Technol. 2017, 174, 183–193. [Google Scholar] [CrossRef]
- Butewicz, A.; Gavilan, K.C.; Pestov, A.; Yatluk, Y.; Trochimczuk, A.; Guibal, E. Palladium and platinum sorption on a thiocarbamoyl-derivative of chitosan. J. Appl. Polym. Sci. 2010, 116, 3318–3330. [Google Scholar] [CrossRef]
- Morisada, S.; Kim, Y.-H.; Ogata, T.; Marutani, Y.; Nakano, Y. Improved adsorption behaviors of amine-modified tannin gel for palladium and platinum ions in acidic chloride solutions. Ind. Eng. Chem. Res. 2011, 50, 1875–1880. [Google Scholar] [CrossRef]
- Guibal, E.; Sweeney, N.V.O.; Zikan, M.; Vincent, T.; Tobin, J. Competitive sorption of platinum and palladium on chitosan derivatives. Int. J. Biol. Macromol. 2001, 28, 401–408. [Google Scholar] [CrossRef]
- Won, S.W.; Kwak, I.S.; Yun, Y.-S. The role of biomass in polyethylenimine-coated chitosan/bacterial biomass composite biosorbent fiber for removal of Ru from acetic acid waste solution. Bioresour. Technol. 2014, 160, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Mahdavinia, G.R.; Bazmizeynabad, F.; Seyyedi, B. kappa-Carrageenan beads as new adsorbent to remove crystal violet dye from water: Adsorption kinetics and isotherm. Desalin. Water Treat. 2015, 53, 2529–2539. [Google Scholar] [CrossRef]
- Mahdavinia, G.R.; Iravani, S.; Zoroufi, S.; Hosseinzadeh, H. Magnetic and K+-cross-linked kappa-carrageenan nanocomposite beads and adsorption of crystal violet. Iran. Polym. J. 2014, 23, 335–344. [Google Scholar] [CrossRef]
- Bertagnolli, C.; Grishin, A.; Vincent, T.; Guibal, E. Boron removal by a composite sorbent: Polyethylenimine/tannic acid derivative immobilized in alginate hydrogel beads. J. Environ. Sci. Health Part A 2017, 52, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Vincent, T.; Roux, J.-C.; Faur, C.; Guibal, E. Innovative conditioning of algal-based sorbents: Macro-porous discs for palladium sorption. Chem. Eng. J. 2017, 325, 521–532. [Google Scholar] [CrossRef]
- Mahdavinia, G.R.; Massoudi, A.; Baghban, A.; Shokri, E. Study of adsorption of cationic dye on magnetic kappa-carrageenan/PVA nanocomposite hydrogels. J. Environ. Chem. Eng. 2014, 2, 1578–1587. [Google Scholar] [CrossRef]
- Willner, I.; Eichen, Y.; Frank, A.J.; Fox, M.A. Photoinduced electron-transfer processes using organized redox-functionalized bipyridinium-polyethylenimine-titania colloids and particulate assemblies. J. Phys. Chem. 1993, 97, 7264–7271. [Google Scholar] [CrossRef]
- Elnashar, M.M.; Yassin, M.A.; Kahil, T. Novel thermally and mechanically stable hydrogel for enzyme immobilization of penicillin G acylase via covalent technique. J. Appl. Polym. Sci. 2008, 109, 4105–4111. [Google Scholar] [CrossRef]
- Zaak, H.; Fernandez-Lopez, L.; Otero, C.; Sassi, M.; Fernandez-Lafuente, R. Improved stability of immobilized lipases via modification with polyethylenimine and glutaraldehyde. Enzyme Microb. Technol. 2017, 106, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Kononova, O.; Kholmogorov, A.; Mikhlina, E. Palladium sorption on vinylpyridine ion exchangers from chloride solutions obtained from spent catalysts. Hydrometallurgy 1998, 48, 65–72. [Google Scholar] [CrossRef]
- Ricoux, Q.; Méricq, J.; Bouyer, D.; Bocokić, V.; Hernandez-Juarez, L.; van Zutphen, S.; Faur, C. A selective dynamic sorption-filtration process for separation of Pd (II) ions using an aminophosphine oxide polymer. Sep. Purif. Technol. 2017, 174, 159–165. [Google Scholar] [CrossRef]
- Lagergren, S. About the theory of so-called adsorption of soluble substances. Kungliga Sven. Vetenskapsakad. 1898, 24, 1–39. [Google Scholar]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process. Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Weber, W.J.; Morris, J.C. Kinetics of adsorption on carbon from solution. J. Sanit. Eng. Div. 1963, 89, 31–60. [Google Scholar]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Freundlich, H. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 1100–1107. [Google Scholar]
- Sips, R. Combined form of Langmuir and Freundlich equations. J. Chem. Phys. 1948, 16, 490–495. [Google Scholar] [CrossRef]
- Schiewer, S.; Balaria, A. Biosorption of Pb2+ by original and protonated citrus peels: Equilibrium, kinetics, and mechanism. Chem. Eng. J. 2009, 146, 211–219. [Google Scholar] [CrossRef]
- Naebe, M.; Wang, J.; Amini, A.; Khayyam, H.; Hameed, N.; Li, L.H.; Chen, Y.; Fox, B. Mechanical property and structure of covalent functionalised graphene/epoxy nanocomposites. Sci. Rep. 2014, 4, 4375. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wang, L.; Mott, D.; Njoki, P.N.; Kariuki, N.; Zhong, C.-J.; He, T. Ternary alloy nanoparticles with controllable sizes and composition and electrocatalytic activity. J. Mater. Chem. 2006, 16, 1665–1673. [Google Scholar] [CrossRef]
- Hofmann, M.P.; Young, A.M.; Gbureck, U.; Nazhat, S.N.; Barralet, J.E. FTIR-monitoring of a fast setting brushite bone cement: Effect of intermediate phases. J. Mater. Chem. 2006, 16, 3199–3206. [Google Scholar] [CrossRef]
- Theras, J.E.M.; Kalaivani, D.; Jayaraman, D.; Joseph, V. Growth and spectroscopic, thermodynamic and nonlinear optical studies of L-threonine phthalate crystal. J. Cryst. Growth 2015, 427, 29–35. [Google Scholar] [CrossRef]
- Wang, L.; Ji, Q.; Glass, T.; Ward, T.; McGrath, J.; Muggli, M.; Burns, G.; Sorathia, U. Synthesis and characterization of organosiloxane modified segmented polyether polyurethanes. Polymer 2000, 41, 5083–5093. [Google Scholar] [CrossRef]
- Wang, X.; Li, D.; Wang, W.; Feng, Q.; Cui, F.; Xu, Y.; Song, X.; van der Werf, M. Crosslinked collagen/chitosan matrix for artificial livers. Biomaterials 2003, 24, 3213–3220. [Google Scholar] [CrossRef]
- Lawrie, G.; Keen, I.; Drew, B.; Chandler-Temple, A.; Rintoul, L.; Fredericks, P.; Grøndahl, L. Interactions between alginate and chitosan biopolymers characterized using FTIR and XPS. Biomacromolecules 2007, 8, 2533–2541. [Google Scholar] [CrossRef] [PubMed]
- Zawadzki, J.; Kaczmarek, H. Thermal treatment of chitosan in various conditions. Carbohyd. Polym. 2010, 80, 394–400. [Google Scholar] [CrossRef]
- Rosu, D.; Rosu, L.; Cascaval, C.N. IR-change and yellowing of polyurethane as a result of UV irradiation. Polym. Degrad. Stabil. 2009, 94, 591–596. [Google Scholar] [CrossRef]
- Tarulli, S.; Quinzani, O.; Piro, O.E.; Castellano, E.E.; Baran, E. Structural and spectroscopic characterization of bis(thiosaccharinato) bis(benzimidazole) cadmium (II). J. Mol. Struct. 2006, 797, 56–60. [Google Scholar] [CrossRef]
- Qiao, J.; Hamaya, T.; Okada, T. New highly proton-conducting membrane poly(vinylpyrrolidone)(PVP) modified poly(vinyl alcohol)/2-acrylamido-2-methyl-1-propanesulfonic acid (PVA–PAMPS) for low temperature direct methanol fuel cells (DMFCs). Polymer 2005, 46, 10809–10816. [Google Scholar] [CrossRef]
- Ríos-Gómez, J.; Lucena, R.; Cárdenas, S. Paper supported polystyrene membranes for thin film microextraction. Microchem. J. 2017, 133, 90–95. [Google Scholar] [CrossRef]
- Lindén, J.B.; Larsson, M.; Kaur, S.; Skinner, W.M.; Miklavcic, S.J.; Nann, T.; Kempson, I.M.; Nydén, M. Polyethyleneimine for copper absorption II: Kinetics, selectivity and efficiency from seawater. RSC Adv. 2015, 5, 51883–51890. [Google Scholar] [CrossRef]
- Tan, I.S.; Lee, K.T. Immobilization of β-glucosidase from Aspergillus niger on κ-carrageenan hybrid matrix and its application on the production of reducing sugar from macroalgae cellulosic residue. Bioresour. Technol. 2015, 184, 386–394. [Google Scholar] [CrossRef] [PubMed]
- Xiaohong, G.; Yang, C.Q. FTIR spectroscopy study of the formation of cyclic anhydride intermediates of polycarboxylic acids catalyzed by sodium hypophosphite. Text. Res. J. 2000, 70, 64–70. [Google Scholar] [CrossRef]
- Haug, A. Affinity of some divalent metals to different types of alginates. Acta Chem. Scand. 1961, 15, 1794. [Google Scholar] [CrossRef]
- Sadeghi, M. Synthesis of a biocopolymer carrageenan-g-poly (AAm-co-IA)/montmorilonite superabsorbent hydrogel composite. Braz. J. Chem. Eng. 2012, 29, 295–305. [Google Scholar] [CrossRef]
- Sekkal, M.; Legrand, P.; Huvenne, J.P.; Verdus, M.C. The use of FTIR microspectrometry as a new tool for the identification in situ of polygalactanes in red seaweeds. J. Mol. Struct. 1993, 294, 227–230. [Google Scholar] [CrossRef]
- Ricoux, Q.; Bocokić, V.; Méricq, J.; Bouyer, D.; Zutphen, S.; Faur, C. Selective recovery of palladium using an innovative functional polymer containing phosphine oxide. Chem. Eng. J. 2015, 264, 772–779. [Google Scholar] [CrossRef]
- Choong, T.S.; Chuah, T. Comment on “Separation of vitamin E from palm fatty acid distillate using silica: I. Equilibrium of batch adsorption by BS Chu et al. [Journal of Food Engineering 62 (2004) 97–103]”. J. Food Eng. 2005, 67, 379. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Hosseini-Bandegharaei, A.; Chao, H.-P. Mistakes and inconsistencies regarding adsorption of contaminants from aqueous solutions: A critical review. Water Res. 2017, 120, 88–116. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Chen, X.; Wu, F.; Ji, Y. High adsorption capability and selectivity of ZnO nanoparticles for dye removal. Coll. Surf. A 2016, 509, 474–483. [Google Scholar] [CrossRef]
- Modwi, A.; Khezami, L.; Taha, K.; Al-Duaij, O.; Houas, A. Fast and high efficiency adsorption of Pb (II) ions by Cu/ZnO composite. Mater. Lett. 2017, 195, 41–44. [Google Scholar] [CrossRef]
- Fleurence, J. Seaweed proteins: Biochemical, nutritional aspects and potential uses. Trends Food Sci. Technol. 1999, 10, 25–28. [Google Scholar] [CrossRef]
- Foo, K.; Hameed, B. Insights into the modeling of adsorption isotherm systems. Chem. Eng. J. 2010, 156, 2–10. [Google Scholar] [CrossRef]
- Sari, A.; Mendil, D.; Tuzen, M.; Soylak, M. Biosorption of palladium (II) from aqueous solution by moss (Racomitrium lanuginosum) biomass: Equilibrium, kinetic and thermodynamic studies. J. Hazard. Mater. 2009, 162, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Vincent, T.; Roux, J.-C.; Faur, C.; Guibal, E. Pd (II) and Pt (IV) sorption using alginate and algal-based beads. Chem. Eng. J. 2017, 313, 567–579. [Google Scholar] [CrossRef]
- Cataldo, S.; Muratore, N.; Orecchio, S.; Pettignano, A. Enhancement of adsorption ability of calcium alginate gel beads towards Pd (II) ion. A kinetic and equilibrium study on hybrid Laponite and Montmorillonite–alginate gel beads. Appl. Clay Sci. 2015, 118, 162–170. [Google Scholar] [CrossRef]
- Kimuro, T.; Gandhi, M.R.; Kunda, U.M.R.; Hamada, F.; Yamada, M. Palladium (II) sorption of a diethylphosphate-modified thiacalix [6] arene immobilized on amberlite resin. Hydrometallurgy 2017, 171, 254–261. [Google Scholar] [CrossRef]
- Navarro, R.; Saucedo, I.; Gonzalez, C.; Guibal, E. Amberlite XAD-7 impregnated with Cyphos IL-101 (tetraalkylphosphonium ionic liquid) for Pd (II) recovery from HCl solutions. Chem. Eng. J. 2012, 185, 226–235. [Google Scholar] [CrossRef]
- Gandhi, M.R.; Yamada, M.; Kondo, Y.; Shibayama, A.; Hamada, F. p-Sulfonatothiacalix [6] arene-impregnated resins for the sorption of platinum group metals and effective separation of palladium from automotive catalyst residue. J. Ind. Eng. Chem. 2015, 30, 20–28. [Google Scholar] [CrossRef]
- Cataldo, S.; Gianguzza, A.; Pettignano, A. Sorption of Pd (II) ion by calcium alginate gel beads at different chloride concentrations and pH. A kinetic and equilibrium study. Arab. J. Chem. 2016, 9, 656–667. [Google Scholar] [CrossRef]
- Nagireddi, S.; Golder, A.K.; Uppaluri, R. Investigation on Pd (II) removal and recovery characteristics of chitosan from electroless plating solutions. J. Water Process Eng. 2017, 19, 8–17. [Google Scholar] [CrossRef]
- Sharma, S.; Rajesh, N. Augmenting the adsorption of palladium from spent catalyst using a thiazole ligand tethered on an amine functionalized polymeric resin. Chem. Eng. J. 2016, 283, 999–1008. [Google Scholar] [CrossRef]
- Su, C.; Puls, R.W. Arsenate and arsenite removal by zerovalent iron: Effects of phosphate, silicate, carbonate, borate, sulfate, chromate, molybdate, and nitrate, relative to chloride. Environ. Sci. Technol. 2001, 35, 4562–4568. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, V.R.; Samanta, C.; Choudhary, T. Factors influencing decomposition of H2O2 over supported Pd catalyst in aqueous medium. J. Mol. Catal. A Chem. 2006, 260, 115–120. [Google Scholar] [CrossRef]










| Model | Parameter | LD/PEI | CC/PEI |
|---|---|---|---|
| Experiment | qeq,exp (mmol g−1) | 0.81 | 1.36 |
| PFORE | qeq,calc (mmol g−1) | 0.77 (0.041) | 1.30 (0.038) |
| k1 × 103 (min−1) | 7.51 (1.07) | 3.03 (0.32) | |
| R2 | 0.94 | 0.97 | |
| PSORE | qeq,calc (mmol g−1) | 0.82 (0.037) | 1.42 (0.032) |
| k2 × 103 (g mmol−1 min−1) | 14.42 (2.13) | 3.08 (0.42) | |
| R2 | 0.97 | 0.99 | |
| IPDE | kid,1 (mmol g−1 h−0.5) | 0.267 (0.008) | 0.319 (0.006) |
| C1 | 0.047 (0.008) | 0.022 (0.01) | |
| R12 | 0.99 | 0.99 | |
| kid,2 (mmol g−1 h−0.5) | 0.105 (0.001) | 0.041 (0.003) | |
| C2 | 0.400 (0.001) | 1.047 (0.02) | |
| R22 | 0.99 | 0.99 |
| Model | Parameter | LD | CC | LD/PEI | CC/PEI |
|---|---|---|---|---|---|
| qeq,exp (mmol Pd g−1) | 0.11 | 0.04 | 0.85 | 1.34 | |
| Langmuir | qeq,cal (mmol Pd g−1) | 0.11 (0.01) | 0.04 (0.00) | 0.81 (0.03) | 1.31 (0.05) |
| b (L mmol−1) | 112.67 (30.47) | 26.79 (7.69) | 262.71 (102.8) | 129.01 (32.3) | |
| R2 | 0.95 | 0.91 | 0.88 | 0.96 | |
| Freundlich | KF (mmol g−1)/(mmol L−1)n | 0.14 (0.03) | 0.05 (0.01) | 0.90 (0.04) | 1.47 (0.09) |
| n | 0.16 (0.04) | 0.24 (0.03) | 0.16 (0.03) | 0.16 (0.03) | |
| R2 | 0.77 | 0.97 | 0.90 | 0.89 | |
| Sips | qeq,cal (mmol Pd g−1) | 0.11 (0.01) | 0.08 (0.01) | 0.88 (0.08) | 1.44 (0.05) |
| b (L mmol−1) | 382.54 (93.44) | 1.18 (0.43) | 16.64 (5.72) | 15.66 (5.69) | |
| n | 0.80 (0.02) | 2.66 (1.07) | 1.72 (0.07) | 1.71 (0.06) | |
| R2 | 0.96 | 0.96 | 0.95 | 0.99 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Vincent, T.; Faur, C.; Guibal, E. A Comparison of Palladium Sorption Using Polyethylenimine Impregnated Alginate-Based and Carrageenan-Based Algal Beads. Appl. Sci. 2018, 8, 264. https://doi.org/10.3390/app8020264
Wang S, Vincent T, Faur C, Guibal E. A Comparison of Palladium Sorption Using Polyethylenimine Impregnated Alginate-Based and Carrageenan-Based Algal Beads. Applied Sciences. 2018; 8(2):264. https://doi.org/10.3390/app8020264
Chicago/Turabian StyleWang, Shengye, Thierry Vincent, Catherine Faur, and Eric Guibal. 2018. "A Comparison of Palladium Sorption Using Polyethylenimine Impregnated Alginate-Based and Carrageenan-Based Algal Beads" Applied Sciences 8, no. 2: 264. https://doi.org/10.3390/app8020264
APA StyleWang, S., Vincent, T., Faur, C., & Guibal, E. (2018). A Comparison of Palladium Sorption Using Polyethylenimine Impregnated Alginate-Based and Carrageenan-Based Algal Beads. Applied Sciences, 8(2), 264. https://doi.org/10.3390/app8020264