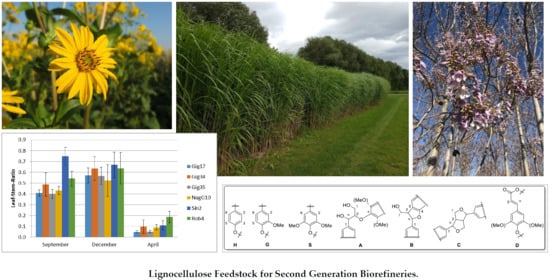
Low-Input Crops as Lignocellulosic Feedstock for Second-Generation Biorefineries and the Potential of Chemometrics in Biomass Quality Control
Abstract
:Featured Application
Abstract
1. Introduction
2. Lignocellulose Feedstock Biorefineries
2.1. First- and Second-Generation LCF Biorefineries
2.2. Reported Techno-Economic Analysis Studies
2.3. Low-Input Crops: Sources and Availability
2.3.1. C4 Grasses: Miscanthus
2.3.2. Fast Growing Trees: Paulownia, Eucalyptus, and Pinus
2.3.3. Cup Plants: Silphium Perfoliatum
3. LCF Structure Analysis and Quality Control
3.1. Spectroscopic Data Processing Using Chemometric Methods for Biomass Analysis
3.2. Chemometrics Used for Ligocellulose Feedstock Specification
3.3. Future Aspects Using Chemometrics for LCF Quality Control
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. In A Sustainable Bioeconomy for Europe: Strengthening the Connection between Economy, Society and the Environment; Reference No. 11.10.2018 COM; The European Commission: Brussels, Belgium, 2018.
- Vanhamaki, S.; Medkova, K.; Malamakis, A.; Kontogianni, S.; Marisova, E.; Huisman-Dellago, D.; Moussiopoulos, N. Bio-based Circular Economy in European National and Regional Strategies. Int. J. Sustain. Dev. Plan. 2019, 14, 31–43. [Google Scholar] [CrossRef]
- Hassan, S.S.; Williams, G.A.; Jaiswal, A.K. Lignocellulosic Biorefineries in Europe: Current State and Prospects. Trend Biotechnol. 2018. [Google Scholar] [CrossRef]
- Bren, C.; Reitsma, F.; Baiocchi, G.; Barthel, S.; Güneralp, B.; Erb, K.-H. Future urban land expansion and implications for global croplands. Proc. Natl. Acad. Sci. USA 2017, 114, 8939–8944. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–361. [Google Scholar] [CrossRef]
- Fiorentino, G.; Zucaro, A.; Ulgiati, S. Towards an energy efficient chemistry. Switching from fossil to bio-based products in a life cycle perspective. Energy 2019, 170, 720–729. [Google Scholar] [CrossRef]
- Biorefineries Roadmap as Part of the German Federal Government Action Plans for the Material and Energetic Utilization of Renewable Raw Materials; The Federal Government Germany: Bonn, Germany, 2012. Available online: https://www.bmbf.de/upload_filestore/pub/Roadmap_Biorefineries_eng.pdf (accessed on 7 May 2019).
- Kamm, B.; Kamm, M.; Hirth, T.; Schulze, M. Lignocelluloses Based Chemical Products and Product Family Trees. In Biorefineries-Industrial Processes and Products; Kamm, M., Kamm, B., Gruber, P.C., Eds.; Wiley-VCH: Weinheim, Germany, 2006; pp. 97–150. ISBN 3-527-31027-4. [Google Scholar]
- Kamm, B.; Gruber, P.R.; Kamm, M. Biorefineries-Industrial Processes and Products. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2016; ISBN 9783527306732. [Google Scholar]
- Alzagameem, A.; El Khaldi-Hansen, B.; Kamm, B.; Schulze, M. Lignocellulosic biomass for energy, biofuels, biomaterials, and chemicals. In Biomass and Green Chemistry, 1st ed.; Vaz, S., Jr., Ed.; Springer International Publishing: Basel, Switzerland, 2018; pp. 95–132. ISBN 978-3-319-66736-2. [Google Scholar] [CrossRef]
- Hansen, B.; Kamm, B.; Schulze, M. Qualitative and quantitative analysis of lignin produced from beech wood by different conditions of the Organosolv process. J. Polym. Environ. 2016, 24, 85–97. [Google Scholar] [CrossRef]
- Hansen, B.; Kamm, B.; Schulze, M. Qualitative and quantitative analysis of lignins from different sources and isolation methods for an application as a biobased chemical resource and polymeric material. In Analytical Techniques and Methods for Biomass Products; Vaz, S., Jr., Seidl, P., Eds.; Springer: Berlin, Germany, 2017; pp. 15–44. ISBN 978-3-319-41414-0. [Google Scholar] [CrossRef]
- Hassan, S.S.; Williams, G.A.; Jaiswala, A.K. Moving towards the second generation of lignocellulosic biorefineries in the EU: Drivers, challenges, and opportunities. Renew. Sustain. Environ. Rev. 2019, 101, 590–599. [Google Scholar] [CrossRef]
- Van Meerbeek, K.; Muysa, B.; Hermya, M. Lignocellulosic biomass for bioenergy beyond intensive cropland and forests. Renew. Sustain. Environ. Rev. 2019, 102, 139–149. [Google Scholar] [CrossRef]
- Zucaro, A.; Fierro, A.; Forte, A. A Review on Potential Candidate Lignocellulosic Feedstocks for Bio-energy Supply Chain. In Life Cycle Assessment of Energy Systems and Sustainable Energy Technologies, 1st ed.; Basosi, R., Cellura, M., Longo, S., Parisi, M.L., Eds.; Springer: Basel, Switzerland, 2019; pp. 119–138. ISBN 978-3-319-93740-3. [Google Scholar] [CrossRef]
- Song, K.; Chu, Q.; Hu, J.; Bu, Q.; Li, F.; Chen, X.; Shi, A. Two-stage alkali-oxygen pretreatment capable of improving biomass saccharification for bioethanol production and enabling lignin valorization via adsorbents for heavy metal ions under the biorefinery concept. Bioresour. Technol. 2019, 276, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, H.; Liu, Y.; Wang, Y.; Huo, F.; Fan, M.; Zhang, S. Recent progress in theoretical and computational studies on the utilization of lignocellulosic materials. Green Chem. 2019, 21, 9–35. [Google Scholar] [CrossRef]
- Lignin Market—Forecasts from 2018 to 2023; Knowledge Sourcing Intelligence LLP: Noida, India, 2018; 104p.
- Lignin Market Analysis by Product (Lignosulphonates, Kraft Lignin, Organosolv Lignin) by Application (Macromolecules, Aromatics), by Region (North America, Europe, APAC, Central & South America, MEA), and Segment Forecasts, 2014–2025; ID: 4240413 Report; Grand View Research: San Francisco, CA, USA, 2017; 110p.
- Chaleawlertumpon, S.; Berthold, T.; Wang, X.; Antonietti, M.; Liedel, C. Kraft lignin as electrode material for sustainable electrochemical energy storage. Adv. Mater. Interfaces 2017, 4, 1700698. [Google Scholar] [CrossRef]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.; Weckhuysen, B.M. Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis. Angew. Chem. Int. Ed. 2016, 55, 2. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef] [PubMed]
- Lupoi, J.S.; Singh, S.; Parthasarathi, R.; Simmons, B.A.; Henry, R.J. Recent innovations in analytical methods for the qualitative and quantitative assessment of lignin. Renew. Sustain. Energy. Rev. 2015, 49, 871–906. [Google Scholar] [CrossRef] [Green Version]
- Lan, W.; Yue, F.; Rencoret, J.; del Río, J.; Boerjan, W.; Lu, F.; Ralph, J. Elucidating Tricin-Lignin Structures: Assigning Correlations in HSQC Spectra of Monocot Lignins. Polymers 2018, 10, 916. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhao, C.; Zha, Y.; Wan, C.; Si, S.; Liu, F.; Xu, N. The minor wall-networks between monolignols and interlinked-phenolics predominantly affect biomass enzymatic digestibility in Miscanthus. PLoS ONE 2014, 9, e105115. [Google Scholar] [CrossRef] [PubMed]
- Bergs, M.; Einfluss von Miscanthus, G. Erntezeit auf Gehalt und Struktur von Lignin aus Organosolv-Verfahren. Ph.D. Thesis, Rheinische Friedrich-Wilhelms-University, Bonn, Germany, 20 December 2018. [Google Scholar]
- Bergs, M.; Völkering, G.; Kraska, T.; Do, X.T.; Kusch, P.; Monakhova, Y.; Konow, C.; Pude, R.; Schulze, M. Miscanthus × giganteus Stem versus Leave-derived Lignins Differing in Monolignol Ratio and Linkage. Int. J. Mol. Sci. 2019, 20, 1200. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, H.; Akiyama, T.; Tamai, A.; Nawawi, D.S.; Syafii, W.; Yokoyama, T.; Matsumoto, Y. Variation of the contents of biphenyl structures in lignins among wood species. Holzforschung 2019. [Google Scholar] [CrossRef]
- Jia, Z.; Lu, C.; Zhou, P.; Wang, L. Preparation and characterization of high boiling solvent lignin-based polyurethane film with lignin as the only hydroxyl group provider. RSC Adv. 2015, 5, 53949–53955. [Google Scholar] [CrossRef]
- Griffini, G.; Passoni, V.; Suriano, R.; Levi, M.; Turri, S. Polyurethane Coatings Based on Chemically Unmodified Fractionated Lignin. ACS Sustain. Chem. Eng. 2015, 3, 1145–1154. [Google Scholar] [CrossRef]
- García, A.; Toledano, A.; Serrano, A.; Egüés, I.; González, M.; Marín, F.; Labidi, J. Preparation and characterization of lignin demethylated at atmospheric pressure and its application in fast curing biobased phenolic resins. Sep. Purif. Technol. 2009, 68, 193–198. [Google Scholar] [CrossRef]
- Klein, S.E.; Rumpf, J.; Kusch, P.; Albach, R.; Rehahn, M.; Witzleben, S.; Schulze, M. Utilization of Unmodified Kraft Lignin for the Preparation of Highly Flexible and Transparent Polyurethane Coatings. RSC Adv. 2018, 8, 40765. [Google Scholar] [CrossRef]
- Klein, S.E.; Rumpf, J.; Rehahn, M.; Witzleben, S.; Schulze, M. Biobased Flexible Polyurethane Coatings Prepared from Kraft Lignin: One-Pot Synthesis and Antioxidant Activity. J. Coat. Technol. Res. 2019. [Google Scholar] [CrossRef]
- Witzleben, S.T.; Walbrück, K.; Klein, S.E.; Schulze, M. Investigation of Temperature Dependency of Morphological Properties of Thermoplastic Polyurethane Using WAXS and SAXS Monitoring. J. Chem. Chem. Eng. 2016, 9, 494–499. [Google Scholar]
- Vinardell, M.P.; Mitjans, M. Lignins and Their Derivatives with Beneficial Effects on Human Health. Int. J. Mol. Sci. 2017, 18, 1219. [Google Scholar] [CrossRef]
- Figueiredo, P.; Lintinen, K.; Hirvonen, J.T.; Kostiainen, M.A.; Santos, H.A. Properties and chemical modifications of lignin: Towards lignin-based nanomaterials for biomedical applications. Prog. Mater. Sci. 2018, 93, 233–269. [Google Scholar] [CrossRef]
- Witzler, M.; Alzagameem, A.; Bergs, M.; El Khaldi-Hansen, B.; Klein, S.E.; Hielscher, D.; Kamm, B.; Kreyenschmidt, J.; Tobiasch, E.; Schulze, M. Lignin-Derived Biomaterials for Drug Release and Tissue Engineering. Molecules 2018, 23, 1885. [Google Scholar] [CrossRef] [PubMed]
- Alzagameem, A.; El Khaldi-Hansen, B.; Büchner, D.; Larkins, M.; Kamm, B.; Witzleben, S.; Schulze, M. Lignocellulosic Biomass as Source for Lignin-Based Environmentally Benign Antioxidants. Molecules 2018, 23, 2664. [Google Scholar] [CrossRef]
- Do, X.T.; Nöster, J.; Weber, M.; Nietsch, A.; Jung, C.; Witzleben, S.; Schulze, M. Comparative Studies of Lignin Depolymerisation: Photolysis versus Ozonolysis in Alkaline Medium. In Proceedings of the Annual Conference of the GDCh Division Sustainable Chemistry, Aachen, Germany, 17–19 September 2018. [Google Scholar]
- Do, X.T.; Nietzsch, A.; Jung, C.; Witzleben, S.; Schulze, M. Lignin-Depolymerisation via UV-Photolysis and Titanium Dioxide Photocatalysis. Preprints 2017, 2017100128. [Google Scholar] [CrossRef]
- Renders, T.; Van den Bossche, G.; Vangeel, T.; Van Aelst, K.; Sels, B. Reductive catalytic fractionation: State of the art of the lignin-first biorefinery. Curr. Opin. Biotechnol. 2019, 56, 193–201. [Google Scholar] [CrossRef]
- Rabaçal, M.; Ferreira, A.F.; Costa, M. (Eds.) Biorefineries: Targeting Energy, High Value Products and Waste Valorisation, 1st ed.; Springer: Basel, Switzerland, 2017; ISBN 978-3-319-48288-0. [Google Scholar]
- Borregard Chemicals Company. Welcome to Borregard’s Capital Markets. Available online: https://www.borregaard.com/content/download/109093/19584546/file/Borregaard%20CMD%202018%20-%20presentations%20[Read-Only].pdf Oslo (accessed on 18 September 2018).
- Rodsrud, G. Borregaard history. In Cascading Use of Woody Biomass; European Commission Workshop: Brussels, Belgium, 2018. [Google Scholar]
- Panoutsou, C.; Uslu, A.; van Stralen, J.; Elbersen, B.; Bottcher, H.; Fritsche, U.; Kretschmer, B. How much can biomass contribute to meet the demand for 2020 & which market segments are more promising? In Proceedings of the 20th EU Biomass Conference and Exhibition, Milano, Italy, 18–22 June 2012; ISBN 978-88-89407-54-7. [Google Scholar] [CrossRef]
- Chen, M.; Smith, P.M.; Wolcott, M.P. U.S. Biofuels Industry: A Critical Review of Opportunities and Challenges. Bioprod. Bus. 2016, 1, 42–59. [Google Scholar]
- Bozell, J.J.; Holladay, J.E.; Johnson, D.; White, J.F. Top Value Added Chemicals from Biomass, Volume II: Results of Screening for Potential Candiates from Biorefinery Lignin; Report PNNL-16983 Produced by Pacific Northwest National Laboratory (PNNL) and the National Renewable Energy Laboratory (NREL) for the USA Department of Energy; PNNL: Richland, WA, USA; NREL: Lakewood, CO, USA, 2007. [Google Scholar]
- Littlejohns, J.; Rehmann, L.; Murdy, R.; Oo, A.; Neill, S. Current state and future prospects for liquid biofuels in Canada. Biofuel Res. J. 2018, 17, 759–779. [Google Scholar] [CrossRef]
- Shao, X.; DiMarco, K.; Richard, T.L.; Lynd, L.R. Winter rye as a bioenergy feedstock: Impact of crop maturity on composition, biological solubilization and potential revenue. Biotechnol. Biofuels 2015, 8, 35–45. [Google Scholar] [CrossRef]
- Tomani, P. The LignoBoost Process. Cellul. Chem. Technol. 2010, 44, 53–58. [Google Scholar]
- Strassberger, Z.; Prinsen, P.; van der Klis, F.; van Es, D.S.; Tanasea, S.; Rothenberg, G. Lignin solubilisation and gentle fractionation in liquid ammonia. Green Chem. 2014, 17, 325–334. [Google Scholar] [CrossRef] [Green Version]
- Stoklosa, R.J.; Velez, J.; Kelkar, S.; Saffron, C.M.; Thies, M.C.; Hodge, D.B. Correlating lignin structural features to phase partitioning behavior in a novel aqueous fractionation of softwood Kraft black liquor. Green Chem. 2013, 15, 2904–2912. [Google Scholar] [CrossRef]
- Velez, J.; Thies, M.C. Solvated liquid-lignin fractions from a Kraft black liquor. Bioresour. Technol. 2013, 148, 586–590. [Google Scholar] [CrossRef] [PubMed]
- Lilja, K.; Loukola-Ruskeeniemi, K. (Eds.) Wood-Based Bioeconomy Solving Global Challenges, 1st ed.; Ministry of Economic Affairs and Employment of Finland: Helsinki, Finland, 2017; ISSN 2342-7914 (Printed), ISBN 978-952-327-214-9, ISSN 2342-7922 (Web), ISBN 978-952-327-215-6.
- Thomas, M.H.; Wright, J.K.; Stone, B.; Beer, T.; Lamb, D.; Edye, L.; Rogers, P.; Batten, D.F.; Tegart, G.; Campbell, P.K. Biofuels for Transport: A Roadmap for Development in Australia, 1st ed.; The Australian Academy of Technological Sciences and Engineering (ATSE): Melbourne, Australia, 2008; ISBN 187561897X. [Google Scholar]
- Valdivia, M.; Galan, J.L.; Laffarga, J.; Ramos, J.-L. Biofuels 2020: Biorefineries based on lignocellulosic Materials. Microb. Biotechnol. 2016, 9, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, W.W.; Johnson, J.M.F.; Karlen, D.L.; Lightle, D.T. Corn Stover to Sustain Soil Organic Carbon Further Constrains Biomass Supply. Agron. J. 2007, 99, 1665–1667. [Google Scholar] [CrossRef] [Green Version]
- Schulze, P.; Seidel-Morgenstern, A.; Lorenz, H.; Leschinsky, M.; Unkelbach, G. Advanced process for precipitation of lignin from ethanol organosolv spent liquors. Bioresour. Technol. 2016, 199, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Michels, J.; Wagemann, K. The german lignocellulose feedstock biorefinery project. Biofuels Bioprod. Biorefin. 2010, 4, 263–267. [Google Scholar] [CrossRef]
- Cuellar Soares M on behalf of the BPF. The Bioprocess Pilot Facility (BPF) Enabling the Transition to a Bio-Based Economy. EU Grant Agreement No 760802. Available online: http://biocatpolymers.eu (accessed on 19 April 2019).
- Treusch, K.; Ritzberger, J.; Schwaiger, N.; Pucher, P.; Siebenhofer, M. Diesel production from lignocellulosic feed: The bioCRACK process. R. Soc. Open Sci. 2017, 4, 171122. [Google Scholar] [CrossRef]
- Da Silva, A.R.G.; Giuliano, A.; Errico, M.; Rong, B.G.; Barletta, D. Economic value and environmental impact analysis of lignocellulosic ethanol production: Assessment of different pretreatment processes. Clean Technol. Env. Policy 2019, 21, 637–654. [Google Scholar] [CrossRef]
- Patel, M.; Oyedun, A.O.; Kumar, A.; Gupta, R. What is the production cost of renewable diesel from woody biomass and agricultural residue based on experimentation? A comparative assessment. Fuel Proc. Technol. 2019, 191, 79–92. [Google Scholar] [CrossRef]
- Albashabsheh, N.T.; Heier Stamm, J.L. Optimization of lignocellulosic biomass-to-biofuel supply chains with mobile pelleting. Transp. Res. Part E 2019, 122, 545–562. [Google Scholar] [CrossRef]
- Srivastava, N.; Kharwar, R.K.; Mishra, P.K. Cost Economy Analysis of Biomass-Based Biofuel Production. In New and Future Developments in Microbial Biotechnology and Bioengineering. From Cellulose to Cellulase: Strategies to Improve Biofuel Production; Elsevier: Amsterdam, The Netherlands, 2019; pp. 1–10. [Google Scholar] [CrossRef]
- Karkee, A. Optimization and Cost Analysis of Lignocellulosic Biomass Feedstocks Supply Chains for Biorefineries. Ph.D. Thesis, Iowa State University, Armes, IA, USA, 2016. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, X.; Xu, J.; Ou, X.; Chang, S.; Wu, M. Techno-Economic Analysis of Bioethanol Production from Lignocellulosic Biomass in China: Dilute-Acid Pretreatment and Enzymatic Hydrolysis of Corn Stover. Energies 2015, 8, 4096–4117. [Google Scholar] [CrossRef]
- Gnansounou, E.; Dauriat, A. Technoeconomic Analysis of Lignocellulosic Ethanol, in: Biofuels. Alternative Feedstocks and Conversion Processes. In Biofuels: Alternative Feedstocks and Conversion Processes; Elsevier: Amsterdam, The Netherlands, 2011; pp. 123–148. [Google Scholar] [CrossRef]
- Emmerling, C.; Pude, R. Introducing Miscanthus to the greening measures of the EU Common Agricultural Policy. GCB Bioenergy 2017, 9, 274–279. [Google Scholar] [CrossRef]
- Baum, S.; Bolte, A.; Weih, M. Short rotation coppice (SRC) plantations provide additional habitats for vascular plant species in agricultural mosaic landscapes. Bioenergy Res. 2012, 5, 573–583. [Google Scholar] [CrossRef]
- Don, A.; Osborne, B.; Hastings, A.; Skiba, U.; Carter, M.S.; Drewer, J.; Lanigan, G.J. Land-use change to bioenergy production in Europe: Implications for the greenhouse gas balance and soil carbon. Bioenergy Res. 2012, 4, 372–391. [Google Scholar] [CrossRef]
- Felten, D.; Froba, N.; Fries, J.; Emmerling, C. Energy balances and CO2-mitigation potentials of bioenergy crops (Miscanthus, rapeseed, maize) based on farming conditions in Western Germany. Renew. Energy 2013, 55, 160–174. [Google Scholar] [CrossRef]
- McCalmont, J.P.; Hastings, A.; McNamara, N.P.; Richter, G.M.; Robson, P.; Donnison, I.S.; Clifton-Brown, J. Environmental costs and benefits of growing Miscanthus for bioenergy in the UK. GCB Bioenergy 2015, 9, 489–507. [Google Scholar] [CrossRef]
- Milner, S.; Holland, R.A.; Lovett, A.; Sunnenberg, G.; Hastings, A.; Smith, P.; Taylor, G. Potential impacts on ecosystem services of land use transitions to second-generation bioenergy crops in GB. GCB Bioenergy 2015, 8, 317–333. [Google Scholar] [CrossRef] [Green Version]
- Clifton-Brown, J.; Harfouche, A.; Casler, M.D. Breeding progress and preparedness for mass-scale deployment of perennial lignocellulosic biomass crops switchgrass, miscanthus, willow and poplar. GCB Bioenergy 2019, 11, 118–151. [Google Scholar] [CrossRef]
- Iqbal, Y.; Lewandowski, I. Lignocellulosic Energy Grasses for Combustion, Production, and Provision. In Energy from Organic Materials (Biomass), 1st ed.; Springer: New York, NY USA, 2019; pp. 89–99, Online; ISBN 978-1-4939-7813-7. [Google Scholar]
- Kellogg, E.A. C4 photosynthesis. Curr. Biol. 2013, 23, R594–R599. [Google Scholar] [CrossRef] [Green Version]
- Villaverde, J.J.; Ligero, P.; Vega, A. Miscanthus × giganteus as a Source of Biobased Products through Organosolv Fractionation: A Mini Review. TOAJ 2010, 4, 102–110. [Google Scholar] [CrossRef]
- Teese, P. Intraspecific variation for CO2 compensation point and differential growth among variants in a C3-C4 intermediate plant. Oecologia 1995, 102, 371–376. [Google Scholar] [CrossRef]
- Lewandowski, I.; Clifton-Brown, J.; Trindade, L.M.; van der Linden, G.C.; Schwarz, K.-U.; Müller-Sämann, K.; Anisimov, A.; Chen, C.-L.; Dolstra, O.; Donnison, I.S.; et al. Progress on optimizing miscanthus biomass production for the European bioeconomy: Results of the EU FP7 project OPTIMISC. Front. Plant Sci. 2016, 7, 1620–1632. [Google Scholar] [CrossRef]
- Pude, R.; Treseler, C.H.; Noga, G. Morphological, chemical and technical parameters of Miscanthus genotypes. J. Appl. Bot. 2004, 78, 58–63. [Google Scholar]
- Pude, R.; Treseler, C.H.; Trettin, R.; Noga, G. Suitability of Miscanthus genotypes for lightweight concrete. Die Bodenkult. 2005, 56, 61–69. [Google Scholar]
- Dahmen, N.; Lewandowski, I.; Zibek, S.; Weidtmann, A. Integrated lignocellulosic value chains in a growing bioeconomy: Status quo and perspectives. GCB Bioenergy 2019, 11, 107–117. [Google Scholar] [CrossRef]
- Clifton-Brown, J.; Breuer, J.; Jones, M.B. Carbon mitigation by the energy crop Miscanthus. Glob. Chang. Biol. 2007, 13, 2296–2307. [Google Scholar] [CrossRef]
- Clifton-Brown, J.C.; Lewandowski, I.; Andersson, B.; Basch, G.; Christian, D.G.; Kjeldsen, J.B.; Jørgensen, U.; Mortensene, J.V.; Riched, A.B.; Schwarz, K.U.; et al. Performance of 15 Miscanthus Genotypes at Five Sites in Europe. Agron. J. 2011, 93, 1013–1019. [Google Scholar] [CrossRef]
- Lewandowski, I.; Clifton-Brown, J.C.; Scurlock, J.M.O.; Huisman, W. Miscanthus: European experience with a novel energy crop. Biomass Bioenergy 2000, 19, 209–227. [Google Scholar] [CrossRef]
- Kraska, T.; Kleinschmidt, B.; Weinand, J.; Pude, R. Cascading use of Miscanthus as growing substrate in soilless cultivation of vegetables (tomatoes, cucumbers) and subsequent direct combustion. Sci. Hortic. 2018, 235, 205–213. [Google Scholar] [CrossRef]
- Winzer, F.; Kraska, T.; Elsenberger, C.; Kötter, T.; Pude, R. Biomass from fruit trees for combined energy and food production. Biomass Bioenergy 2017, 107, 279–286. [Google Scholar] [CrossRef]
- Hemansi, R.G.; Yadav, G.; Kumar, G.; Yadav, A.; Saini, J.K.; Kuhad, R.C. Second Generation Bioethanol Production: The State of Art. In Sustainable Approaches for Biofuels Production Technologies, 1st ed.; Srivastava, N., Srivastava, M., Mishra, P.K., Upadhyay, S.N., Ramteke, P.W., Gupta, V.K., Eds.; Springer: Basel, Switzerland, 2019; Volume 7, pp. 121–146. ISBN 978-3-319-94796-9. [Google Scholar] [CrossRef]
- De Vrije, T.; de Haas, G.G.; Tan, G.B.; Keijsers, E.R.P.; Claassen, P.A.M. Pretreatment of Miscanthus for hydrogen production by Thermotoga elfii. Int. J. Hydrogen Energy 2002, 27, 1381–1390. [Google Scholar] [CrossRef]
- Kjeldsen, J.B.; Joergensen, U.; Kristensen, E.F. Miscanthus for Thatching; DJF Rapport; Danmarks Jordbrugsforskning: Foulum, Tjele, Denmark, 1999; pp. 1–59. Available online: https://www.feadk.dk/uf/30000_39999/32791/8e13981ccbd4a10a35c844b6f52d7fea.pdf (accessed on 7 May 2019).
- Wagenaar, B.M.; van den Heuvel, E. Co-combustion of Miscanthus in a pulverised coal combustor: Experiments in a droptube furnace. Biomass. Bioenergy 1997, 12, 185–197. [Google Scholar] [CrossRef] [Green Version]
- Khelfa, A.; Sharypov, V.; Finqueneisel, G.; Weber, J.V. Catalytic pyrolysis and gasification of Miscanthus Giganteus: Haematite (Fe2O3) a versatile catalyst. J. Anal. Appl. Pyrolysis 2009, 84, 84–88. [Google Scholar] [CrossRef]
- Velasquez, J.A.; Ferrando, F.; Farriol, X.; Salvado, J. Binderless fiberboard from steam exploded Miscanthus sinensis. Wood Sci. Technol. 2003, 37, 269–278. [Google Scholar] [CrossRef]
- Vega, A.; Bao, M.; Lamas, J. Application of factorial design to the modelling of organosolv delignification of Miscanthus sinensis (elephant grass) with phenol and dilute acid solutions. Bioresour. Technol. 1997, 61, 1–7. [Google Scholar] [CrossRef]
- El Hage, R.; Brosse, N.; Chrusciel, L.; Sanchez, C.; Sannigrahi, P.; Ragauskas, A. Characterization of milled wood lignin and ethanol organosolv lignin from miscanthus. Polym. Degrad. Stab. 2009, 94, 1632–1638. [Google Scholar] [CrossRef]
- El Hage, R.; Perrin, D.; Brosse, N. Effect of the pre-treatment severity on the antioxidant properties of ethanol organosolv Miscanthus × giganteus lignin. Nat. Resour. 2012, 3, 29–34. [Google Scholar] [CrossRef]
- El Hage, R.; Brosse, N.; Sannigrahi, P.; Ragauskas, A. Effects of process severity on the chemical structure of Miscanthus ethanol organosolv lignin. Polym. Degrad. Stab. 2010, 95, 997–1003. [Google Scholar] [CrossRef]
- Chan, J.M.W.; Bauer, S.; Sorek, H.; Sreekumar, S.; Wang, K.; Toste, F.D. Studies on the vanadium-catalyzed nonoxidative depolymerization of Miscanthus giganteus-derived lignin. ACS Catal. 2013, 3, 1369–1377. [Google Scholar] [CrossRef]
- Luo, H.; Klein, I.M.; Jiang, Y.; Zhu, H.; Liu, B.; Kenttämaa, H.I.; Abu-Omar, M.M. Total Utilization of Miscanthus Biomass, Lignin and Carbohydrates, Using Earth Abundant Nickel Catalyst. ACS Sustain. Chem. Eng. 2016, 4, 2316–2322. [Google Scholar] [CrossRef]
- Vanderghem, C.; Richel, A.; Jacquet, N.; Blecker, C.; Paquot, M. Impact of formic/acetic acid and ammonia pre-treatments on chemical structure and physico-chemical properties of Miscanthus × giganteus lignins. Polym. Degrad. Stab. 2011, 96, 1761–1770. [Google Scholar] [CrossRef]
- Timilsena, Y.P.; Abeywickrama, C.J.; Rakshit, S.K.; Brosse, N. Effect of different pretreatments on delignification pattern and enzymatic hydrolysability of miscanthus, oil palm biomass and typha grass. Bioresour. Technol. 2013, 135, 82–88. [Google Scholar] [CrossRef]
- Jin, X.; Chen, X.; Shi, C.; Li, M.; Guan, Y.; Yeon, C.; Yamada, Y.T.; Sacks, E.J.; Peng, J. Determination of hemicellulose, cellulose and lignin content using visible and near infrared spectroscopy in Miscanthus sinensis. Bioresour. Technol. 2017, 241, 603–609. [Google Scholar] [CrossRef]
- Van der Weijde, T.; Kiesel, A.; Iqbal, Y.; Muylle, H.; Dolstra, O.; Visser, R.G.F.; Lewandowski, I.; Trindade, L.M. Evaluation of Miscanthus sinensis biomass quality as feedstock for conversion into different bioenergy products. GCB Bioenergy 2016, 9, 176–190. [Google Scholar] [CrossRef]
- Baker, P.W.; Winters, A.; Hale, M.D.C. Biodegradation of Different Genotypes of Miscanthus by Wood Rot Fungi. BioResources 2016, 11, 4379–4391. [Google Scholar] [CrossRef]
- Ion, S.; Opris, C.; Cojocaru, B.; Tudorache, M.; Zgura, I.; Galca, A.C.; Bodescu, A.M.; Enache, M.; Maria, G.; Parvulescu, V.I. One-Pot Enzymatic Production of Lignin-Composites. Front. Chem. 2018, 6, 124. [Google Scholar] [CrossRef]
- Sonnenberg, A.M.; Baars, J.J.P.; Visser, M.H.M.; Lavrijssen, B.; Hendrickx, P.M. Evaluation of Shiitake Strains (Lentinula edodes) on Selective Lignin Degradation in Miscanthus × giganteus; Wageningen UR, Plant Breeding: Wageningen, The Netherlands, 2016. [Google Scholar] [CrossRef]
- Pereira, J.S.; Landsberg, J.J. (Eds.) Biomass Production by Fast-Growing Trees; Springer: Dordrecht, The Netherlands, 1989; ISBN 978-94-010-7557-2. [Google Scholar] [CrossRef]
- Ye, X.; Chen, K.; Chen, Y. Kinetics Study of Enzymatic Hydrolysis of Paulownia by Dilute Acid, Alkali, and Ultrasonic-assisted Alkali Pretreatments. Biotechnol. Bioprocess Eng. 2015, 20, 242–248. [Google Scholar] [CrossRef]
- Ayrilmis, N.; Kaymakci, A. Fast growing biomass as reinforcing filler in thermoplastic composites: Paulownia elongate wood. Ind. Crops Prod. 2013, 43, 457–464. [Google Scholar] [CrossRef]
- Cheng, J.; Sun, Y.; Chen, Y. Optimization of dilute acid pretreatment of Paulownia for the production of bioethanol by respond surface methodology. Adv. Mater. Res. 2012, 250–553. [Google Scholar] [CrossRef]
- Ashori, A.; Nourbakhsh, A. Studies on Iranian cultivated paulownia–a potential source of fibrous raw material for paper industry. Eur. J. Wood Prod. 2009, 67, 323–327. [Google Scholar] [CrossRef]
- Zahedi, M.; Tabarsa, T.; Ashori, A.; Madhoushi, M.; Shakeri, A. A Comparative Study on Some Properties of Wood Plastic Composites Using Canola Stalk, Paulownia, and Nanoclay. J. Appl. Polym. Sci. 2013, 1491–1499. [Google Scholar] [CrossRef]
- Pertuzzatti, A.; Missio, A.L.; de Cademartori, P.H.G.; Santini, E.J.; Haselein, C.R.; Berger, C.; Gatto, D.A.; Tondi, G. Effect of Process Parameters in the Thermomechanical Densification of Pinus elliottii and Eucalyptus grandis Fast-growing Wood. BioResources 2018, 13, 1576–1590. [Google Scholar] [CrossRef]
- Conrad, M.; Biertümpfel, A.; Vetter, A. Durchwachsene Silphie (Silphiumperfoliatum, L.)–von der Futterpflanze zum Koferment, F.N.R. Gülzower Fachgespräche. Presented at the 2nd Symposium Energiepflanzen, Fachagentur Nachwachsende Rohstoffe, Gülzow, Germany, 17–18 November 2009; pp. 281–289. [Google Scholar]
- Aurbacher, J.; Benke, M.; Formowitz, B.; Glauert, T.; Heiermann, M.; Herrmann, C.; Idler, C.; Kornatz, P.; Nehring, A.; Rieckmann, C.; et al. Energiepflanzen für Biogasanlagen (Broschüre No. 553); Fachagentur Nachwachsende Rohstoffe e.V.: Rostock, Germany, 2012; pp. 1–84. [Google Scholar]
- Stolzenburg, K.; Monkos, A. Erste Versuchsergebnisse mit der Durchwachsenen Silphie (Silphium perfoliatum L.) in Baden-Württemberg; Landwirtschaftliches Technologiezentrum Augustenberg: Karlsruhe, Germany, 2012. [Google Scholar]
- Gansberger, M.; Montgomery, L.F.R.; Liebhard, P. Botanical characteristics, crop management and potential of Silphium perfoliatum L. as a renewable resource for biogas production: A review. Ind. Crops Prod. 2015, 63, 362–372. [Google Scholar] [CrossRef]
- Šiaudinis, A.G.; Jasinskas, A.; Šleptiene, A.; Karcauskiene, D. The evaluation of biomass and energy productivity of common mugwort (Artemisia vulgaris L.) and cup plant (Silphium perfoliatum L.). Žemdirbystė Agriculture 2012, 99, 357–362. [Google Scholar]
- Kowalski, R. Silphium trifoliatum L.–a new alternative cultivation herbal plant? Acta Agric. Scand. B Soil Plant Sci. 2007, 57, 155–166. [Google Scholar]
- Klímek, P.; Meinlschmidt, P.; Wimmer, R.; Plinke, B.; Schirp, A. Using sunflower (Helianthus annuus L.), topinambour (Helianthustuberosus L.) and cup-plant (Silphium perfoliatum L.) stalks as alternative raw materials for particle boards. Ind. Crops Prod. 2016, 92, 157–164. [Google Scholar] [CrossRef]
- Papadopoulos, A.N.; Kyzas, G.Z.; Mitropoulos, A.C. Lignocellulosic Composites from Acetylated Sunflower Stalks. Appl. Sci. 2019, 9, 646. [Google Scholar] [CrossRef]
- Krakowska, B.; Custers, D.; Deconinck, E.; Daszykowski, M. Chemometrics and the identification of counterfeit medicines-A review. J. Pharm. Biomed. Anal. 2016, 127, 112–122. [Google Scholar] [CrossRef]
- Kamiloglu, S. Authenticity and traceability in beverages. Food Chem. 2019, 277, 12. [Google Scholar] [CrossRef]
- Panchuk, V.; Yaroshenko, I.; Legin, A.; Semenov, V.; Kirsanov, D. Application of chemometric methods to XRF-data—A tutorial review. Anal. Chim. Acta 2018, 1040, 19. [Google Scholar] [CrossRef]
- Monakhova, Y.B.; Holzgrabe, U.; Diehl, B.W.K. Current role and future perspectives of multivariate (chemometric) methods in NMR spectroscopic analysis of pharmaceutical products. J. Pharm. Biomed. Anal. 2018, 147, 580–589. [Google Scholar] [CrossRef]
- Monakhova, Y.; Diehl, B.W.K.; Do, X.T.; Witzleben, S.; Schulze, M. Novel method for the determination of average molecular weight of natural polymers based on 2D DOSY NMR and chemometrics: Example of heparin. J. Pharm. Biomed. Anal. 2018, 149, 128–132. [Google Scholar] [CrossRef]
- Iqbal, Y.; Lewandowski, I. Inter-annual variation in biomass combustion quality traits over five years in fifteen Miscanthus genotypes in south Germany. Fuel Proc. Technol. 2014, 121, 47–55. [Google Scholar] [CrossRef]
- Boeriu, C.G.; Bravo, D.; Gosselink, R.J.A.; van Dam, J.E.G. Characterisation of structure-dependent functional properties of Lignin with infrared spectroscopy. Ind. Crops Prod. 2004, 20, 205–218. [Google Scholar] [CrossRef]
- Chen, H.; Ferrari, C.; Angiuli, M.; Yao, J.; Raspi, C.; Bramanti, E. Qualitative and quantitative analysis of wood samples by Fourier transform infrared spectroscopy and multivariate analysis. Carbohydr. Polym. 2010, 82, 772–778. [Google Scholar] [CrossRef]
- Lancefield, C.S.; Constant, S.; de Peinder, P.; Bruijnincx, P.C.A. Linkage Abundance and Molecular Weight Characteristics of Technical Lignins by Attenuated Total Reflection-FTIR Spectroscopy Combined with Multivariate Analysis. ChemSusChem 2019, 12, 1139–1146. [Google Scholar] [CrossRef]
- Lange, H.; Rulli, F.; Crestini, C. Gel permeation chromatography in determining molecular weights of lignins: Critical aspects revisited for improved utility in the development of novel materials. ACS Sustain. Chem. Eng. 2016, 4, 5167–5180. [Google Scholar] [CrossRef]
- Morgan, T.J.; George, A.; Boulamanti, A.K.; Álvarez, P.; Adanouj, I.; Dean, C.; Andersen, L.K. Quantitative X-ray fluorescence analysis of biomass (switchgrass, corn stover, eucalyptus, beech, and pine wood) with a typical commercial multi-element method on a WD-XRF spectrometer. Energy Fuels 2015, 29, 1669. [Google Scholar] [CrossRef]
- Da Costa, R.M.F.; Lee, S.J.; Allison, G.G.; Hazen, S.P.; Winters, A.; Bosch, M. Genotype, development and tissue-derived variation of cell-wall properties in the lignocellulosic energy crop Miscanthus. Ann. Bot. 2014, 114, 1265–1277. [Google Scholar] [CrossRef] [Green Version]
- Schäfer, J.; Sattler, M.; Iqbal, Y.; Lewandowski, I.; Bunzel, M. Characterization of Miscanthus cell wall polymers. GCB Bioenergy 2019, 11, 191–205. [Google Scholar] [CrossRef]
- Li, X.; Wei, Y.; Xu, J.; Xu, N.; He, Y. Quantitative visualization of lignocellulose components in transverse sections of moso bamboo based on FTIR macro- and micro-spectroscopy coupled with chemometrics. Biotechnol. Biofuels 2018, 11, 263–279. [Google Scholar] [CrossRef]
- Uddin, M.N.; Nayeem, J.; Islam, M.S.; Jahan, M.S. Rapid determination method of dissolving pulp properties by spectroscopic data and chemometrics. Biomass Convers. Biorefin. 2019, 9, 35. [Google Scholar] [CrossRef]
- Colares, C.J.G.; Pastore, T.C.M.; Coradin, V.T.R.; Camargos, J.A.A.; Moreira, A.C.O.; Rubimb, J.C.; Braga, J.W.B. Exploratory Analysis of the Distribution of Lignin and Cellulose in Woods by Raman Imaging and Chemometrics. J. Braz. Chem. Soc. 2015, 1–10. [Google Scholar] [CrossRef]
- Aguilera-Saeza, L.M.; Arrabal-Camposa, F.M.; Callejon-Ferreb, A.J.; Suarez Medina, M.D.; Fernandez, I. Use of multivariate NMR analysis in the content prediction of hemicellulose, cellulose and lignin in greenhouse crop residues. Phytochemistry 2019, 158, 110–119. [Google Scholar] [CrossRef]
- Mancini, M.; Duca, D.; Toscano, G. Laboratory customized online measurements for the prediction of the key-parameters of biomass quality control. J. Near Infrared Spectr. 2019, 1–11. [Google Scholar] [CrossRef]
- Christou, C.; Agapiou, A.; Kokkinofta, R. Use of FTIR spectroscopy and chemometrics for the classification of carobs origin. J. Adv. Res. 2018, 10, 1–8. [Google Scholar] [CrossRef]
- Boeriu, C.G.; Fitigau, F.I.; Gosselink, R.J.A.; Frissen, A.E.; Stoutjesdijk, J.; Peter, F. Fractionation of five technical lignins by selective extraction in green solvents and characterization of isolated fractions. Ind. Crops Prod. 2014, 62, 481–490. [Google Scholar] [CrossRef]
- Gosselink, R.J.A.; van Dam, J.E.G.; de Jong, E.; Scott, E.L.; Sanders, J.P.M.; Li, J.; Gellerstedt, G. Fractionation, analysis, and PCA modeling of properties of four technical lignins for prediction of their application potential in binders. Holzforschung 2010, 64, 193–200. [Google Scholar] [CrossRef]
- Kirsanov, D.; Panchuk, V.; Agafonova-Moroz, M.; Khaydukova, M.; Lumpov, A.; Semenov, V.; Legin, A. A sample-effective calibration design for multiple components. Analyst 2014, 139, 4303–4309. [Google Scholar] [CrossRef]
- Monakhova, Y.B.; Diehl, B.W. Combining 1H NMR spectroscopy and multivariate regression techniques to quantitatively determine falsification of porcine heparin with bovine species. J. Pharm. Biomed. Anal. 2015, 115, 543–551. [Google Scholar] [CrossRef]
- Debus, B.; Kirsanov, D.O.; Panchuk, V.V.; Semenov, V.G.; Legin, A. Three-point multivariate calibration models by correlation constrained MCR-ALS: A feasibility study for quantitative analysis of complex mixtures. Talanta 2017, 163, 39–47. [Google Scholar] [CrossRef]
- Monakhova, Y.B.; Diehl, B.W. Transfer of multivariate regression models between high-resolution NMR instruments: Application to authenticity control of sunflower lecithin. Magn. Res. Chem. 2016, 54, 712–717. [Google Scholar] [CrossRef]














| Linkage | Hard Wood H/G/S traces/25-50/50–75 | Soft Wood H/G/S 0.5–3.4/90–95/0–1 | Miscanthus H/G/S 24/49/27 |
|---|---|---|---|
| β-O-4′ | 50–65 | 43–50 | 93 |
| A-O-4′ | 4–8 | 6–8 | ns* |
| β-β′ | 3–7 | 2–4 | 4 |
| β-5′ | 4–6 | 9–12 | 3 |
| β-1′ | 5–7 | 3–7 | traces |
| 4-O-5′ | 6–7 | 4 | ns* |
| 5-5′ | 4–10 | 10–25 | ns* |
| Company/Institution | Location | Production Scale | Feedstock and Products | Reference |
|---|---|---|---|---|
| Borregaard LignoTech | Sarpsborg, Norway | Industrial scale | World leader in lignin-based products (lignins and lignosulfonates and lignin-derived chemicals). In Fernandina Beach, FL, USA: Southern yellow pine-based lignin utilizing a coproduct of RYAM’s sulfite pulping process | [18,19,42,43,44,45] |
| Tembec/Rayonier Advanced Materials | Jacksonville, FL, USA | Industrial scale | Paper, pulp and lignin production | [18,19,46,47] |
| Domtar Corporation | Montreal, QC, Canada | Industrial scale | LignoBoost plant in Plymouth. Pine-based BioChoice® | [18,19,48,49,50] |
| Asian Lignin Manufacturing Pvt. Ltd. | Chandigarh, India | Industrial scale | Paper, pulp and lignin production | [18,19] |
| Northway Lignin Chemical | Sturgeon Falls, ON, Canada | Industrial scale | Paper, pulp and lignin production | [18,19,48] |
| GreenValue SA | Orbe, Switzerland | Industrial scale | Sulfur-free lignin. Feedstock: wheat straw, flax, sugar cane. Aqueous alkaline extraction. | [18,19,45,51] |
| Domsjö Fabriker AB (world’s 2nd largest producer of powder lignin). Domsjö is part of the Aditya Birla Group. | Örnsköldsvik, Sweden/Aditya Birla Headquarter Mumbai, India | Industrial scale | Powder lignin. Domsjö is the world’s 2nd largest producer of Lignin powder with its origin from sustainable Swedish forestry. | [18,19,42] |
| Changzhou Shanfeng Chemical Industry Co. Ltd. | Changzhou, Jiangsu, China | Industrial scale | Lignin polyether polyols | [18,19] |
| The Dallas Group of America | Whitehouse, NJ, USA | Industrial scale | Lignosulfonates | [18,19,46,47] |
| Nippon Paper Ind. Co. Ltd. | Tokyo, Japan | Industrial scale | Lignosulfonates | [18,19,45] |
| Liquid Lignin Company, LLC | Clemson, SC, USA | Industrial scale | Liquid Lignin Company develops and commercializes new lignin-based technologies. | [18,19,46,52,53] |
| Metsä Group | Espoo, Finland | Industrial scale | Forests and wood-based bioproducts | [18,19,45,54] |
| Fibria | Sao Paulo, Brasilia | Industrial scale | Forests and wood-based bioproducts. World’s leader in Eucalyptus-derived pulp. | [18,19,45] |
| Lenzing AG | Lenzing, Austria | Industrial scale | Forests and wood-based bioproducts. European leader in pulp production. | [18,19,45] |
| Stora Enso | Helsinki, Finland | Industrial scale | LignoBoost plant at Sunila mill. Lineo™® (wood-based). Kraft pulping process of Nordic softwood, pine and spruce. The refined kraft lignin is available as a stable, free-flowing brown powder or a moist powder block. 50,000 tons of dry lignin per year. | [18,19,45,50,54] |
| Weyerhaeuser Company (in collaboration with Lignol Energy Corp./Fibria Cellulose SA) | Seattle, WA, USA | Industrial scale | Second generation biofuels and chemicals | [18,19,46,47,48,49] |
| GreenField | Boucherville, Quebec, Canada | Industrial scale | Biobased alcohols | [18,19,45,48] |
| Enchi Corp. | Lebanon, NH, USA | Industrial scale | Bioenergy and biofuels | [18,19,46,49] |
| Microbiogen | Lane Cove West, Australia | Industrial scale | Bioethanol and bioethanol producing yeast. | [18,19,55] |
| DuPont/VERBIO North America Corporation (VNA), Grand Rapids, Michigan, U.S. | Nevada, IA, USA | Industrial scale | Second generation biofuels and chemicals. World’s largest cellulosic ethanol and biofuel facility (30 million gallons per year). Corn stover feedstock. | [18,19,46,56] |
| POET-DSM | Sioux Falls, SD, USA | Industrial scale | Second generation biofuels and chemicals | [18,19,56,57] |
| IOGEN Corp. | Ottawa, ON, Canada | Demonstration | Second generation biofuels and chemicals. Cellulosic Ethanol. Crop Residue Feedstock. | [18,19,48] |
| Fraunhofer Center for Chemical-Biotechnological Processes (CBP) | Leuna, Germany | Pilot plant | Wood-based organosolv lignin: debarked beech wood (Fagus sylvatica) chips by ethanol–water-pulping in a batch process (70 kg dry biomass). | [18,19,58,59] |
| Bioprocess | Delft, The Netherlands | Pilot plant | Biomass hydrolysis and fermentation | [18,19,60] |
| bioCRACK | Schwechat, Austria | Pilot plant | Second generation biofuels | [18,19,61] |
| Feedstock/Biomass Used | Data Analyzed | Experimental Methods Used | Chemometric Methods Used | References |
|---|---|---|---|---|
| 25 Miscanthus genotype samples, i.e., M. x giganteus, M. sinensis, M. Sacchari florussaccroflorus | Cell-wall composition and lignin content of different Miscanthus stem and leaf samples. | FTIR and NMR spectroscopy | FTIR spectra processing using MatLab (regions 1900–800 cm−1), transformation via Savitzky–Golay algorithm; PCA and Eigenvector PLS (version 7.0.3); statistics via Statistica (version 8.0 StatSoft, Tulsa, OK, USA). | [134] |
| Miscanthus, Switchgrass, Reed Canary Grass | Element analysis including N, S, P, Si, Cl, Na, K, Ca, and heating value | FTIR, NMR spectroscopy | Principal component analysis (PCA) | [135] |
| Moso bamboo samples from three sites in China: 15 culms of 5 physiological ages (1–5 years). Furthermore, samples obtained from 4 positions from each culm (base, middle, top, and middle node sections). 180 samples in total. | Quantitative visualization of lignocellulose components. | FTIR macro- and micro-spectroscopy | Partial least-squares regression (PLSR) and a Montecarlo sampling method (MSM) were used to establish the quantitative determination model of lignocelluloses. | [136] |
| Dissolving pulp | Pulp composition determined including pentosan, α-cellulose, viscosity, and brightness. | Wet chemical methods (TAPPI 2003-4), UV- and Fourier transformed near-infrared (FTNIR) spectroscopy | Pre-treatment by mean normalization, smoothing with moving average, Standard Normal Variate, Savitzky-Golay smoothing with first/second derivatives, and combinations. Raw and treated data processing using principal component regression (PCR) and partial least square regression (PLSR). | [137] |
| Swietenia macrophylla King (Mahogany) and Eucalyptus hybrid (E. urophylla × E. camaldulensis). | Determination of cellulose and lignin distribution in wood surfaces of Swietenia macrophylla King (Mahogany) and Eucalyptus hybrid (E. urophylla × E. camaldulensis). | Raman image spectroscopy (RIS) | The multivariate curve resolution-alternating least squares method is based on the bilinear model. The relative concentration maps were obtained by applying a multivariate curve resolution procedure. | [138] |
| 8 evaluated biomasses from greenhouse crop residues (Cucurbita pepo, Cucumis sativus, Solanum melongena, Solanum lycopersicum, Phaseoulus vulgaris, Capsicum annuum, Citrillus vulgaris Schrad, Cucumis melo). | Crop content prediction of hemicellulose, cellulose (sugar content) and lignin. | 1D and 2D NMR spectroscopy (i.e., as 1H-1H TOCSY, 1H-13C HSQC, 1H-13C HMBC) | The experimental NMR data were processed using the PLS-DA model. The prediction of hemicellulose showed errors up to 22%, while for the other two components the errors are in all the cases below 1%. Discriminant buckets from a PLS-DA model combined with linear models provided a useful and rapid tool for the determination of cell wall composition. | [139] |
| 94 woodchip samples and 70 pellet samples from different Italian power plants (March-May 2017 and February-May 2018). | Prediction of different chemical-physical parameters of woodchip and pellet samples, such as moisture content, net calorific value, ash content and gross calorific value of woodchip samples. | Vis-NIR spectroscopy with and without sample pre-treatment (i.e., grinding or stabilization at 40 °C for 24 h) | Visible NIR data were processed using partial least square regression to predict various chemical-physical parameters of wood-chips and pellets correlated to biofuel quality. Best results were obtained considering only the near IR region. | [140] |
| Carob samples (flesh and seed) from seven different Mediterranean countries (Cyprus, Greece, Italy, Spain, Turkey, Jordan and Palestine) | Crop origin determination via functional group analysis to be assigned to polysaccharides, lipids and proteins. | FTIR spectroscopy (recorded in transmittance mode) | Experimental data were processed statistically using multivariate chemometric techniques, including Principal Component Analysis (PCA), Cluster Analysis (CA), Partial Least Squares (PLS) and Orthogonal Partial Least Square-Discriminant Analysis (OPLSDA). Results confirmed that PCA was most useful to differentiate the studied carob samples, in particular the contribution of the geographical origin. | [141] |
| Lignins from different origin: i.e., soda-derived lignins (wheat straw and Sarkanda grass/wheat mixture), Organosolv lignin from maple/birch/poplar hard wood mixture, a pine-derived kraft lignin (Indulin AT) and an alkaline-isolated wheat straw-lignin. | Crop origin determination via functional group content analysis. | Fourier Transform Infrared (FTIR) and quantitative 31P NMR spectroscopy. | Principal component analysis and partial least squares regression analysis were used for data processing (Unscrambler® 7.6, Camo, Norway). PCA results showed differences of the studied lignin fractions. PLS could correlate 31P-NMR and FT-IR data with the chemical composition of lignin fractions. Authors reported a calibration model to predict the chemical parameters. PCA and the PLS model were validated using a new set of data (i.e., cross validation set). | [129,142,143] |
| Lignins from different Miscanthus genotypes including M. giganteus, M. robustus and M. sinensis harvested at different seasons and years, respectively, separated into leaf and stem | Genotype composition, monolignol ratio (G, H, versus S) and corresponding linkages. | FTIR, UV-Vis and NMR (HSQC) spectroscopy, GPC, Pyrolysis-GC/MS, composition analysis via NREL | Principal component analysis (PCA) | [26,27] |
| 54 different technical lignin samples (including kraft, soda and organosolv pulping). | Linkage abundance and molecular weight characteristics of technical lignins | Attenuated Total Reflection-FTIR, gel-permeation chromatography (GPC) and nuclear magnetic resonance (NMR) for structure analysis of technical lignins. | Principal component analysis and partial least square modelling (using PLS_Toolbox v. 8.6, Eigenvector) in Matlab. Spectra were pre-processed using baseline correction, normalization and mean-centering. Results clearly showed similarities and deviations for the 54 lignins correlating to their botanic origin and pulping process (used for isolation). | [131] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzagameem, A.; Bergs, M.; Do, X.T.; Klein, S.E.; Rumpf, J.; Larkins, M.; Monakhova, Y.; Pude, R.; Schulze, M. Low-Input Crops as Lignocellulosic Feedstock for Second-Generation Biorefineries and the Potential of Chemometrics in Biomass Quality Control. Appl. Sci. 2019, 9, 2252. https://doi.org/10.3390/app9112252
Alzagameem A, Bergs M, Do XT, Klein SE, Rumpf J, Larkins M, Monakhova Y, Pude R, Schulze M. Low-Input Crops as Lignocellulosic Feedstock for Second-Generation Biorefineries and the Potential of Chemometrics in Biomass Quality Control. Applied Sciences. 2019; 9(11):2252. https://doi.org/10.3390/app9112252
Chicago/Turabian StyleAlzagameem, Abla, Michel Bergs, Xuan Tung Do, Stephanie Elisabeth Klein, Jessica Rumpf, Michael Larkins, Yulia Monakhova, Ralf Pude, and Margit Schulze. 2019. "Low-Input Crops as Lignocellulosic Feedstock for Second-Generation Biorefineries and the Potential of Chemometrics in Biomass Quality Control" Applied Sciences 9, no. 11: 2252. https://doi.org/10.3390/app9112252
APA StyleAlzagameem, A., Bergs, M., Do, X. T., Klein, S. E., Rumpf, J., Larkins, M., Monakhova, Y., Pude, R., & Schulze, M. (2019). Low-Input Crops as Lignocellulosic Feedstock for Second-Generation Biorefineries and the Potential of Chemometrics in Biomass Quality Control. Applied Sciences, 9(11), 2252. https://doi.org/10.3390/app9112252