Effect of Mother’s Age and Pathology on Functional Behavior of Amniotic Mesenchymal Stromal Cells—Hints for Bone Regeneration
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. hAMSC Isolation
2.2. hAMSC Growth and Phenotype Analysis
2.3. Differentiation of hAMSCs
2.3.1. Osteogenic Differentiation
2.3.2. Chondrogenic Differentiation
2.4. Adhesion to Titanium Disks
2.5. Statistical Analysis
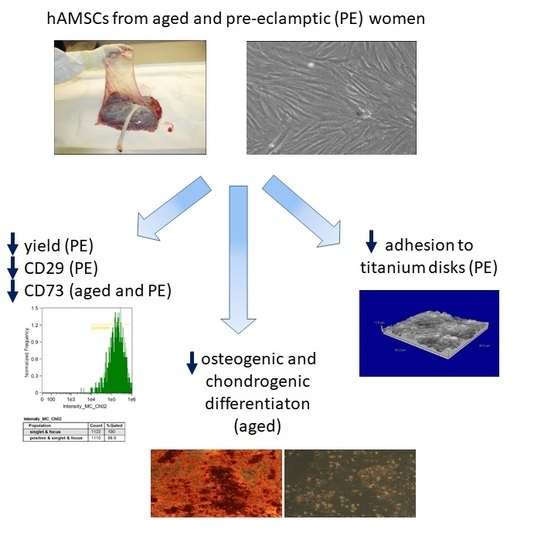
3. Results
3.1. Characteristics of Donors
3.2. Age and Pathology Affect Hamsc Yield, Viability, and Cell Proliferation
3.3. hAMSC Marker Expression is Modified by Age and Pathology
3.4. Differentiation Capacity of hAMSCs
3.5. Pre-Eclampsia Affects hAMSCs Adhesion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mao, X.; Liu, Y.; Chen, C.; Shi, S. Mesenchymal Stem Cells and Their Role in Dental Medicine. Dent. Clin. North. Am. 2017, 61, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Egusa, H.; Sonoyama, W.; Nishimura, M.; Atsuta, I.; Akiyama, K. Stem cells in dentistry—Part II: Clinical applications. J. Prosthodont. Res. 2012, 56, 229–248. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z. Calcium Phosphate-Based Osteoinductive Materials. Chem. Rev. 2008, 108, 4742–4753. [Google Scholar] [CrossRef] [PubMed]
- Olivares-Navarrete, R.; Hyzy, S.L.; Hutton, D.L.; Erdman, C.P.; Wieland, M.; Boyan, B.D.; Schwartz, Z. Direct and Indirect Effects of Microstructured Titanium Substrates on the Induction of Mesenchymal Stem Cell Differentiation towards the Osteoblast Lineage. BioMat. 2010, 31, 2728–2735. [Google Scholar] [CrossRef] [PubMed]
- Egusa, H.; Sonoyama, W.; Nishimura, M.; Atsuta, I.; Akiyama, K. Stem cells in dentistry—Part I: Stem cell sources. J. Prosthodont. Res. 2012, 56, 151–165. [Google Scholar] [CrossRef] [PubMed]
- Toda, A.; Okabe, M.; Yoshida, T.; Nikaido, T. The potential of amniotic membrane/amnion-derived cells for regeneration of various tissues. J. Pharmacol. Sci. 2007, 105, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Parolini, O.; Alviano, F.; Bagnara, G.P.; Bilic, G.; Buhring, H.J.; Evangelista, M.; Hennerbichler, S.; Liu, B.; Magatti, M.; Mao, N.; et al. Concise review: Isolation and characterization of cells from human term placenta: Outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells 2008, 26, 300–311. [Google Scholar] [CrossRef] [PubMed]
- Portmann-Lanz, C.B.; Schoeberlein, A.; Huber, A.; Sager, R.; Malek, A.; Holzgreve, W.; Surbek, D.V. Placental mesenchymal stem cells as potential autologous graft for pre- and perinatal neuroregeneration. Am. J. Obstet. Gynecol. 2006, 194, 664–673. [Google Scholar] [CrossRef]
- Alviano, F.; Fossati, V.; Marchionni, C.; Arpinati, M.; Bonsi, L.; Franchina, M.; Lanzoni, G.; Cantoni, S.; Cavallini, C.; Bianchi, F.; et al. Term amniotic membrane is a high throughput source for multipotent mesenchymal stem cells with the ability to differentiate into endothelial cells in vitro. BMC Dev. Boil. 2007, 7, 11. [Google Scholar]
- Soncini, M.; Vertua, E.; Gibelli, L.; Zorzi, F.; Denegri, M.; Albertini, A.; Wengler, G.S.; Parolini, O. Isolation and characterization of mesenchymal cells from human fetal membranes. J. Tissue Eng. Regen. Med. 2007, 1, 296–305. [Google Scholar] [CrossRef]
- Manuelpillai, U.; Moodley, Y.; Borlongan, C.; Parolini, O.; Borlongan, C. Amniotic membrane and amniotic cells: Potential therapeutic tools to combat tissue inflammation and fibrosis? Placenta 2011, 32, S320–S325. [Google Scholar] [CrossRef]
- Rennie, K.; Gruslin, A.; Hengstschläger, M.; Pei, D.; Cai, J.; Nikaido, T.; Bani-Yaghoub, M. Applications of Amniotic Membrane and Fluid in Stem Cell Biology and Regenerative Medicine. Stem Cells Int. 2012, 2012, 1–13. [Google Scholar] [CrossRef]
- Carbone, A.; Paracchini, V.; Castellani, S.; Gioia, S.; Seia, M.; Colombo, C.; Conese, M. Human Amnion-Derived Cells: Prospects for the Treatment of Lung Diseases. Curr. Stem Cell Res. Ther. 2014, 9, 297–305. [Google Scholar] [CrossRef]
- Kmiecik, G.; Spoldi, V.; Silini, A.; Parolini, O. Current View on Osteogenic Differentiation Potential of Mesenchymal Stromal Cells Derived from Placental Tissues. Stem. Cell. Rev. 2015, 11, 570–585. [Google Scholar] [CrossRef]
- Caplan, A. Why are MSCs therapeutic? New data: New insight. J. Pathol. 2009, 217, 318–324. [Google Scholar] [CrossRef]
- Stolzing, A.; Jones, E.; McGonagle, D.; Scutt, A. Age-related changes in human bone marrow-derived mesenchymal stem cells: Consequences for cell therapies. Mech. Ageing Dev. 2008, 129, 163–173. [Google Scholar] [CrossRef]
- Zhou, S.; Greenberger, J.S.; Epperly, M.W.; Goff, J.P.; Adler, C.; LeBoff, M.S.; Glowacki, J. Age-Related Intrinsic Changes in Human Bone Marrow-Derived Mesenchymal Stem Cells and Their Differentiation to Osteoblasts. Aging Cell 2008, 7, 335–343. [Google Scholar] [CrossRef]
- D’Ippolito, G.; Schiller, P.C.; Ricordi, C.; Roos, B.A.; Howard, G.A. Age-Related Osteogenic Potential of Mesenchymal Stromal Stem Cells from Human Vertebral Bone Marrow. J. Bone Miner. Res. 1999, 14, 1115–1122. [Google Scholar] [CrossRef]
- Scheubel, R.J.; Zorn, H.; Silber, R.-E.; Kuss, O.; Morawietz, H.; Holtz, J.; Simm, A. Age-dependent depression in circulating endothelial progenitor cells in patients undergoing coronary artery bypass grafting. J. Am. Coll. Cardiol. 2003, 42, 2073–2080. [Google Scholar] [CrossRef]
- Heeschen, C.; Lehmann, R.; Honold, J.; Assmus, B.; Aicher, A.; Walter, D.H.; Martin, H.; Zeiher, A.M.; Dimmeler, S.; Oudit, G.Y.; et al. Profoundly Reduced Neovascularization Capacity of Bone Marrow Mononuclear Cells Derived From Patients With Chronic Ischemic Heart Disease. Circulation 2004, 109, 1615–1622. [Google Scholar] [CrossRef] [Green Version]
- Powe, C.E.; Levine, R.J.; Karumanchi, S.A. Preeclampsia, a disease of the maternal endothelium: The role of anti-angiogenic factors and implications for later cardiovascular disease. Circulation 2011, 123, 2856–2869. [Google Scholar] [CrossRef]
- Banek, C.T.; Gilbert, J.S. Approaching the threshold for predicting preeclampsia: Monitoring angiogenic balance during pregnancy. Hypertension 2011, 58, 774–775. [Google Scholar] [CrossRef]
- Nelson, D.M.; Burton, G.J. A technical note to improve the reporting of studies of the human placenta. Placenta 2011, 32, 195–196. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar]
- Paracchini, V.; Carbone, A.; Colombo, F.S.; Castellani, S.; Mazzucchelli, S.; Di Gioia, S.; DeGiorgio, D.; Seia, M.; Porretti, L.; Colombo, C.; et al. Amniotic Mesenchymal Stem Cells: A New Source for Hepatocyte-Like Cells and Induction of CFTR Expression by Coculture with Cystic Fibrosis Airway Epithelial Cells. J. Biomed. Biotechnol. 2012, 2012, 1–15. [Google Scholar] [CrossRef]
- Carbone, A.; Valente, M.; Annacontini, L.; Castellani, S.; Di Gioia, S.; Parisi, D.; Rucci, M.; Belgiovine, G.; Colombo, C.; Di Benedetto, A.; et al. Adipose-derived mesenchymal stromal (stem) cells differentiate to osteoblast and chondroblast lineages upon incubation with conditioned media from dental pulp stem cell-derived osteoblasts and auricle cartilage chondrocytes. J. Boil. Regul. Homeost. Agents 2016, 30, 111–122. [Google Scholar]
- Lepore, S.; Milillo, L.; Trotta, T.; Castellani, S.; Porro, C.; Panaro, M.A.; Santarelli, A.; Bambini, F.; Muzio, L.L.; Conese, M.; et al. Adhesion and growth of osteoblast-like cells on laser-engineered porous titanium surface: Expression and localization of N-cadherin and beta-catenin. J. Boil. Regul. Homeost. Agents 2013, 27, 531–541. [Google Scholar]
- Yu, K.R.; Kang, K.S. Aging-Related Genes in Mesenchymal Stem Cells: A Mini-Review. Gerontology 2013, 59, 557–563. [Google Scholar] [CrossRef]
- Block, T.J.; Marinkovic, M.; Tran, O.N.; Gonzalez, A.O.; Marshall, A.; Dean, D.D.; Chen, X.D. Restoring the quantity and quality of elderly human mesenchymal stem cells for autologous cell-based therapies. Stem Cell Res. Ther. 2017, 8, 239. [Google Scholar] [CrossRef]
- Mueller, S.M.; Glowacki, J. Age-related decline in the osteogenic potential of human bone marrow cells cultured in three-dimensional collagen sponges. J. Cell. Biochem. 2001, 82, 583–590. [Google Scholar] [CrossRef]
- Ganguly, P.; El-Jawhari, J.; Ponchel, F. age related changes in bone marrow mesenchymal stromal cells: a potential impact on osteoporosis and osteoarthritis development. Cell Transplant. 2017, 26, 1520–1529. [Google Scholar] [CrossRef]
- Moore, R.; Silver, R.; Moore, J. Physiological Apoptotic Agents have Different Effects upon Human Amnion Epithelial and Mesenchymal Cells. Placenta 2003, 24, 173–180. [Google Scholar] [CrossRef]
- Zhao, P.; Ise, H.; Hongo, M.; Ota, M.; Konishi, I.; Nikaido, T. Human amniotic mesenchymal cells have some characteristics of cardiomyocytes. Transplantation 2005, 79, 528–535. [Google Scholar] [CrossRef]
- Liu, H.; Xia, X.; Li, B. Mesenchymal stem cell aging: Mechanisms and influences on skeletal and non-skeletal tissues. Exp. Boil. Med. 2015, 240, 1099–1106. [Google Scholar] [CrossRef] [Green Version]
- Khan, H.; Mafi, P.; Mafi, R.; Khan, W. The Effects of Ageing on Differentiation and Characterisation of Human Mesenchymal Stem Cells. Curr. Stem. Cell. Res. Ther. 2018, 13, 378–383. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wu, Q.; Wang, Y.; Li, L.; Bu, H.; Bao, J. Senescence of mesenchymal stem cells. Int. J. Mol. Med. 2017, 39, 775–782. [Google Scholar] [CrossRef]
- Alrefaei, G.I.; Ayuob, N.N.; Ali, S.S.; Al-Karim, S. Effects of maternal age on the expression of mesenchymal stem cell markers in the components of human umbilical cord. Folia Histochem. Cytobiol. 2015, 53, 259–271. [Google Scholar] [CrossRef]
- Young, B.C.; Levine, R.J.; Karumanchi, S.A. Pathogenesis of preeclampsia. Annu. Rev. Pathol. 2010, 5, 173–192. [Google Scholar] [CrossRef]
- Gathiram, P.; Moodley, J. Pre-eclampsia: Its pathogenesis and pathophysiolgy. Cardiovasc. J. Afr. 2016, 27, 71–78. [Google Scholar] [CrossRef]
- Phipps, E.; Prasanna, D.; Brima, W.; Jim, B. Preeclampsia: Updates in Pathogenesis, Definitions, and Guidelines. Clin. J. Am. Soc. Nephrol. 2016, 11, 1102–1113. [Google Scholar] [CrossRef] [Green Version]
- Pianta, S.; Magatti, M.; Vertua, E.; Bonassi Signoroni, P.; Muradore, I.; Nuzzo, A.M.; Rolfo, A.; Silini, A.; Quaglia, F.; Todros, T.; et al. Amniotic mesenchymal cells from pre-eclamptic placentae maintain immunomodulatory features as healthy controls. J. Cell. Mol. Med. 2016, 20, 157–169. [Google Scholar] [CrossRef]
- Miao, L.; Xin, X.; Xin, H.; Shen, X.; Zhu, Y.Z. Hydrogen Sulfide Recruits Macrophage Migration by Integrin beta1-Src-FAK/Pyk2-Rac Pathway in Myocardial Infarction. Sci. Rep. 2016, 6, 22363. [Google Scholar] [CrossRef]
- Suto, E.G.; Mabuchi, Y.; Suzuki, N.; Suzuki, K.; Ogata, Y.; Taguchi, M.; Muneta, T.; Sekiya, I.; Akazawa, C. Prospectively isolated mesenchymal stem/stromal cells are enriched in the CD73+ population and exhibit efficacy after transplantation. Sci. Rep. 2017, 7, 4838. [Google Scholar] [CrossRef]
- Ip, J.E.; Wu, Y.; Huang, J.; Zhang, L.; Pratt, R.E.; Dzau, V.J. Mesenchymal stem cells use integrin beta1 not CXC chemokine receptor 4 for myocardial migration and engraftment. Mol. Biol. Cell. 2007, 18, 2873–2882. [Google Scholar] [CrossRef]
- Pytela, R.; Pierschbacher, M.D.; Ruoslahti, E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell 1985, 40, 191–198. [Google Scholar] [CrossRef]
- Olivares-Navarrete, R.; Raz, P.; Zhao, G.; Chen, J.; Wieland, M.; Cochran, D.L.; Chaudhri, R.A.; Ornoy, A.; Boyan, B.D.; Schwartz, Z. Integrin alpha2beta1 plays a critical role in osteoblast response to micron-scale surface structure and surface energy of titanium substrates. Proc. Natl. Acad. Sci. USA 2008, 105, 15767–15772. [Google Scholar] [CrossRef]
- Lavenus, S.; Berreur, M.; Trichet, V.; Pilet, P.; Louarn, G.; Layrolle, P. Adhesion and osteogenic differentiation of human mesenchymal stem cells on titanium nanopores. Eur. Cell. Mater 2011, 22, 84–96. [Google Scholar] [CrossRef]
- Zimmermann, H. 5′-Nucleotidase: Molecular structure and functional aspects. Biochem. J. 1992, 285, 345–365. [Google Scholar] [CrossRef]
- Ode, A.; Kopf, J.; Kurtz, A.; Schmidt-Bleek, K.; Schrade, P.; Kolar, P.; Buttgereit, F.; Lehmann, K.; Hutmacher, D.W.; Duda, G.N.; et al. CD73 and CD29 concurrently mediate the mechanically induced decrease of migratory capacity of mesenchymal stromal cells. Eur. Cell. Mater. 2011, 22, 26–42. [Google Scholar] [CrossRef]
- Spriano, S.; Yamaguchi, S.; Baino, F.; Ferraris, S. A critical review of multifunctional titanium surfaces: New frontiers for improving osseointegration and host response, avoiding bacteria contamination. Acta Biomater. 2018, 79, 1–22. [Google Scholar] [CrossRef]
- Olivares-Navarrete, R.; Hyzy, S.L.; Park, J.H.; Dunn, G.R.; Haithcock, D.A.; Wasilewski, C.E.; Boyan, B.D.; Schwartz, Z. Mediation of osteogenic differentiation of human mesenchymal stem cells on titanium surfaces by a Wnt-integrin feedback loop. Biomaterials 2011, 32, 6399–6411. [Google Scholar] [CrossRef] [Green Version]
- Gittens, R.A.; Olivares-Navarrete, R.; Cheng, A.; Anderson, D.M.; McLachlan, T.; Stephan, I.; Geis-Gerstorfer, J.; Sandhage, K.H.; Fedorov, A.G.; Rupp, F.; et al. The roles of titanium surface micro/nanotopography and wettability on the differential response of human osteoblast lineage cells. Acta Biomater. 2013, 9, 6268–6277. [Google Scholar] [CrossRef]
- Boyan, B.; Cheng, A.; Olivares-Navarrete, R.; Schwartz, Z. Implant Surface Design Regulates Mesenchymal Stem Cell Differentiation and Maturation. Adv. Dent. Res. 2016, 28, 10–17. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. On implant surfaces: A review of current knowledge and opinions. Int. J. Oral Maxillofac. Implant. 2010, 25, 63–74. [Google Scholar]





| Study Group | Mother’s Age (years) | Gestational Age (weeks) | Newborn Sex | Child Weight (gr) |
|---|---|---|---|---|
| Group 1 <40 years (n = 7) | 28.7 ± 6.7 | 39.6 ± 1.1 | M =4 F = 3 | 3327 ± 468 |
| Group 2 >40 years (n = 6) | 41.8 ± 2.0 ** | 40.3 ± 0.9 | M = 3 F = 3 | 3375 ± 185 |
| Group 3 Pre-eclampsia (n = 5) | 30.6 ± 2.4 * | 37.3 ± 2.2 * | M = 3 F = 2 | 2740 ± 439 |
| Study Group | CD14 | CD34 | CD45 |
|---|---|---|---|
| Group 1 (<40) | |||
| p0 | 3.4 ± 1.2 | 3.4 ± 1.9 | 3.9 ± 2.5 |
| p2 | 2.9 ± 1.3 | 3.6 ± 1.7 | 3.8 ± 2.0 |
| Group 2 (>40) | |||
| p0 | 0.8 ± 0.3 | 0.6 ± 0.7 | 0.9 ± 1.0 |
| p2 | 2.8 ± 1.6 | 0.9 ± 0.6 | 1.8 ± 1.8 |
| Group 3 (PE) | |||
| p0 | 0.8 ± 0.4 | 1.3 ± 0.2 | 2.1 ± 1.4 |
| p2 | 1.5 ± 1.2 | 1.0 ± 0.2 | 2.0 ± 1.5 |
| Time/Titanium Disk | Group 1 | Group 2 |
|---|---|---|
| 3 h/machined | 0.011 | 0.0358 |
| 6 h/machined | 0.0049 | 0.0195 |
| 24 h/machined | 0.0076 | 0.0130 |
| 48 h/machined | 0.0087 | 0.0246 |
| 3 h/sandblasted | 0.0013 | 0.0148 |
| 6 h/sandblasted | <0.0001 | 0.0064 |
| 24 h/sandblasted | 0.0007 | 0.0122 |
| 48 h/sandblasted | 0.0005 | 0.0716 |
| 72 h/sandblasted | 0.0199 | 0.0096 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matteo, M.; Beccia, E.; Carbone, A.; Castellani, S.; Milillo, L.; Lauritano, D.; Di Gioia, S.; Angiolillo, A.; Conese, M. Effect of Mother’s Age and Pathology on Functional Behavior of Amniotic Mesenchymal Stromal Cells—Hints for Bone Regeneration. Appl. Sci. 2019, 9, 3471. https://doi.org/10.3390/app9173471
Matteo M, Beccia E, Carbone A, Castellani S, Milillo L, Lauritano D, Di Gioia S, Angiolillo A, Conese M. Effect of Mother’s Age and Pathology on Functional Behavior of Amniotic Mesenchymal Stromal Cells—Hints for Bone Regeneration. Applied Sciences. 2019; 9(17):3471. https://doi.org/10.3390/app9173471
Chicago/Turabian StyleMatteo, Maria, Elisa Beccia, Annalucia Carbone, Stefano Castellani, Lucio Milillo, Dorina Lauritano, Sante Di Gioia, Antonella Angiolillo, and Massimo Conese. 2019. "Effect of Mother’s Age and Pathology on Functional Behavior of Amniotic Mesenchymal Stromal Cells—Hints for Bone Regeneration" Applied Sciences 9, no. 17: 3471. https://doi.org/10.3390/app9173471
APA StyleMatteo, M., Beccia, E., Carbone, A., Castellani, S., Milillo, L., Lauritano, D., Di Gioia, S., Angiolillo, A., & Conese, M. (2019). Effect of Mother’s Age and Pathology on Functional Behavior of Amniotic Mesenchymal Stromal Cells—Hints for Bone Regeneration. Applied Sciences, 9(17), 3471. https://doi.org/10.3390/app9173471