Polyisobutylene-Based Thermoplastic Elastomers for Manufacturing Polymeric Heart Valve Leaflets: In Vitro and In Vivo Results
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. SIBS Synthesis
2.2. SIBS and Gore-TexTM Samples
2.3. Mechanical Properties
2.4. Assessment of Surface Properties
2.5. In Vitro Cytotoxicity and Cell Viability Assessment
2.6. In Vitro Calcification Assessment
2.7. In Vivo Hemocompatibility Assessment
2.8. In Vitro Hemocompatibility Assessment
2.9. Degree of Hemolysis
2.10. Platelet Aggregation
2.11. Platelet Adhesion
2.12. Statistical Analysis
3. Results
3.1. Gel Permeation Chromatography
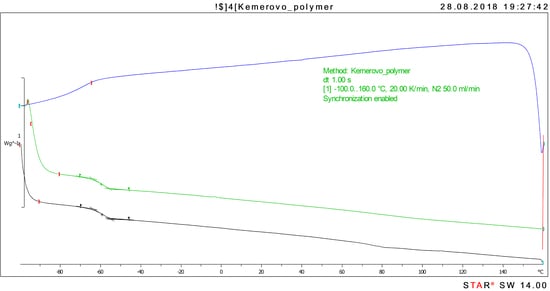
3.2. Differential Scanning Calorimetry
3.3. Physical and Mechanical Properties
3.4. Surface Topography
3.5. In Vitro Biocompatibility
3.6. In Vitro Calcification
3.7. Tissue Response in the Rat Model
3.8. In Vivo Calcification
3.9. Hemolysis
3.10. Platelet Aggregation
3.11. Platelet Adhesion
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Chen, Y.; Yiu, K.H. Growing importance of valvular heart disease in the elderly. J. Thorac. Dis. 2016, 8, E1701–E1703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Arcy, J.L.; Coffey, S.; Loudon, M.A.; Kennedy, A.; Pearson-Stuttard, J.; Birks, J.; Frangou, E.; Farmer, A.J.; Mant, D.; Wilson, J.; et al. Large-scale community echocardiographic screening reveals a major burden of undiagnosed valvular heart disease in older people: The OxVALVE Population Cohort Study. Eur. Heart J. 2016, 37, 3515–3522. [Google Scholar] [CrossRef] [PubMed]
- Manji, R.A.; Ekser, B.; Menkis, A.H.; Cooper, D.K.C. Bioprosthetic heart valves of the future. Xenotransplantation 2014, 21, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jaffer, I.H.; Whitlock, R.P. A mechanical heart valve is the best choice. Heart Asia 2016, 8, 62–64. [Google Scholar] [CrossRef] [Green Version]
- Poli, D.; Antonucci, E.; Pengo, V.; Migliaccio, L.; Testa, S.; Lodigiani, C.; Coffetti, N.; Facchinetti, R.; Serricchio, G.; Falco, P.; et al. Italian Federation of Anticoagulation Clinics. Mechanical prosthetic heart valves: Quality of anticoagulation and thromboembolic risk. The observational multicenter PLECTRUM study. Int. J. Cardiol. 2018, 267, 68–73. [Google Scholar] [CrossRef]
- Lee, S.; Levy, R.J.; Christian, A.J.; Hazen, S.L.; Frick, N.E.; Lai, E.K.; Grau, J.B.; Bavaria, J.E.; Ferrari, G. Calcification and Oxidative Modifications Are Associated with Progressive Bioprosthetic Heart Valve Dysfunction. J. Am. Heart Assoc. 2017, 6, e005648. [Google Scholar] [CrossRef]
- Cartlidge, T.R.G.; Doris, M.K.; Sellers, S.L.; Pawade, T.A.; White, A.C.; Pessotto, R.; Kwiecinski, J.; Fletcher, A.; Alcaide, C.; Lucatelli, C.; et al. Detection and Prediction of Bioprosthetic Aortic Valve Degeneration. J. Am. Coll. Cardiol. 2019, 73, 1107–1119. [Google Scholar] [CrossRef]
- Yang, J.M.; Olanrele, O.S.; Zhang, X.; Hsu, C.C. Fabrication of Hydrogel Materials for Biomedical Applications. In Novel Biomaterials for Regenerative Medicine (Advances in Experimental Medicine and Biology); Chun, H., Park, K., Kim, C.H., Khang, G., Eds.; Springer: Singapore, 2018; Volume 1077, pp. 197–224. [Google Scholar]
- Hasan, A.; Soliman, S.; El Hajj, F.; Tseng, Y.T.; Yalcin, H.C.; Marei, H.E. Fabrication and In Vitro Characterization of a Tissue Engineered PCL-PLLA Heart Valve. Sci. Rep. 2018, 8, 8187. [Google Scholar] [CrossRef]
- Demetzos, C.; Pippa, N. (Eds.) Thermodynamics and Biophysics of Biomedical Nanosystems: Applications and Practical Considerations; Springer: Singapore, 2019; p. 475. [Google Scholar]
- Cohn, D.; Sloutski, A.; Elyashiv, A.; Varma, V.B.; Ramanujan, R. In situ generated medical devices. Adv. Healthc. Mater. 2019, 8, 1801066. [Google Scholar] [CrossRef]
- Piconi, C. Bioinert Ceramics: State-of-the-Art. Key Eng. Mater. 2017, 758, 3–13. [Google Scholar] [CrossRef]
- Vellayappan, M.V.; Balaji, A.; Subramanian, A.P.; John, A.A.; Jaganathan, S.K.; Murugesan, S.; Mohandas, H.; Supriyanto, E.; Yusof, M. Tangible nanocomposites with diverse properties for heart valve application. Sci. Technol. Adv. Mater. 2015, 16, 033504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozeik, M.M.; Wheatley, D.J.; Gourlay, T. Investigating the Suitability of Carbon Nanotube Reinforced Polymer in Transcatheter Valve Applications. Cardiovasc. Eng. Technol. 2017, 8, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Yang, J.; Loh, X.J.; Li, Z. Polymeric Janus Nanoparticles: Recent Advances in Synthetic Strategies, Materials Properties, and Applications. Macromol. Rapid Commun. 2019, 40, e1800203. [Google Scholar] [CrossRef] [PubMed]
- Amadeus, S.Z.; Grande-Allen, K.J. Heart valve tissue engineering for valve replacement and disease modeling. Curr. Opin. Biomed. Eng. 2018, 5, 35–41. [Google Scholar]
- Blum, K.M.; Drews, J.D.; Breuer, C.K. Tissue-Engineered Heart Valves: A Call for Mechanistic Studies. Tissue Eng. Part B Rev. 2018, 24, 240–253. [Google Scholar] [CrossRef]
- Zeng, H.; Jarvik, R.; Catausan, G.; Moldovan, N.; Carlisle, J. Diamond coated artificial cardiovascular devices. Surf. Coat. Technol. 2016, 302, 420–425. [Google Scholar] [CrossRef] [Green Version]
- Bogacz, W.; Lemanowicz, M.; Al-Rashed, M.H.; Nakonieczny, D.; Piotrowski, T.; Wójcik, J. Impact of roughness, wettability and hydrodynamic conditions on the incrustation on stainless steel surfaces. Appl. Therm. Eng. 2017, 112, 352–361. [Google Scholar] [CrossRef]
- De Avila, E.D.; Avila-Campos, M.J.; Vergani, C.E.; Spolidório, D.M.; de Assis Mollo, F., Jr. Structural and quantitative analysis of a mature anaerobic biofilm on different implant abutment surfaces. J. Prosthet. Dent. 2016, 115, 428–436. [Google Scholar] [CrossRef]
- Singhal, P.; Adriana, L.; Butany, J. Bioprosthetic Heart Valves: Impact of Implantation on Biomaterials. ISRN Biomater. 2014, 2013, 728791. [Google Scholar] [CrossRef]
- Hasan, A.; Ragaert, K.; Swieszkowski, W.; Selimovic, S.; Paul, A.; Camci-Unal, G.; Mofrad, M.R.K.; Khademhosseini, A. Biomechanical properties of native and tissue engineered heart valve constructs. J. Biomech. 2014, 47, 1949–1963. [Google Scholar] [CrossRef]
- Rotman, O.M.; Kovarovic, B.; Chiu, W.C.; Bianchi, M.; Marom, G.; Slepian, M.J.; Bluestein, D. Novel Polymeric Valve for Transcatheter Aortic Valve Replacement Applications: In Vitro Hemodynamic Study. Ann. Biomed. Eng. 2019, 47, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Quintessenza, J.A.; Jacobs, J.P.; Chai, P.J.; Morell, V.O.; Lindberg, H. Polytetrafluoroethylene bicuspid pulmonary valve implantation: Experience with 126 patients. World J. Pediatric. Congenit. Heart Surg. 2010, 1, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Hawreliak, J.A.; Lind, J.; Maddox, B.; Barham, M.; Messner, M.; Barton, N.; Jensen, B.J.; Kumar, M. Dynamic Behavior of Engineered Lattice Materials. Sci. Rep. 2016, 6, 28094. [Google Scholar] [CrossRef] [PubMed]
- Chetta, G.E.; Lloyd, J.R. The design, fabrication and evaluation prosthetic heart valve. J. Biomech. Eng. 1980, 102, 34–41. [Google Scholar] [CrossRef]
- Daebritz, S.H.; Fausten, B.; Hermanns, B.; Franke, A.; Schroeder, J.; Groetzner, J.; Autschbach, R.; Messmer, B.J.; Sachweh, J.S. New flexible polymeric heart valve prostheses for the mitral and aortic positions. Heart Surg. Forum 2004, 7, 525–532. [Google Scholar] [CrossRef]
- Jiang, H.; Campbell, G.; Boughner, D.; Wand, W.K.; Quantz, M. Design and manufacture of a polyvinyl alcohol (PVA) cryogel tri-leaflet heart valve prosthesis. Med. Eng. Phys. 2004, 26, 269–277. [Google Scholar] [CrossRef]
- Kidane, A.G.; Burriesci, G.; Edirisinghe, M.; Ghanbari, H.; Bonhoeffer, P.; Seifalian, A.M. A novel nanocomposite polymer for development of synthetic heart valve leaflets. Acta Biomater. 2009, 5, 2409–2417. [Google Scholar] [CrossRef]
- Claiborne, T.E.; Sheriff, J.; Kuetting, M.; Steinseifer, U.; Slepian, M.J.; Bluestein, D.J. In vitro evaluation of a novel hemodynamically optimized trileaflet polymeric prosthetic heart valve. Biomech. Eng. 2013, 135, 021021. [Google Scholar] [CrossRef]
- Pinchuk, L.; Wilson, G.J.; Barry, J.J.; Schoephoerster, R.T.; Parel, J.M.; Kennedy, J.P. Medical applications of poly(styrene-block-isobutylene-block-styrene) (“SIBS”). Biomaterials 2008, 29, 448–460. [Google Scholar] [CrossRef]
- Yin, W.; Gallocher, S.; Pinchuk, L.; Schoephoerster, R.T.; Jesty, J.; Bluestein, D. Flow-induced Platelet Activation in a St. Jude Mechanical Heart Valve, a Trileaflet Polymeric Heart Valve, and a St. Jude Tissue Valve. Artif. Organs 2005, 29, 826–831. [Google Scholar] [CrossRef]
- Wang, Q.; McGoron, A.J.; Bianco, R.; Kato, Y.; Pinchuk, L.; Schoephoerster, R.T. In-vivo assessment of a novel polymer (SIBS) trileaflet heart valve. J. Heart Valve Dis. 2010, 19, 499–505. [Google Scholar] [PubMed]
- Mishra, M.K.; Sar-Mishra, B.; Kennedy, J.P. New telechelic polymers and sequential copolymers by polyfunctional initiator-transfer agents (inifers) LI. Synthesis and characterization of anisole-terminated polyisobutylenes. Polym. Bull. 1986, 16, 47–53. [Google Scholar] [CrossRef]
- Xue, L.; Greisler, H.P. Biomaterials in the development and future of vascular grafts. J. Vasc. Surg. 2003, 37, 472–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Tran, R.T.; Gyawali, D.; Yang, J. Development of Photocrosslinkable Urethane-Doped Polyester Elastomers for Soft Tissue Engineering. Int. J. Biomater. Res. Eng. 2011, 1, 18–31. [Google Scholar] [CrossRef]
- Ayoub, S.; Ferrari, G.; Gorman, R.C.; Gorman, J.H.; Schoen, F.J.; Sacks, M.S. Heart Valve Biomechanics and Underlying Mechanobiology. Compr. Physiol. 2016, 6, 1743–1780. [Google Scholar] [Green Version]
- Aguiari, P.; Fiorese, M.; Iop, L.; Gerosa, G.; Bagno, A. Mechanical testing of pericardium for manufacturing prosthetic heart valves. Interact Cardiovasc. Thorac. Surg. 2016, 22, 72–84. [Google Scholar] [CrossRef]
- Bianco, A.; Di Federico, E.; Cacciotti, I. Electrospun poly(ε-caprolactone)-based composites using synthesized β-tricalcium phosphate. Polym. Adv. Technol. 2011, 22, 1832–1841. [Google Scholar] [CrossRef]
- Falde, E.J.; Yohe, S.T.; Colson, Y.L.; Grinstaff, M.W. Superhydrophobic materials for biomedical applications. Biomaterials 2016, 104, 87–103. [Google Scholar] [CrossRef] [Green Version]
- Brubert, J.; Krajewski, S.; Wendel, H.P.; Nair, S.; Stasiak, J.; Moggridge, G.D. Hemocompatibility of styrenic block copolymers for use in prosthetic heart valves. J. Mater. Sci. Mater. Med. 2016, 27, 32. [Google Scholar] [CrossRef]
- Bezuidenhout, D.; Williams, D.F.; Zilla, P. Polymeric heart valves for surgical implantation, catheter-based technologies and heart assist devices. Biomaterials 2015, 36, 6–25. [Google Scholar] [CrossRef]
- Lam, M.T.; Wu, J.C. Biomaterial applications in cardiovascular tissue repair and regeneration. Expert Rev. Cardiovasc. Ther. 2012, 10, 1039–1049. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, B.; Schricker, S.R. A review of block copolymer-based biomaterials that control protein and cell interactions. J. Biomed. Mater. Res. Part A 2014, 102, 2467–2480. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Lu, W.; Goodwin, A.; Wang, H.; Yin, P.; Kang, N.-G.; Hong, K.; Mays, J.W. Recent Advances in Thermoplastic Elastomers from Living Polymerizations: Macromolecular Architectures and Supramolecular Chemistry. Prog. Polym. Sci. 2019, 95, 1–31. [Google Scholar] [CrossRef]
- Storey, R.F.; Chisholmt, B.J. Morphology and physical properties of poly (styrene-b-isobutylene-b-styrene) block copolymers. Polymer 1996, 37, 2925–2938. [Google Scholar] [CrossRef]
- Vogler, E.A. Protein adsorption in three dimensions. Biomaterials 2012, 33, 1201–1237. [Google Scholar] [CrossRef] [Green Version]
- Nosal, M.; Poruban, R.; Valentík, P.; Sagat, M.; Nagi, A.S.; Kantorova, A. Initial experience with polytetrafluoroethylene leaflet extensions for aortic valve repair. Eur. J. Cardiothorac. Surg. 2012, 41, 1255–1257. [Google Scholar] [CrossRef]
- Yuan, S.; Li, Y.; Luan, S.; Shi, H.; Yan, S.; Yin, J. Infection-resistant styrenic thermoplastic elastomers that can switch from bactericidal capability to anti-adhesion. J. Mater. Chem. B 2016, 4, 1081–1089. [Google Scholar] [CrossRef]
- Ulery, B.D.; Nair, L.S.; Laurencin, C.T. Biomedical Applications of Biodegradable Polymers. J. Polym. Sci. Part B Polym. Phys. 2011, 49, 832–864. [Google Scholar] [CrossRef]
- Chung, T.W.; Liu, D.Z.; Wang, S.Y.; Wang, S.S. Enhancement of the growth of human endothelial cells by surface roughness at nanometer scale. Biomaterials 2003, 24, 4655–4661. [Google Scholar] [CrossRef]
- Fray, M.E.; Prowans, P.; Puskas, J.E.; Altsta, V. Biocompatibility and Fatigue Properties of Polystyrene-Polyisobutylene-Polystyrene, an Emerging Thermoplastic Elastomeric Biomaterial. Biomacromolecules 2006, 7, 844–850. [Google Scholar] [CrossRef]
- Yuan, S.; Li, Z.; Zhao, J.; Luan, S.; Ma, J.; Song, L.; Shi, H.; Jin, J.; Yin, J. Enhanced biocompatibility of biostable poly(styrene-b-isobutylene-b-styrene) elastomer via poly(dopamine)-assisted chitosan/hyaluronic acid immobilization. RSC Adv. 2014, 4, 31481–31488. [Google Scholar] [CrossRef]
- Lu, S.; Zhang, P.; Sun, X.; Gong, F.; Yang, S.; Shen, L.; Huang, Z.; Wang, C. Synthetic ePTFE grafts coated with an anti-CD133 antibody-functionalized heparin/collagen multilayer with rapid in vivo endothelialization properties. ACS Appl. Mater. Interfaces 2013, 5, 7360–7369. [Google Scholar] [CrossRef] [PubMed]
- Thevenot, P.; Hu, W.; Tang, L. Surface chemistry influences implant biocompatibility. Curr. Top. Med. Chem. 2008, 8, 270–280. [Google Scholar] [PubMed]
- Wiggins, M.J.; Wilkoff, B.; Anderson, J.M.; Hiltner, A. Biodegradation of polyether polyurethane inner insulation in bipolar pacemaker leads. J. Biomed. Mater. Res. 2001, 58, 302–307. [Google Scholar] [CrossRef]
- Knoll, A.; Magerle, R.; Krausch, G. Tapping Mode Atomic Force Microscopy on Polymers: Where Is the True Sample Surface? Macromolecules 2001, 34, 4159–4165. [Google Scholar] [CrossRef]
- Claiborne, T.E.; Slepian, M.J.; Hossainy, S.; Bluestein, D. Polymeric trileaflet prosthetic heart valves: Evolution and path to clinical reality. Expert Rev. Med. Devices 2012, 9, 577–594. [Google Scholar] [CrossRef]
- Jee, K.S.; Kim, Y.S.; Park, K.D.; Kim, Y.H. A novel chemical modification of bioprosthetic tissues using L-arginine. Biomaterials 2003, 24, 3409–3416. [Google Scholar] [CrossRef]
- Hilbert, S.; Ferrans, V.; Tomita, Y.; Eidbo, E.; Jones, M. Evaluation of explanted polyurethane trileaflet cardiac valve prostheses. J. Thorac. Cardiovasc. Surg. 1987, 94, 419–429. [Google Scholar]
- Kakavand, M.; Yazdanpanah, G.; Ahmadiani, A.; Niknejad, H. Blood compatibility of human amniotic membrane compared with heparin-coated ePTFE for vascular tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 1701–1709. [Google Scholar] [CrossRef]
- Ye, X.; Wang, Z.; Zhang, X.; Zhou, M.; Cai, L. Hemocompatibility research on the micro-structure surface of a bionic heart valve. Biomed. Mater. Eng. 2014, 24, 2361–2369. [Google Scholar] [Green Version]









| Sample | Min | 25% | Me | 75% | Max |
|---|---|---|---|---|---|
| SIBS | 0.23 | 0.23 | 0.39 | 0.51 | 0.55 |
| Gore-tex™ | 0.49 | 0.53 | 1.25 | 2.70 | 2.95 |
| GA-treated xenopericardium | 1.77 | 2.57 | 93.79 | 155.30 | 159.80 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ovcharenko, E.; Rezvova, M.; Nikishau, P.; Kostjuk, S.; Glushkova, T.; Antonova, L.; Trebushat, D.; Akentieva, T.; Shishkova, D.; Krivikina, E.; et al. Polyisobutylene-Based Thermoplastic Elastomers for Manufacturing Polymeric Heart Valve Leaflets: In Vitro and In Vivo Results. Appl. Sci. 2019, 9, 4773. https://doi.org/10.3390/app9224773
Ovcharenko E, Rezvova M, Nikishau P, Kostjuk S, Glushkova T, Antonova L, Trebushat D, Akentieva T, Shishkova D, Krivikina E, et al. Polyisobutylene-Based Thermoplastic Elastomers for Manufacturing Polymeric Heart Valve Leaflets: In Vitro and In Vivo Results. Applied Sciences. 2019; 9(22):4773. https://doi.org/10.3390/app9224773
Chicago/Turabian StyleOvcharenko, Evgeny, Maria Rezvova, Pavel Nikishau, Sergei Kostjuk, Tatiana Glushkova, Larisa Antonova, Dmitry Trebushat, Tatiana Akentieva, Daria Shishkova, Evgeniya Krivikina, and et al. 2019. "Polyisobutylene-Based Thermoplastic Elastomers for Manufacturing Polymeric Heart Valve Leaflets: In Vitro and In Vivo Results" Applied Sciences 9, no. 22: 4773. https://doi.org/10.3390/app9224773
APA StyleOvcharenko, E., Rezvova, M., Nikishau, P., Kostjuk, S., Glushkova, T., Antonova, L., Trebushat, D., Akentieva, T., Shishkova, D., Krivikina, E., Klyshnikov, K., Kudryavtseva, Y., & Barbarash, L. (2019). Polyisobutylene-Based Thermoplastic Elastomers for Manufacturing Polymeric Heart Valve Leaflets: In Vitro and In Vivo Results. Applied Sciences, 9(22), 4773. https://doi.org/10.3390/app9224773