Role of Oligodendrocytes and Myelin in the Pathophysiology of Autism Spectrum Disorder
Abstract
:1. Introduction
2. Pathophysiological Basis of ASD
3. Role of Oligodendrocytes in ASD: Cellular and Molecular Evidence
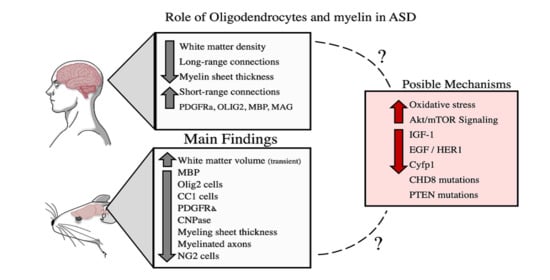
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Parellada, M.; Penzol, M.J.; Pina, L.; Moreno, C.; González-Vioque, E.; Zalsman, G.; Arango, C. The neurobiology of autism spectrum disorders. Eur. Psychiatry 2014, 29, 11–19. [Google Scholar] [CrossRef]
- Carlisi, C.O.; Norman, L.J.; Lukito, S.S.; Radua, J.; Mataix-Cols, D.; Rubia, K. Comparative multimodal meta-analysis of structural and functional brain abnormalities in autism spectrum disorder and obsessive-compulsive disorder. Biol. Psychiatry 2017, 82, 83–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsabbagh, M.; Divan, G.; Koh, Y.J.; Kim, Y.S.; Kauchali, S.; Marcín, C.; Montiel-Nava, C.; Patel, V.; Paula, C.S.; Wang, C.; et al. Global prevalence of autism and other pervasive developmental disorders. Autism Res. 2012, 5, 160–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baxter, A.J.; Brugha, T.S.; Erskine, H.E.; Scheurer, R.W.; Vos, T.; Scott, J.G. The epidemiology and global burden of autism spectrum disorders. Psychol. Med. 2015, 45, 601–613. [Google Scholar] [CrossRef] [PubMed]
- Rice, C.E.; Rosanoff, M.; Dawson, G.; Durkin, M.S.; Croen, L.A.; Singer, A.; Yeargin-Allsopp, M. Evaluating changes in the prevalence of the autism spectrum disorders (ASDs). Public Health Rev. 2012, 34, 1–22. [Google Scholar] [CrossRef] [Green Version]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5); American Psychiatric Publishing: Washington, DC, USA, 2013; ISBN 9780890425541. [Google Scholar]
- Harstad, E.B.; Fogler, J.; Sideridis, G.; Weas, S.; Mauras, C.; Barbaresi, W.J. Comparing diagnostic outcomes of autism spectrum disorder using DSM-IV-TR and DSM-5 criteria. J. Autism Dev. Disord. 2015, 45, 1437–1450. [Google Scholar] [CrossRef]
- Masi, A.; DeMayo, M.M.; Glozier, N.; Guastella, A.J. An overview of autism spectrum disorder, heterogeneity and treatment options. Neurosci. Bull. 2017, 33, 183–193. [Google Scholar] [CrossRef] [Green Version]
- Balasco, L.; Provenzano, G.; Bozzi, Y. Sensory abnormalities in autism spectrum disorders: A focus on the tactile domain, from genetic mouse models to the clinic. Front. Psychiatry 2020, 10, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Mosconi, M.W.; Sweeney, J.A. Sensorimotor dysfunctions as primary features of autism spectrum disorders. Sci. China Life Sci. 2015, 58, 1016–1023. [Google Scholar] [CrossRef] [Green Version]
- Baum, S.H.; Stevenson, R.A.; Wallace, M.T. Behavioral, perceptual, and neural alterations in sensory and multisensory function in autism spectrum disorder. Prog. Neurobiol. 2015, 134, 140–160. [Google Scholar] [CrossRef] [Green Version]
- Girirajan, S.; Rosenfeld, J.A.; Coe, B.P.; Parikh, S.; Friedman, N.; Goldstein, A.; Filipink, R.A.; McConnell, J.S.; Angle, B.; Meschino, W.S.; et al. Phenotypic heterogeneity of genomic disorders and rare copy-number variants. N. Engl. J. Med. 2012, 367, 1321–1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rylaarsdam, L.; Guemez-Gamboa, A. Genetic causes and modifiers of autism spectrum disorder. Front. Cell. Neurosci. 2019, 13. [Google Scholar] [CrossRef] [PubMed]
- Courchesne, E.; Karns, C.M.; Davis, H.R.; Ziccardi, R.; Carper, R.A.; Tigue, Z.D.; Chisum, H.J.; Moses, P.; Pierce, K.; Lord, C.; et al. Unusual brain growth patterns in early life in patients with autistic disorder: An MRI study. Neurology 2001, 57, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Courchesne, E.; Carper, R.; Akshoomoff, N. Evidence of brain overgrowth in the first year of life in autism. J. Am. Med. Assoc. 2003, 290, 337–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Courchesne, E.; Pierce, K. Why the frontal cortex in autism might be talking only to itself: Local over-connectivity but long-distance disconnection. Curr. Opin. Neurobiol. 2005, 15, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Courchesne, E.; Pierce, K.; Schumann, C.M.; Redcay, E.; Buckwalter, J.A.; Kennedy, D.P.; Morgan, J. Mapping early brain development in autism. Neuron 2007, 56, 399–413. [Google Scholar] [CrossRef] [Green Version]
- Courchesne, E.; Campbell, K.; Solso, S. Brain growth across the life span in autism: Age-specific changes in anatomical pathology. Brain Res. 2011, 1380, 138–145. [Google Scholar] [CrossRef] [Green Version]
- Morgan, J.T.; Barger, N.; Amaral, D.G.; Schumann, C.M. Stereological study of amygdala glial populations in adolescents and adults with autism spectrum disorder. PLoS ONE 2014, 9, e110356. [Google Scholar] [CrossRef] [Green Version]
- McAlonan, G.M.; Daly, E.; Kumari, V.; Critchley, H.D.; Van Amelsvoort, T.; Suckling, J.; Simmons, A.; Sigmundsson, T.; Greenwood, K.; Russell, A.; et al. Brain anatomy and sensorimotor gating in Asperger’s syndrome. Brain 2002, 127, 1594–1606. [Google Scholar] [CrossRef]
- Bloemen, O.J.N.; Deeley, Q.; Sundram, F.; Daly, E.M.; Barker, G.J.; Jones, D.K.; Van Amelsvoort, T.A.M.J.; Schmitz, N.; Robertson, D.; Murphy, K.C.; et al. White matter integrity in Asperger syndrome: A preliminary diffusion tensor magnetic resonance imaging study in adults. Autism Res. 2010, 3, 203–213. [Google Scholar] [CrossRef]
- Schmitz, N.; Daly, E.; Murphy, D. Frontal anatomy and reaction time in Autism. Neurosci. Lett. 2007, 412, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Rashid, B.; Blanken, L.M.E.; Muetzel, R.L.; Miller, R.; Damaraju, E.; Arbabshirani, M.R.; Erhardt, E.B.; Verhulst, F.C.; van der Lugt, A.; Jaddoe, V.W.V.; et al. Connectivity dynamics in typical development and its relationship to autistic traits and autism spectrum disorder. Hum. Brain Mapp. 2018, 39, 3127–3142. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.E.; Hernandez, L.M.; Bookheimer, S.Y.; Dapretto, M. Atypical longitudinal development of functional connectivity in adolescents with autism spectrum disorder. Autism Res. 2019, 12, 53–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chauhan, A.; Chauhan, V. Oxidative stress in autism. Pathophysiology 2006, 13, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Zeidán-Chuliá, F.; Salmina, A.B.; Malinovskaya, N.A.; Noda, M.; Verkhratsky, A.; Moreira, J.C.F. The glial perspective of autism spectrum disorders. Neurosci. Biobehav. Rev. 2014, 38, 160–172. [Google Scholar] [CrossRef] [PubMed]
- Casanova, M.F.; Buxhoeveden, D.P.; Switala, A.E.; Roy, E. Minicolumnar pathology in autism. Neurology 2002, 58, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Wegiel, J.; Kuchna, I.; Nowicki, K.; Imaki, H.; Wegiel, J.; Marchi, E.; Ma, S.Y.; Chauhan, A.; Chauhan, V.; Bobrowicz, T.W.; et al. The neuropathology of autism: Defects of neurogenesis and neuronal migration, and dysplastic changes. Acta Neuropathol. 2010, 119, 755–770. [Google Scholar] [CrossRef] [Green Version]
- Zeidán-Chuliá, F.; de Oliveira, B.H.N.; Casanova, M.F.; Casanova, E.L.; Noda, M.; Salmina, A.B.; Verkhratsky, A. Up-regulation of oligodendrocyte lineage markers in the cerebellum of autistic patients: Evidence from network analysis of gene expression. Mol. Neurobiol. 2016, 53, 4019–4025. [Google Scholar] [CrossRef]
- Herbert, M.R.; Ziegler, D.A.; Makris, N.; Filipek, P.A.; Kemper, T.L.; Normandin, J.J.; Sanders, H.A.; Kennedy, D.N.; Caviness, V.S. Localization of white matter volume increase in autism and developmental language disorder. Ann. Neurol. 2004, 55, 530–540. [Google Scholar] [CrossRef]
- Hong, S.J.; Hyung, B.; Paquola, C.; Bernhardt, B.C. The superficial white matter in autism and its role in connectivity anomalies and symptom severity. Cereb. Cortex 2019, 29, 4415–4425. [Google Scholar] [CrossRef]
- Mengler, L.; Khmelinskii, A.; Diedenhofen, M.; Po, C.; Staring, M.; Lelieveldt, B.P.F.; Hoehn, M. Brain maturation of the adolescent rat cortex and striatum: Changes in volume and myelination. Neuroimage 2014, 84, 35–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomassy, G.S.; Dershowitz, L.B.; Arlotta, P. Diversity matters: A revised guide to myelination. Trends Cell Biol. 2016, 26, 135–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herbert, M.R.; Ziegler, D.A.; Deutsch, C.K.; O’Brien, L.M.; Lange, N.; Bakardjiev, A.; Hodgson, J.; Adrien, K.T.; Steele, S.; Makris, N.; et al. Dissociations of cerebral cortex, subcortical and cerebral white matter volumes in autistic boys. Brain 2003, 126, 1182–1192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmody, D.P.; Lewis, M. Regional white matter development in children with autism spectrum disorders. Dev. Psychobiol. 2010, 52, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Dimond, D.; Schuetze, M.; Smith, R.E.; Dhollander, T.; Cho, I.; Vinette, S.; Ten Eycke, K.; Lebel, C.; McCrimmon, A.; Dewey, D.; et al. Reduced white matter fiber density in autism spectrum disorder. Cereb. Cortex 2019, 29, 1–11. [Google Scholar] [CrossRef]
- DiCicco-Bloom, E.; Lord, C.; Zwaigenbaum, L.; Courchesne, E.; Dager, S.R.; Schmitz, C.; Schultz, R.T.; Crawley, J.; Young, L.J. The developmental neurobiology of autism spectrum disorder. J. Neurosci. 2006, 26, 6897–6906. [Google Scholar] [CrossRef] [Green Version]
- Yassin, W.; Kojima, M.; Owada, K.; Kuwabara, H.; Gonoi, W.; Aoki, Y.; Takao, H.; Natsubori, T.; Iwashiro, N.; Kasai, K.; et al. Paternal age contribution to brain white matter aberrations in autism spectrum disorder. Psychiatry Clin. Neurosci. 2019, 73, 649–659. [Google Scholar] [CrossRef] [Green Version]
- Swanson, M.R.; Hazlett, H.C. White matter as a monitoring biomarker for neurodevelopmental disorder intervention studies. J. Neurodev. Disord. 2019, 11, 1–11. [Google Scholar] [CrossRef]
- Hughes, J.R. Autism: The first firm finding = underconnectivity? Epilepsy Behav. 2007, 11, 20–24. [Google Scholar] [CrossRef]
- Boger-Megiddo, I.; Shaw, D.W.W.; Friedman, S.D.; Sparks, B.F.; Artru, A.A.; Giedd, J.N.; Dawson, G.; Dager, S.R. Corpus callosum morphometrics in young children with autism spectrum disorder. J. Autism Dev. Disord. 2006, 36, 733–739. [Google Scholar] [CrossRef]
- Dawson, G.; Munson, J.; Webb, S.J.; Nalty, T.; Abbott, R.; Toth, K. Rate of head growth decelerates and symptoms worsen in the second year of life in autism. Biol Psychiatry 2007, 61, 458–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolff, J.J.; Gu, H.; Gerig, G.; Elison, J.T.; Styner, M.; Gouttard, S.; Botteron, K.N.; Dager, S.R.; Dawson, G.; Estes, A.M.; et al. Differences in white matter fiber tract development present from 6 to 24 months in infants with autism. Am. J. Psychiatry 2012, 169, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Lee, J.; Kim, E. Excitation/inhibition imbalance in animal models of autism spectrum disorders. Biol. Psychiatry 2017, 81, 838–847. [Google Scholar] [CrossRef] [Green Version]
- Ecker, C.; Ronan, L.; Feng, Y.; Daly, E.; Murphy, C.; Ginestet, C.E.; Brammer, M.; Fletcher, P.C.; Bullmore, E.T.; Suckling, J.; et al. Intrinsic gray-matter connectivity of the brain in adults with autism spectrum disorder. Proc. Natl. Acad. Sci. USA 2013, 110, 13222–13227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Reilly, C.; Lewis, J.D.; Elsabbagh, M. Is functional brain connectivity atypical in autism? A systematic review of EEG and MEG studies. PLoS ONE 2017, 12, e0175870. [Google Scholar] [CrossRef]
- Lee, H.; Thacker, S.; Sarn, N.; Dutta, R.; Eng, C. Constitutional mislocalization of Pten drives precocious maturation in oligodendrocytes and aberrant myelination in model of autism spectrum disorder. Transl. Psychiatry 2019, 9, 13. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.X.; Kim, G.H.; Tan, J.W.; Riso, A.E.; Sun, Y.; Xu, E.Y.; Liao, G.Y.; Xu, H.; Lee, S.H.; Do, N.Y.; et al. Elevated protein synthesis in microglia causes autism-like synaptic and behavioral aberrations. Nat. Commun. 2020, 11. [Google Scholar] [CrossRef]
- Kim, Y.S.; Choi, J.; Yoon, B.E. Neuron-glia interactions in neurodevelopmental disorders. Cells 2020, 9, 2176. [Google Scholar] [CrossRef]
- Chauhan, A.; Chauhan, W.; Brown, W.T. Autism oxidative stress, inflamation and Immune abnormalities. In CRC Press; Evans, T.A., Perry, G., Smith, M.A., Salomon, R.G., McGinnis, W.R., Sajdel-Sulkowska, M., Zhu, X., Eds.; CRC Press: Boca Raton, FL, USA, 2010; pp. 1–34. ISBN 9781420068818. [Google Scholar]
- Yui, K.; Kawasaki, Y.; Yamada, H.; Ogawa, S. Oxidative stress and nitric oxide in autism spectrum disorder and other neuropsychiatric disorders. CNS Neurol. Disord. Drug Targets 2016, 15, 587–596. [Google Scholar] [CrossRef]
- Back, S.A. White matter injury in the preterm infant: Pathology and mechanisms. Acta Neuropathol. 2017, 134, 331–349. [Google Scholar] [CrossRef]
- Thorburne, S.K.; Juurlink, B.H.J. Low glutathione and high iron govern the susceptibility of oligodendroglial precursors to oxidative stress. J. Neurochem. 1996, 67, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- McTigue, D.M.; Tripathi, R.B. The life, death, and replacement of oligodendrocytes in the adult CNS. J. Neurochem. 2008, 107, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Graciarena, M.; Seiffe, A.; Nait-Oumesmar, B.; Depino, A.M. Hypomyelination and oligodendroglial alterations in a mouse model of autism spectrum disorder. Front. Cell. Neurosci. 2019, 12, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Khanbabaei, M.; Hughes, E.; Ellegood, J.; Qiu, L.R.; Yip, R.; Dobry, J.; Murari, K.; Lerch, J.P.; Rho, J.M.; Cheng, N. Precocious myelination in a mouse model of autism. Transl. Psychiatry 2019, 9, 251. [Google Scholar] [CrossRef] [Green Version]
- Cartocci, V.; Catallo, M.; Tempestilli, M.; Segatto, M.; Pfrieger, F.W.; Bronzuoli, M.R.; Scuderi, C.; Servadio, M.; Trezza, V.; Pallottini, V. Altered brain cholesterol/isoprenoid metabolism in a rat model of autism spectrum disorders. Neuroscience 2018, 372, 27–37. [Google Scholar] [CrossRef]
- Pacey, L.K.K.; Xuan, I.C.Y.; Guan, S.; Sussman, D.; Henkelman, R.M.; Chen, Y.; Thomsen, C.; Hampson, D.R. Delayed myelination in a mouse model of fragile X syndrome. Hum. Mol. Genet. 2013, 22, 3920–3930. [Google Scholar] [CrossRef] [PubMed]
- Meikle, L.; Talos, D.M.; Onda, H.; Pollizzi, K.; Rotenberg, A.; Sahin, M.; Jensen, F.E.; Kwiatkowski, D.J. A mouse model of tuberous sclerosis: Neuronal loss of Tsc1 causes dysplastic and ectopic neurons, reduced myelination, seizure activity, and limited survival. J. Neurosci. 2007, 27, 5546–5558. [Google Scholar] [CrossRef] [PubMed]
- Noriuchi, M.; Kikuchi, Y.; Yoshiura, T.; Kira, R.; Shigeto, H.; Hara, T.; Tobimatsu, S.; Kamio, Y. Altered white matter fractional anisotropy and social impairment in children with autism spectrum disorder. Brain Res. 2010, 1362, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Zikopoulos, B.; García-Cabezas, M.Á.; Barbas, H. Parallel trends in cortical gray and white matter architecture and connections in primates allow fine study of pathways in humans and reveal network disruptions in autism. PLoS Biol. 2018, 16, 1–43. [Google Scholar] [CrossRef] [Green Version]
- Ha, S.; Sohn, I.; Kim, N.; Sim, H.J.; Cheon, K. Characteristics of brains in autism spectrum disorder: Structure, function and connectivity across the lifespan. Exp. Neurobiol. 2015, 24, 273–284. [Google Scholar] [CrossRef] [Green Version]
- Wolff, J.J.; Gerig, G.; Lewis, J.D.; Soda, T.; Styner, M.A.; Vachet, C.; Botteron, K.N.; Elison, J.T.; Dager, S.R.; Estes, A.M.; et al. Altered corpus callosum morphology associated with autism over the first 2 years of life. Brain 2015, 138, 2046–2058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheon, K.A.; Kim, Y.S.; Oh, S.H.; Park, S.Y.; Yoon, H.W.; Herrington, J.; Nair, A.; Koh, Y.J.; Jang, D.P.; Kim, Y.B.; et al. Involvement of the anterior thalamic radiation in boys with high functioning autism spectrum disorders: A diffusion tensor imaging study. Brain Res. 2011, 1417, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Sundaram, S.K.; Sivaswamy, L.; Behen, M.E.; Makki, M.I.; Ager, J.; Janisse, J.; Chugani, H.T.; Chugani, D.C. Alterations in frontal lobe tracts and corpus callosum in young children with autism spectrum disorder. Cereb. Cortex 2010, 20, 2103–2113. [Google Scholar] [CrossRef] [PubMed]
- Schaer, M.; Ottet, M.-C.; Scariati, E.; Dukes, D.; Franchini, M.; Eliez, S.; Glaser, B. Decreased frontal gyrification correlates with altered connectivity in children with autism. Front. Hum. Neurosci. 2013, 7, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikuta, T.; Shafritz, K.M.; Bregman, J.; Peters, B.D.; Gruner, P.; Malhotra, A.K.; Szeszko, P.R. Abnormal cingulum bundle development in autism: A probabilistic tractography study. Psychiatry Res. Neuroimaging 2014, 221, 63–68. [Google Scholar] [CrossRef] [Green Version]
- Nair, A.; Treiber, J.M.; Shukla, D.K.; Shih, P.; Müller, R.A. Impaired thalamocortical connectivity in autism spectrum disorder: A study of functional and anatomical connectivity. Brain 2013, 136, 1942–1955. [Google Scholar] [CrossRef]
- Pardini, M.; Elia, M.; Garaci, F.G.; Guida, S.; Coniglione, F.; Krueger, F.; Benassi, F.; Emberti Gialloreti, L. Long-term cognitive and behavioral therapies, combined with augmentative communication, are related to uncinate fasciculus integrity in autism. J. Autism Dev. Disord. 2012, 42, 585–592. [Google Scholar] [CrossRef] [Green Version]
- Pardini, M.; Garaci, F.G.; Bonzano, L.; Roccatagliata, L.; Palmieri, M.G.; Pompili, E.; Coniglione, F.; Krueger, F.; Ludovici, A.; Floris, R.; et al. White matter reduced streamline coherence in young men with autism and mental retardation. Eur. J. Neurol. 2009, 16, 1185–1190. [Google Scholar] [CrossRef]
- McAlonan, G.M.; Cheung, C.; Cheung, V.; Wong, N.; Suckling, J.; Chua, S.E. Differential effects on white-matter systems in high-functioning autism and Asperger’s syndrome. Psychol. Med. 2009, 39, 1885–1893. [Google Scholar] [CrossRef] [Green Version]
- Barnea-Goraly, N.; Lotspeich, L.J.; Reiss, A.L. Similar white matter aberrations in children with autism and their unaffected siblings. Arch. Gen. Psychiatry 2010, 67, 1052. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, P.T.; Whitaker, R.T.; Tao, R.; DuBray, M.B.; Froehlich, A.; Ravichandran, C.; Alexander, A.L.; Bigler, E.D.; Lange, N.; Lainhart, J.E. Microstructural connectivity of the arcuate fasciculus in adolescents with high-functioning autism. Neuroimage 2010, 51, 1117–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, J.W.; Kumar, A.K.; Sundaram, S.K.; Chugani, H.T.; Chugani, D.C. Sharp curvature of frontal lobe white matter pathways in children with autism spectrum disorders: Tract-based morphometry analysis. Am. J. Neuroradiol. 2011, 32, 1600–1606. [Google Scholar] [CrossRef] [PubMed]
- Lo, Y.C.; Soong, W.T.; Gau, S.S.F.; Wu, Y.Y.; Lai, M.C.; Yeh, F.C.; Chiang, W.Y.; Kuo, L.W.; Jaw, F.S.; Tseng, W.Y.I. The loss of asymmetry and reduced interhemispheric connectivity in adolescents with autism: A study using diffusion spectrum imaging tractography. Psychiatry Res. Neuroimaging 2011, 192, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.; Chua, S.E.; Cheung, V.; Khong, P.L.; Tai, K.S.; Wong, T.K.W.; Ho, T.P.; McAlonan, G.M. White matter fractional anisotrophy differences and correlates of diagnostic symptoms in autism. J. Child Psychol. Psychiatry Allied Discip. 2009, 50, 1102–1112. [Google Scholar] [CrossRef]
- Barnea-Goraly, N.; Kwon, H.; Menon, V.; Eliez, S.; Lotspeich, L.; Reiss, A.L. White matter structure in autism: Preliminary evidence from diffusion tensor imaging. Biol. Psychiatry 2004, 55, 323–326. [Google Scholar] [CrossRef]
- Ecker, C.; Rocha-Rego, V.; Johnston, P.; Mourao-Miranda, J.; Marquand, A.; Daly, E.M.; Brammer, M.J.; Murphy, C.; Murphy, D.G. Investigating the predictive value of whole-brain structural MR scans in autism: A pattern classification approach. Neuroimage 2010, 49, 44–56. [Google Scholar] [CrossRef]
- Shukla, D.K.; Keehn, B.; Lincoln, A.J.; Müller, R.A. White matter compromise of callosal and subcortical fiber tracts in children with autism spectrum disorder: A diffusion tensor imaging study. J. Am. Acad. Child Adolesc. Psychiatry 2010, 49, 1269–1278. [Google Scholar] [CrossRef] [Green Version]
- Sivaswamy, L.; Kumar, A.; Rajan, D.; Behen, M.; Muzik, O.; Chugani, D.; Chugani, H. A diffusion tensor imaging study of the cerebellar pathways in children with autism spectrum disorder. J. Child Neurol. 2010, 25, 1223–1231. [Google Scholar] [CrossRef]
- Yu, Q.; Peng, Y.; Kang, H.; Peng, Q.; Ouyang, M.; Slinger, M.; Hu, D.; Shou, H.; Fang, F.; Huang, H. Differential white matter maturation from birth to 8 years of age. Cereb. Cortex 2020, 30, 2674–2690. [Google Scholar] [CrossRef]
- Brito, A.R.; Vasconcelos, M.M.; Domingues, R.C.; Hygino Da Cruz, L.C.; Rodrigues, L.D.S.; Gasparetto, E.L.; Calçada, C.A.B.P. Diffusion tensor imaging findings in school-aged autistic children. J. Neuroimaging 2009, 19, 337–343. [Google Scholar] [CrossRef]
- Jou, R.J.; Mateljevic, N.; Kaiser, M.D.; Sugrue, D.R.; Volkmar, F.R.; Pelphrey, K.A. Structural neural phenotype of autism: Preliminary evidence from a diffusion tensor imaging study using tract-based spatial statistics. Am. J. Neuroradiol. 2011, 32, 1607–1613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shukla, D.K.; Keehn, B.; Müller, R.A. Tract-specific analyses of diffusion tensor imaging show widespread white matter compromise in autism spectrum disorder. J. Child Psychol. Psychiatry Allied Discip. 2011, 52, 286–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahyoun, C.P.; Belliveau, J.W.; Mody, M. White matter integrity and pictorial reasoning in high-functioning children with autism. Brain Cogn. 2010, 73, 180–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ameis, S.H.; Fan, J.; Rockel, C.; Voineskos, A.N.; Lobaugh, N.J.; Soorya, L.; Wang, A.T.; Hollander, E.; Anagnostou, E. Impaired structural connectivity of socio-emotional circuits in autism spectrum disorders: A diffusion tensor imaging study. PLoS ONE 2011, 6, e28044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, C.; Humphreys, K.; Jung, K.J.; Minshew, N.; Behrmann, M. The anatomy of the callosal and visual-association pathways in high-functioning autism: A DTI tractography study. Cortex 2011, 47, 863–873. [Google Scholar] [CrossRef] [Green Version]
- Itahashi, T.; Yamada, T.; Nakamura, M.; Watanabe, H.; Yamagata, B.; Jimbo, D.; Shioda, S.; Kuroda, M.; Toriizuka, K.; Kato, N.; et al. Linked alterations in gray and white matter morphology in adults with high-functioning autism spectrum disorder: A multimodal brain imaging study. Neuroimage Clin. 2015, 7, 155–169. [Google Scholar] [CrossRef] [Green Version]
- Roine, U.; Salmi, J.; Roine, T.; Wendt, T.N.-v.; Leppämäki, S.; Rintahaka, P.; Tani, P.; Leemans, A.; Sams, M. Constrained spherical deconvolution-based tractography and tract-based spatial statistics show abnormal microstructural organization in Asperger syndrome. Mol. Autism 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Karahanoğlu, F.I.; Baran, B.; Nguyen, Q.T.H.; Meskaldji, D.E.; Yendiki, A.; Vangel, M.; Santangelo, S.L.; Manoach, D.S. Diffusion-weighted imaging evidence of altered white matter development from late childhood to early adulthood in autism spectrum disorder. Neuroimage Clin. 2018, 19, 840–847. [Google Scholar] [CrossRef]
- Poustka, L.; Jennen-Steinmetz, C.; Henze, R.; Vomstein, K.; Haffner, J.; Sieltjes, B. Fronto-temporal disconnectivity and symptom severity in children with autism spectrum disorder. World J. Biol. Psychiatry 2012, 13, 269–280. [Google Scholar] [CrossRef]
- Conturo, T.E.; Williams, D.L.; Smith, C.D.; Gultepe, E.; Akbudak, E.; Minshew, N.J. Neuronal fiber pathway abnormalities in autism: An initial MRI diffusion tensor tracking study of hippocampo-fusiform and amygdalo-fusiform pathways. J. Int. Neuropsychol. Soc. 2008, 14, 933–946. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Chou, K.H.; Chen, I.Y.; Fan, Y.T.; Decety, J.; Lin, C.P. Atypical development of white matter microstructure in adolescents with autism spectrum disorders. Neuroimage 2010, 50, 873–882. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, M.; Ben-Sira, L.; Levy, Y.; Zachor, D.A.; Itzhak, E.B.e.n.; Artzi, M.; Tarrasch, R.; Eksteine, P.M.; Hendler, T.; Bashat, D. Ben Abnormal white matter integrity in young children with autism. Hum. Brain Mapp. 2011, 32, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Mengotti, P.; D’Agostini, S.; Terlevic, R.; De Colle, C.; Biasizzo, E.; Londero, D.; Ferro, A.; Rambaldelli, G.; Balestrieri, M.; Zanini, S.; et al. Altered white matter integrity and development in children with autism: A combined voxel-based morphometry and diffusion imaging study. Brain Res. Bull. 2011, 84, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Tang, T.; Hong, S.; Hang, Y.; Zou, B.; Li, H.; Zhou, Z.; Ruan, Z.; Lu, Z.; Tao, G.; et al. White matter impairments in autism, evidence from voxel-based morphometry and diffusion tensor imaging. Brain Res. 2009, 1265, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhou, Z.; Chang, C.; Qian, L.; Li, C.; Xiao, T.; Xiao, X.; Chu, K.; Fang, H.; Ke, X. Anomalies in uncinate fasciculus development and social defects in preschoolers with autism spectrum disorder. BMC Psychiatry 2019, 19, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sahyoun, C.P.; Belliveau, J.W.; Soulières, I.; Schwartz, S.; Mody, M. Neuroimaging of the functional and structural networks underlying visuospatial vs. linguistic reasoning in high-functioning autism. Neuropsychologia 2010, 48, 86–95. [Google Scholar] [CrossRef] [Green Version]
- Vidal, C.N.; Nicolson, R.; DeVito, T.J.; Hayashi, K.M.; Geaga, J.A.; Drost, D.J.; Williamson, P.C.; Rajakumar, N.; Sui, Y.; Dutton, R.A.; et al. Mapping corpus callosum deficits in autism: An index of aberrant cortical connectivity. Biol. Psychiatry 2006, 60, 218–225. [Google Scholar] [CrossRef]
- Frazier, T.W.; Keshavan, M.S.; Minshew, N.J.; Hardan, A.Y. A two-year longitudinal MRI study of the corpus callosum in autism. J. Autism Dev. Disord. 2012, 42, 2312–2322. [Google Scholar] [CrossRef] [Green Version]
- Hardan, A.Y.; Pabalan, M.; Gupta, N.; Bansal, R.; Melhem, N.M.; Fedorov, S.; Keshavan, M.S.; Minshew, N.J. Corpus callosum volume in children with autism. Psychiatry Res. Neuroimaging 2009, 174, 57–61. [Google Scholar] [CrossRef] [Green Version]
- Hong, S.; Ke, X.; Tang, T.; Hang, Y.; Chu, K.; Huang, H.; Ruan, Z.; Lu, Z.; Tao, G.; Liu, Y. Detecting abnormalities of corpus callosum connectivity in autism using magnetic resonance imaging and diffusion tensor tractography. Psychiatry Res. Neuroimaging 2011, 194, 333–339. [Google Scholar] [CrossRef]
- Jou, R.J.; Jackowski, A.P.; Papademetris, X.; Rajeevan, N.; Staib, L.H.; Volkmar, F.R. Diffusion tensor imaging in autism spectrum disorders: Preliminary evidence of abnormal neural connectivity. Aust. N. Z. J. Psychiatry 2011, 45, 153–162. [Google Scholar] [CrossRef] [Green Version]
- Thakkar, K.N.; Polli, F.E.; Joseph, R.M.; Tuch, D.S.; Hadjikhani, N.; Barton, J.J.S.; Manoach, D.S. Response monitoring, repetitive behaviour and anterior cingulate abnormalities in autism spectrum disorders (ASD). Brain 2008, 131, 2464–2478. [Google Scholar] [CrossRef] [Green Version]
- Haigh, S.M.; Keller, T.A.; Minshew, N.J.; Eack, S.M. Reduced white matter integrity and deficits in neuropsychological functioning in adults with autism spectrum disorder. Autism Res. 2020, 13, 702–714. [Google Scholar] [CrossRef]
- Catani, M.; Jones, D.K.; Daly, E.; Embiricos, N.; Deeley, Q.; Pugliese, L.; Curran, S.; Robertson, D.; Murphy, D.G.M. Altered cerebellar feedback projections in Asperger syndrome. Neuroimage 2008, 41, 1184–1191. [Google Scholar] [CrossRef]
- Hardan, A.Y.; Minshew, N.J.; Keshavan, M.S. Corpus callosum size in autism. Neurology 2000, 55, 1033–1036. [Google Scholar] [CrossRef]
- Piven, J.; Bailey, J.; Ranson, B.J.; Arndt, S. An MRI study of the corpus callosum in autism. Am. J. Psychiatry 1997, 154, 1051–1056. [Google Scholar] [CrossRef]
- Langen, M.; Leemans, A.; Johnston, P.; Ecker, C.; Daly, E.; Murphy, C.M.; Dell’Acqua, F.; Durston, S.; Murphy, D.G.M. Fronto-striatal circuitry and inhibitory control in autism: Findings from diffusion tensor imaging tractography. Cortex 2012, 48, 183–193. [Google Scholar] [CrossRef]
- Libero, L.E.; DeRamus, T.P.; Lahti, A.C.; Deshpande, G.; Kana, R.K. Multimodal neuroimaging based classification of autism spectrum disorder using anatomical, neurochemical, and white matter correlates. Cortex 2015, 66, 46–59. [Google Scholar] [CrossRef] [Green Version]
- Haigh, S.M.; Eack, S.M.; Keller, T.; Minshew, N.J.; Behrmann, M. White matter structure in schizophrenia and autism: Abnormal diffusion across the brain in schizophrenia. Neuropsychologia 2019, 135, 107233. [Google Scholar] [CrossRef]
- Bradl, M.; Lassmann, H. Oligodendrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 37–53. [Google Scholar] [CrossRef] [Green Version]
- Dulamea, A. The contribution of oligodendrocytes and oligodendrocyte progenitor cells to central nervous system repair in multiple sclerosis: Perspectives for remyelination therapeutic strategies. Neural Regen. Res. 2017, 12, 1939. [Google Scholar] [CrossRef]
- Tiane, A.; Schepers, M.; Rombaut, B.; Hupperts, R.; Prickaerts, J.; Hellings, N.; van den Hove, D.; Vanmierlo, T. From OPC to oligodendrocyte: An Epigenetic journey. Cells 2019, 8, 1236. [Google Scholar] [CrossRef] [Green Version]
- Alexander, A.L.; Hurley, S.A.; Samsonov, A.A.; Adluru, N.; Hosseinbor, A.P.; Mossahebi, P.; Tromp, D.P.M.; Zakszewski, E.; Field, A.S. Characterization of cerebral white matter properties using quantitative magnetic resonance imaging stains. Brain Connect. 2011, 1, 423–446. [Google Scholar] [CrossRef]
- Menn, B.; Garcia-Verdugo, J.M.; Yaschine, C.; Gonzalez-Perez, O.; Rowitch, D.; Alvarez-Buylla, A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J. Neurosci. 2006, 26, 7907–7918. [Google Scholar] [CrossRef]
- Bergles, D.E.; Roberts, J.D.B.; Somogyl, P.; Jahr, C.E. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature 2000, 405, 187–191. [Google Scholar] [CrossRef]
- Kukley, M.; Capetillo-Zarate, E.; Dietrich, D. Vesicular glutamate release from axons in white matter. Nat. Neurosci. 2007, 10, 311–320. [Google Scholar] [CrossRef]
- Ziskin, J.L.; Nishiyama, A.; Rubio, M.; Fukaya, M.; Bergles, D.E. Vesicular release of glutamate from unmyelinated axons in white matter. Nat. Neurosci. 2007, 10, 321–330. [Google Scholar] [CrossRef]
- Bronzuoli, M.R.; Facchinetti, R.; Ingrassia, D.; Sarvadio, M.; Schiavi, S.; Steardo, L.; Verkhratsky, A.; Trezza, V.; Scuderi, C. Neuroglia in the autistic brain: Evidence from a preclinical model 11 medical and health sciences 1109 neurosciences 17 psychology and cognitive sciences 1701 psychology. Mol. Autism 2018, 9, 1–17. [Google Scholar] [CrossRef]
- Riikonen, R.; Makkonen, I.; Vanhala, R.; Turpeinen, U.; Kuikka, J.; Kokki, H. Cerebrospinal fluid insulin-like growth factors IGF-1 and IGF-2 in infantile autism. Dev. Med. Child Neurol. 2006, 48, 751–755. [Google Scholar] [CrossRef]
- Vanhala, R.; Turpeinen, U.; Riikonen, R. Low levels of insulin-like growth factor-I in cerebrospinal fluid in children with autism. Dev. Med. Child Neurol. 2007, 43, 614–616. [Google Scholar] [CrossRef]
- Suzuki, K.; Hashimoto, K.; Iwata, Y.; Nakamura, K.; Tsujii, M.; Tsuchiya, K.J.; Sekine, Y.; Suda, S.; Sugihara, G.; Matsuzaki, H.; et al. Decreased serum levels of epidermal growth factor in adult subjects with high-functioning autism. Biol. Psychiatry 2007, 62, 267–269. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.J. Decreased epidermal growth factor (EGF) associated with HMGB1 and increased hyperactivity in children with autism. Biomark. Insights 2013, 8, 35–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, A.I.; Ulfarsson, M.O.; Stefansson, H.; Gustafsson, O.; Walters, G.B.; Linden, D.E.J.; Wilkinson, L.S.; Drakesmith, M.; Owen, M.J.; Hall, J.; et al. Reciprocal white matter changes associated with copy number variation at 15q11.2 BP1-BP2: A diffusion tensor imaging study. Biol. Psychiatry 2019, 85, 563–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefansson, H.; Meyer-Lindenberg, A.; Steinberg, S.; Magnusdottir, B.; Morgen, K.; Arnarsdottir, S.; Bjornsdottir, G.; Walters, G.B.; Jonsdottir, G.A.; Doyle, O.M.; et al. CNVs conferring risk of autism or schizophrenia affect cognition in controls. Nature 2014, 505, 361–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domínguez-Iturza, N.; Lo, A.C.; Shah, D.; Armendáriz, M.; Vannelli, A.; Mercaldo, V.; Trusel, M.; Li, K.W.; Gastaldo, D.; Santos, A.R.; et al. The autism- and schizophrenia-associated protein CYFIP1 regulates bilateral brain connectivity and behaviour. Nat. Commun. 2019, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Nir, A.; Barak, B. White matter alterations in Williams syndrome related to behavioral and motor impairments. Glia 2021, 69, 5–19. [Google Scholar] [CrossRef]
- Morris, C.A. Introduction: Williams syndrome. Am. J. Med. Genet. Part C Semin. Med. Genet. 2010, 154, 203–208. [Google Scholar] [CrossRef] [Green Version]
- Osório, A.; Soares, J.M.; Prieto, M.F.; Vasconcelos, C.; Fernandes, C.; Sousa, S.; Carracedo, Á.; Gonçalves, Ó.F.; Sampaio, A. Cerebral and cerebellar MRI volumes in Williams syndrome. Res. Dev. Disabil. 2014, 35, 922–928. [Google Scholar] [CrossRef] [Green Version]
- Marie, C.; Clavairoly, A.; Frah, M.; Hmidan, H.; Yan, J.; Zhao, C.; Van Steenwinckel, J.; Daveau, R.; Zalc, B.; Hassan, B.; et al. Oligodendrocyte precursor survival and differentiation requires chromatin remodeling by Chd7 and Chd8. Proc. Natl. Acad. Sci. USA 2018, 115, E8246–E8255. [Google Scholar] [CrossRef] [Green Version]
- Bernier, R.; Golzio, C.; Xiong, B.; Stessman, H.A.; Coe, B.P.; Penn, O.; Witherspoon, K.; Gerdts, J.; Baker, C.; Vulto-Van Silfhout, A.T.; et al. Disruptive CHD8 mutations define a subtype of autism early in development. Cell 2014, 158, 263–276. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Dong, C.; Frah, M.; Deng, Y.; Marie, C.; Zhang, F.; Xu, L.; Ma, Z.; Dong, X.; Lin, Y.; et al. Dual requirement of CHD8 for chromatin landscape establishment and histone methyltransferase recruitment to promote CNS myelination and repair. Dev. Cell 2018, 45, 753–768. [Google Scholar] [CrossRef] [Green Version]
- Platt, R.J.; Zhou, Y.; Slaymaker, I.M.; Shetty, A.S.; Weisbach, N.R.; Kim, J.A.; Sharma, J.; Desai, M.; Sood, S.; Kempton, H.R.; et al. Chd8 mutation leads to autistic-like behaviors and impaired striatal circuits. Cell Rep. 2017, 19, 335–350. [Google Scholar] [CrossRef] [Green Version]
- Cotney, J.; Muhle, R.A.; Sanders, S.J.; Liu, L.; Willsey, A.J.; Niu, W.; Liu, W.; Klei, L.; Lei, J.; Yin, J.; et al. The autism-associated chromatin modifier CHD8 regulates other autism risk genes during human neurodevelopment. Nat. Commun. 2015, 6, 6404. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, Y.Y.; Wang, X.; Tan, G.H.; Li, H.P.; Hulbert, S.W.; Li, C.Y.; Hu, C.C.; Xiong, Z.Q.; Xu, X.; et al. Autism-associated CHD8 deficiency impairs axon development and migration of cortical neurons. Mol. Autism 2018, 9, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Galvez-Contreras, A.Y.; Campos-Ordonez, T.; Gonzalez-Castaneda, R.E.; Gonzalez-Perez, O. Alterations of growth factors in autism and attention-deficit/hyperactivity disorder. Front. Psychiatry 2017, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Baumann, N.; Pham-Dinh, D. Biology of oligodendrocyte and myelin in the mammalian central nervous system. Physiol. Rev. 2001, 81, 871–927. [Google Scholar] [CrossRef]
- Hsieh, J.; Aimone, J.B.; Kaspar, B.K.; Kuwabara, T.; Nakashima, K.; Gage, F.H. IGF-I instructs multipotent adult neural progenitor cells to become oligodendrocytes. J. Cell Biol. 2004, 164, 111–122. [Google Scholar] [CrossRef] [Green Version]
- Beck, K.D.; Powell-Braxtont, L.; Widmer, H.R.; Valverde, J.; Hefti, F. Igf1 gene disruption results in reduced brain size, CNS hypomyelination, and loss of hippocampal granule and striatal parvalbumin-containing neurons. Neuron 1995, 14, 717–730. [Google Scholar] [CrossRef] [Green Version]
- Ye, P.; Li, L.; Richards, R.G.; DiAugustine, R.P.; D’Ercole, A.J. Myelination is altered in insulin-like growth factor-I null mutant mice. J. Neurosci. 2002, 22, 6041–6051. [Google Scholar] [CrossRef] [Green Version]
- Steinman, G. Plausible etiology of brain dysconnectivity in autism—Review and prospectus. Med. Hypotheses 2015, 85, 405–407. [Google Scholar] [CrossRef]
- Bozdagi, O.; Tavassoli, T.; Buxbaum, J.D. Insulin-like growth factor-1 rescues synaptic and motor deficits in a mouse model of autism and developmental delay. Mol. Autism 2013, 4, 3–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Perez, O.; Romero-Rodriguez, R.; Soriano-Navarro, M.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Epidermal growth factor induces the progeny of subventricular zone type B cells to migrate and differentiate into oligodendrocytes. Stem Cells 2009, 27, 2032–2043. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Perez, O.; Quiñones-Hinojosa, A. Dose-dependent effect of EGF on migration and differentiation of adult subventricular zone astrocytes. Glia 2010, 58, 975–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gonzalez-Perez, O.; Alvarez-Buylla, A. Oligodendrogenesis in the subventricular zone and the role of epidermal growth factor. Brain Res. Rev. 2011, 67, 147–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galvez-Contreras, A.Y.; Quiñones-Hinojosa, A.; Gonzalez-Perez, O. The role of EGFR and ErbB family related proteins in the oligodendrocyte specification in germinal niches of the adult mammalian brain. Front. Cell. Neurosci. 2013, 7, 258. [Google Scholar] [CrossRef] [PubMed]
- Russo, A.J. Increased epidermal growth factor receptor (EGFR) associated with hepatocyte growth factor (HGF) and symptom severity in children with autism spectrum disorders (ASDs). J. Cent. Nerv. Syst. Dis. 2014, 6, 79–83. [Google Scholar] [CrossRef] [Green Version]
- Kaphzan, H.; Hernandez, P.; Jung, J.I.; Cowansage, K.K.; Deinhardt, K.; Chao, M.V.; Abel, T.; Klann, E. Reversal of impaired hippocampal long-term potentiation and contextual fear memory deficits in angelman syndrome model mice by ErbB inhibitors. Biol. Psychiatry 2012, 72, 182–190. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Alberts, I.; Li, X. Dysregulation of the IGF-I/PI3K/AKT/mTOR signaling pathway in autism spectrum disorders. Int. J. Dev. Neurosci. 2014, 35, 35–41. [Google Scholar] [CrossRef]
- Onore, C.; Yang, H.; Van de Water, J.; Ashwood, P. Dynamic Akt/mTOR signaling in children with autism spectrum disorder. Front. Pediatr. 2017, 5, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Cuscó, I.; Medrano, A.; Gener, B.; Vilardell, M.; Gallastegui, F.; Villa, O.; González, E.; Rodríguez-Santiago, B.; Vilella, E.; Del Campo, M.; et al. Autism-specific copy number variants further implicate the phosphatidylinositol signaling pathway and the glutamatergic synapse in the etiology of the disorder. Hum. Mol. Genet. 2009, 18, 1795–1804. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Blundell, J.; Ogawa, S.; Kwon, C.H.; Zhang, W.; Sinton, C.; Powell, C.M.; Parada, L.F. Pharmacological inhibition of mTORCl suppresses anatomical, cellular, and behavioral abnormalities in neural-specific PTEN knock-out mice. J. Neurosci. 2009, 29, 1773–1783. [Google Scholar] [CrossRef] [Green Version]
- Ness, J.K.; Mitchell, N.E.; Wood, T.L. IGF-I and NT-3 signaling pathways in developing oligodendrocytes: Differential regulation and activation of receptors and the downstream effector akt. Dev. Neurosci. 2002, 24, 437–445. [Google Scholar] [CrossRef]
- Butler, M.G.; Dazouki, M.J.; Zhou, X.P.; Talebizadeh, Z.; Brown, M.; Takahashi, T.N.; Miles, J.H.; Wang, C.H.; Stratton, R.; Pilarski, R.; et al. Subset of individuals with autism spectrum disorders and extreme macrocephaly associated with germline PTEN tumour suppressor gene mutations. J. Med. Genet. 2005, 42, 318–321. [Google Scholar] [CrossRef] [Green Version]
- Tilot, A.K.; Gaugler, M.K.; Yu, Q.; Romigh, T.; Yu, W.; Miller, R.H.; Frazier, T.W.; Eng, C. Germline disruption of Pten localization causes enhanced sex-dependent social motivation and increased glial production. Hum. Mol. Genet. 2014, 23, 3212–3227. [Google Scholar] [CrossRef] [Green Version]
- Frazier, T.W.; Embacher, R.; Tilot, A.K.; Koenig, K.; Mester, J.; Eng, C. Molecular and phenotypic abnormalities in individuals with germline heterozygous PTEN mutations and autism. Mol. Psychiatry 2015, 20, 1132–1138. [Google Scholar] [CrossRef] [Green Version]
- Jones, J.I.; Clemmons, D.R. Insulin-like growth factors and their binding proteins: Biological actions. Endocr. Rev. 1995, 16, 3–34. [Google Scholar] [CrossRef]
- Linggi, B.; Carpenter, G. ErbB receptors: New insights on mechanisms and biology. Trends Cell Biol. 2006, 16, 649–656. [Google Scholar] [CrossRef]
- Galvez-Contreras, A.Y.; Campos-Ordonez, T.; Lopez-Virgen, V.; Gomez-Plascencia, J.; Ramos-Zuniga, R.; Gonzalez-Perez, O. Growth factors as clinical biomarkers of prognosis and diagnosis in psychiatric disorders. Cytokine Growth Factor Rev. 2016, 32, 85–96. [Google Scholar] [CrossRef]
- Furusho, M.; Dupree, J.L.; Nave, K.A.; Bansal, R. Fibroblast growth factor receptor signaling in oligodendrocytes regulates myelin sheath thickness. J. Neurosci. 2012, 32, 6631–6641. [Google Scholar] [CrossRef] [Green Version]
- Ishii, A.; Furusho, M.; Bansal, R. Sustained activation of ERK1/2 MAPK in oligodendrocytes and schwann cells enhances myelin growth and stimulates oligodendrocyte progenitor expansion. J. Neurosci. 2013, 33, 175–186. [Google Scholar] [CrossRef] [Green Version]
- Ishii, A.; Fyffe-Maricich, S.L.; Furusho, M.; Miller, R.H.; Bansal, R. ERK1/ERK2 MAPK signaling is required to increase myelin thickness independent of oligodendrocyte differentiation and initiation of myelination. J. Neurosci. 2012, 32, 8855–8864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bibollet-Bahena, O.; Almazan, G. IGF-1-stimulated protein synthesis in oligodendrocyte progenitors requires PI3K/mTOR/Akt and MEK/ERK pathways. J. Neurochem. 2009, 109, 1440–1451. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.L.; Almazan, G. IGF-I-induced oligodendrocyte progenitor proliferation requires PI3K/Akt, MEK/ERK, and Src-like tyrosine kinases. J. Neurochem. 2007, 100, 1480–1493. [Google Scholar] [CrossRef] [PubMed]


| Age | Affected Brain Regions in ASD |
|---|---|
| <15 years | Increased white matter density: Supplementary motor area, left precentral, superior longitudinal fasciculus *, left cingulum *, right cingulate gyrus, prefrontal cortex *, radiate volume, corpus callosum *, right inferior frontal gyrus, putamen, insula, sublobar extranuclear area, right superior temporal gyrus, hippocampus, middle temporal gyrus *, fusiform gyrus, uncinate fasciculus *, inferior longitudinal fasciculus *, bilateral middle *, and left inferior cerebellar peduncle. Reduced white matter density: Superior longitudinal fasciculus *, cingulum *, cingulate gyrus, prefrontal cortex *, corona radiata, middle frontal gyrus, corpus callosum *, arcuate fasciculus, inferior frontal gyrus, forceps minor, fornix, anterior thalamic radiation, internal and external capsule, superior temporal gyrus, superior temporal sulcus, temporoparietal junctions, middle temporal gyrus *, right inferior frontal gyrus-middle temporal gyrus tracts, bilateral inferior frontal gyrus-fusiform gyrus tracts, inferior fronto-occipital fasciculus, occipitotemporal gyrus, uncinate fasciculus *, inferior longitudinal fasciculus *, amygdala, inferior temporal gyrus, bilateral superior, middle * and right inferior cerebellar peduncle, pontine crossing tracts and medial lemniscus, cerebellum, and corticospinal tract. |
| >15 years | Increased white matter density: Corpus callosum *, anterior and posterior thalamic radiation, right insula, bilateral amygdala-fusiform pathways temporal, left hippocampus-fusiform pathways, temporal segment of Superior longitudinal fasciculus *, right lingual gyrus, uncinate fasciculus, inferior fronto-occipital fasciculus *, inferior longitudinal fasciculus, and corticospinal tract *. Reduced white matter density: Superior longitudinal fasciculus, intraparietal sulcus, cingulum, anterior cingulate gyrus, right superior frontal gyrus, prefrontal cortex, middle frontal gyrus, corpus callosum *, left orbitofrontal cortex, inferior frontal gyrus, left putamen tracts, thalamus, forceps minor, anterior thalamic radiation, internal capsule, right hippocampus-fusiform pathway, inferior fronto-occipital fasciculus *, cerebellum, and corticospinal tract *. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galvez-Contreras, A.Y.; Zarate-Lopez, D.; Torres-Chavez, A.L.; Gonzalez-Perez, O. Role of Oligodendrocytes and Myelin in the Pathophysiology of Autism Spectrum Disorder. Brain Sci. 2020, 10, 951. https://doi.org/10.3390/brainsci10120951
Galvez-Contreras AY, Zarate-Lopez D, Torres-Chavez AL, Gonzalez-Perez O. Role of Oligodendrocytes and Myelin in the Pathophysiology of Autism Spectrum Disorder. Brain Sciences. 2020; 10(12):951. https://doi.org/10.3390/brainsci10120951
Chicago/Turabian StyleGalvez-Contreras, Alma Y., David Zarate-Lopez, Ana L. Torres-Chavez, and Oscar Gonzalez-Perez. 2020. "Role of Oligodendrocytes and Myelin in the Pathophysiology of Autism Spectrum Disorder" Brain Sciences 10, no. 12: 951. https://doi.org/10.3390/brainsci10120951
APA StyleGalvez-Contreras, A. Y., Zarate-Lopez, D., Torres-Chavez, A. L., & Gonzalez-Perez, O. (2020). Role of Oligodendrocytes and Myelin in the Pathophysiology of Autism Spectrum Disorder. Brain Sciences, 10(12), 951. https://doi.org/10.3390/brainsci10120951