Acute and Chronic Mental Stress both Influence Levels of Neurotransmitter Precursor Amino Acids and Derived Biogenic Amines
Abstract
:1. Introduction
2. Methods
2.1. Ethics Statement
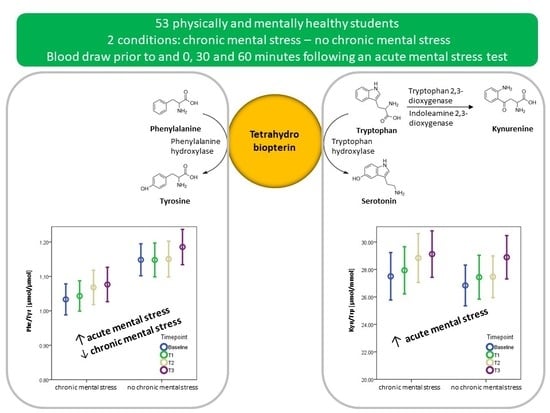
2.2. Study Design
2.3. Participants
2.4. Acute Stress Test
2.5. Assessment of Stress-Related Psychometric Parameters
2.6. Blood Sampling
2.7. Neurotransmitter Precursor Amino Acid Analysis
2.8. Statistical Analysis
3. Results
3.1. Study Population
3.2. Psychometric Stress Assessment
3.3. The Effect of Mental Stress on Serum Neopterin and Nitrite Levels
3.4. The Effect of Acute Mental Stress on Neurotransmitter Precursor Amino Acids
3.5. The Effect of Chronic Mental Stress on Neurotransmitter Precursor Amino Acids
3.6. The Effect of Acute and Chronic Mental Stress on QA and KA Levels
3.7. Correlation Analyses
4. Discussion
4.1. PHE/TYR and KYN/TRP and Acute Mental Stress
4.2. The Effect of Chronic Mental Stress on Neurotransmitter Precursor Amino Acids
4.3. QA and KA in Acute and Chronic Mental Stress
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Selye, H. The evolution of the stress concept. Am. Sci. 1973, 61, 692–699. [Google Scholar] [CrossRef] [Green Version]
- Blugeot, A.; Rivat, C.; Bouvier, E.; Molet, J.; Mouchard, A.; Zeau, B.; Bernard, C.; Benoliel, J.J.; Becker, C. Vulnerability to depression: From brain neuroplasticity to identification of biomarkers. J. Neurosci. 2011, 31, 12889–12899. [Google Scholar] [CrossRef] [PubMed]
- Marchand, A.; Durand, P. Psychosocial and biological indicators in the evaluation of and intervention in mental health problems at work. Healthc. Pap. 2011, 11, 6–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proietti, R.; Mapelli, D.; Volpe, B.; Bartoletti, S.; Sagone, A.; Dal Bianco, L.; Daliento, L. Mental stress and ischemic heart disease: Evolving awareness of a complex association. Future Cardiol. 2011, 7, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Soufer, R.; Jain, H.; Yoon, A.J. Heart-brain interactions in mental stress-induced myocardial ischemia. Curr. Cardiol. Rep. 2009, 11, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Koolhaas, J.M.; Bartolomucci, A.; Buwalda, B.; de Boer, S.F.; Flugge, G.; Korte, S.M.; Meerlo, P.; Murison, R.; Olivier, B.; Palanza, P.; et al. Stress revisited: A critical evaluation of the stress concept. Neurosci. Biobehav. Rev. 2011, 35, 1291–1301. [Google Scholar] [CrossRef]
- Selye, H. The nature of stress. Basal Facts 1985, 7, 3–11. [Google Scholar]
- Bachen, E.A.; Manuck, S.B.; Cohen, S.; Muldoon, M.F.; Raible, R.; Herbert, T.B.; Rabin, B.S. Adrenergic blockade ameliorates cellular immune responses to mental stress in humans. Psychosom. Med. 1995, 57, 366–372. [Google Scholar] [CrossRef]
- Nance, D.M.; Sanders, V.M. Autonomic innervation and regulation of the immune system (1987–2007). Brain Behav. Immun. 2007, 21, 736–745. [Google Scholar] [CrossRef] [Green Version]
- Oxenkrug, G.F. Tryptophan kynurenine metabolism as a common mediator of genetic and environmental impacts in major depressive disorder: The serotonin hypothesis revisited 40 years later. Isr. J. Psychiatry Relat. Sci. 2010, 47, 56–63. [Google Scholar]
- Qin, Y.; Wang, N.; Zhang, X.; Han, X.; Zhai, X.; Lu, Y. IDO and TDO as a potential therapeutic target in different types of depression. Metab. Brain Dis. 2018, 33, 1787–1800. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.A.; Evans, M. Animal liver tryptophan pyrrolases: Absence of apoenzyme and of hormonal induction mechanism from species sensitive to tryptophan toxicity. Biochem. J. 1976, 158, 79–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capuron, L.; Neurauter, G.; Musselman, D.L.; Lawson, D.H.; Nemeroff, C.B.; Fuchs, D.; Miller, A.H. Interferon-alpha-induced changes in tryptophan metabolism. relationship to depression and paroxetine treatment. Biol. Psychiatry 2003, 54, 906–914. [Google Scholar] [CrossRef]
- Pantouris, G.; Serys, M.; Yuasa, H.J.; Ball, H.J.; Mowat, C.G. Human indoleamine 2,3-dioxygenase-2 has substrate specificity and inhibition characteristics distinct from those of indoleamine 2,3-dioxygenase-1. Amino Acids 2014, 46, 2155–2163. [Google Scholar] [CrossRef] [PubMed]
- Myint, A.M. Kynurenines: From the perspective of major psychiatric disorders. FEBS J. 2012, 279, 1375–1385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dantzer, R.; O’Connor, J.C.; Lawson, M.A.; Kelley, K.W. Inflammation-associated depression: From serotonin to kynurenine. Psychoneuroendocrinology 2011, 36, 426–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haroon, E.; Raison, C.L.; Miller, A.H. Psychoneuroimmunology meets neuropsychopharmacology: Translational implications of the impact of inflammation on behavior. Neuropsychopharmacology 2012, 37, 137–162. [Google Scholar] [CrossRef]
- Widner, B.; Laich, A.; Sperner-Unterweger, B.; Ledochowski, M.; Fuchs, D. Neopterin production, tryptophan degradation, and mental depression—What is the link? Brain Behav. Immun. 2002, 16, 590–595. [Google Scholar] [CrossRef]
- Sperner-Unterweger, B.; Kohl, C.; Fuchs, D. Immune changes and neurotransmitters: Possible interactions in depression? Prog. Neuro Psychopharmacol. Biol. Psychiatry 2014, 48, 268–276. [Google Scholar] [CrossRef]
- Werner-Felmayer, G.; Golderer, G.; Werner, E.R. Tetrahydrobiopterin biosynthesis, utilization and pharmacological effects. Curr. Drug Metab. 2002, 3, 159–173. [Google Scholar] [CrossRef] [Green Version]
- Neurauter, G.; Schrocksnadel, K.; Scholl-Burgi, S.; Sperner-Unterweger, B.; Schubert, C.; Ledochowski, M.; Fuchs, D. Chronic immune stimulation correlates with reduced phenylalanine turnover. Curr. Drug Metab. 2008, 9, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Sheehan, D.V.; Lecrubier, Y.; Sheehan, K.H.; Amorim, P.; Janavs, J.; Weiller, E.; Hergueta, T.; Baker, R.; Dunbar, G.C. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 1998, 59 (Suppl. 20), 22–33; quiz 34–57. [Google Scholar] [PubMed]
- Hagströmer, M.; Oja, P.; Sjöström, M. The International Physical Activity Questionnaire (IPAQ): A study of concurrent and construct validity. Public Health Nutr. 2006, 9, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Buysse, D.J.; Reynolds, C.F., 3rd; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Vella, E.J.; Friedman, B.H. Hostility and anger in: Cardiovascular reactivity and recovery to mental arithmetic stress. Int. J. Psychophysiol. 2009, 72, 253–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garde, A.H.; Laursen, B.; Jorgensen, A.H.; Jensen, B.R. Effects of mental and physical demands on heart rate variability during computer work. Eur. J. Appl. Physiol. 2002, 87, 456–461. [Google Scholar] [CrossRef]
- Beck, A.T.; Ward, C.H.; Mendelson, M.; Mock, J.; Erbaugh, J. An inventory for measuring depression. Arch. Gen. Psychiatry 1961, 4, 561–571. [Google Scholar] [CrossRef] [Green Version]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef]
- Schulz, P.; Schlotz, W. Trierer Inventar zur Erfassung von chronischem Stress (TICS): Skalenkonstruktion, teststatistische Überprüfung und Validierung der Skala Arbeitsüberlastung. [The Trier Inventory for the Assessment of Chronic Stress (TICS). Scale construction, statistical testing, and validation of the scale work overload.]. Diagnostica 1999, 45, 8–19. [Google Scholar] [CrossRef]
- Laux, L.; Glanzmann, P.; Schaffner, P.; Spielberger, C.D. Das State-Trait-Angstinventar (STAI), Manual; Beltz Test: Weinheim, Germany, 1981. [Google Scholar]
- Widner, B.; Werner, E.R.; Schennach, H.; Wachter, H.; Fuchs, D. Simultaneous measurement of serum tryptophan and kynurenine by HPLC. Clin. Chem. 1997, 43, 2424–2426. [Google Scholar] [CrossRef] [Green Version]
- Capuron, L.; Schroecksnadel, S.; Feart, C.; Aubert, A.; Higueret, D.; Barberger-Gateau, P.; Laye, S.; Fuchs, D. Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: Role in neuropsychiatric symptoms. Biol. Psychiatry 2011, 70, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Giustarini, D.; Dalle-Donne, I.; Colombo, R.; Milzani, A.; Rossi, R. Adaptation of the Griess reaction for detection of nitrite in human plasma. Free Radic. Res. 2004, 38, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Arnhard, K.; Pitterl, F.; Sperner-Unterweger, B.; Fuchs, D.; Koal, T.; Oberacher, H. A validated liquid chromatography-high resolution-tandem mass spectrometry method for the simultaneous quantitation of tryptophan, kynurenine, kynurenic acid, and quinolinic acid in human plasma. Electrophoresis 2018, 39, 1171–1180. [Google Scholar] [CrossRef] [PubMed]
- Dhabhar, F.S. Effects of stress on immune function: The good, the bad, and the beautiful. Immunol. Res. 2014, 58, 193–210. [Google Scholar] [CrossRef]
- Dhabhar, F.S. The short-term stress response—Mother nature’s mechanism for enhancing protection and performance under conditions of threat, challenge, and opportunity. Front. Neuroendocrinol. 2018, 49, 175–192. [Google Scholar] [CrossRef]
- Hufner, K.; Oberguggenberger, A.; Kohl, C.; Geisler, S.; Gamper, E.; Meraner, V.; Egeter, J.; Hubalek, M.; Beer, B.; Fuchs, D.; et al. Levels in neurotransmitter precursor amino acids correlate with mental health in patients with breast cancer. Psychoneuroendocrinology 2015, 60, 28–38. [Google Scholar] [CrossRef]
- Levy, H.L.; Milanowski, A.; Chakrapani, A.; Cleary, M.; Lee, P.; Trefz, F.K.; Whitley, C.B.; Feillet, F.; Feigenbaum, A.S.; Bebchuk, J.D.; et al. Efficacy of sapropterin dihydrochloride (tetrahydrobiopterin, 6R-BH4) for reduction of phenylalanine concentration in patients with phenylketonuria: A phase III randomised placebo-controlled study. Lancet 2007, 370, 504–510. [Google Scholar] [CrossRef]
- Yılmaz, N.; Herken, H.; Cicek, H.K.; Celik, A.; Yürekli, M.; Akyol, Ö. Increased Levels of Nitric Oxide, Cortisol and Adrenomedullin in Patients with Chronic Schizophrenia. Med. Princ. Pract. 2007, 16, 137–141. [Google Scholar] [CrossRef]
- Simon, G.; Peter, M.; Kathrin, B.; Harald, S.; Dietmar, F.; Johanna, M.G. Serum tryptophan, kynurenine, phenylalanine, tyrosine and neopterin concentrations in 100 healthy blood donors. Pteridines 2015, 26, 31–36. [Google Scholar] [CrossRef]
- Glaser, R.; Kiecolt-Glaser, J.K. Stress-induced immune dysfunction: Implications for health. Nat. Rev. Immunol. 2005, 5, 243–251. [Google Scholar] [CrossRef]
- Curtius, H.C.; Niederwieser, A.; Levine, R.A.; Lovenberg, W.; Woggon, B.; Angst, J. Successful treatment of depression with tetrahydrobiopterin. Lancet 1983, 1, 657–658. [Google Scholar] [CrossRef]
- Fleischhacker, W.W.; Meise, U.; Schubert, H. Re-evaluation of antidepressant effect of tetrahydrobiopterin. Lancet 1985, 2, 387. [Google Scholar] [CrossRef]
- Van Amsterdam, J.G.; Opperhuizen, A. Nitric oxide and biopterin in depression and stress. Psychiatry Res. 1999, 85, 33–38. [Google Scholar] [CrossRef]
- Badawy, A.A. Tryptophan: The key to boosting brain serotonin synthesis in depressive illness. J. Psychopharmacol. 2013, 27, 878–893. [Google Scholar] [CrossRef] [PubMed]
- Lopresti, A.L.; Maker, G.L.; Hood, S.D.; Drummond, P.D. A review of peripheral biomarkers in major depression: The potential of inflammatory and oxidative stress biomarkers. Prog. Neuro Psychopharmacol. Biol. Psychiatry 2014, 48, 102–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, T.W.; Stoy, N.; Darlington, L.G. An expanding range of targets for kynurenine metabolites of tryptophan. Trends Pharmacol. Sci. 2013, 34, 136–143. [Google Scholar] [CrossRef] [Green Version]
- Guillemin, G.J. Quinolinic acid, the inescapable neurotoxin. FEBS J. 2012, 279, 1356–1365. [Google Scholar] [CrossRef]


| Stress Parameter | Chronic Mental Stress | No Chronic Mental Stress | p-Value |
|---|---|---|---|
| PSS (range 0–56 points) | 18.4 (16.3–20.6) | 15.1 (13.4–16.8) | <0.001 * |
| BDI (range 0–63 points) | 3.2 (2.2–4.3) | 2.2 (1.5–2.9) | 0.020 * |
| Trait anxiety scale (STAI, range 20–80 points) | 47.1 (46.4–47.9) | 46.9 (46.2–47.7) | 0.811 |
| State anxiety scale (STAI, range 20–80 points) | 30.7 (31.2–36.1) | 30.2 (28.3–32.0) | <0.001 * |
| TICS chronic stress (range 0–48 points) | 12.7 (10.4–14.9) | 10.3 (8.6–12) | 0.004 * |
| Stress from “Stroop” test (5 Point Likert scale) | 3.3 (3.1–3.6) | 3.1 (2.8–3.3) | 0.042 * |
| Stress from arithmetics test (5 Point Likert scale) | 2.5 (2.3–2.8) | 2.6 (2.3–2.9) | 0.431 |
| Days to exam (days) | 11.8 (11–12.7) | 100.2 (93.3–107.1) | <0.001 * |
| T0 | T1 | T2 | T3 | p-Value | |
|---|---|---|---|---|---|
| PHE/TYR (µmol/µmol) | 1.09 (1.05–1.13) | 1.10 (1.05–1.14) | 1.11 (1.07–1.15) | 1.13 (1.09–1.17) | p = 0.023 * |
| KYN/TRP (µmol/mmol) | 27.1 (25.7–28.6) | 27.7 (26.3–29.1) | 28.1 (26.7–29.5) | 28.9 (27.5–30.3) | p = 0.003 *,2,3 |
| QA (ng/ml) | 58.1 (49.4–66.8) | 56.8 (48.1–66.8) | 54.6 (45.9–63.3) | 54.9 (46.2–63.5) | p = 0.530 2 |
| KA (ng/ml) | 21.1 (19.4–22.8) | 20.4 (18.7–22) | 19.5 (17.8–21.2) | 18.6 (16.9–20.3) | p = 0.002 * |
| Neopterin (nmol/L) | 5.33 (5.01–5.65) | 5.29 (4.97–5.61) | 5.41 (5.09–5.73) | 5.29 (4.97–5.60) | p = 0.243 1,3 |
| Nitrite (µmol/L) | 23.8 (19.2–28.4) | 24.8 (20.2–29.3) | 24.3 (19.7–28.9) | 24.3 (19.8–28.3) | p = 0.269 |
| Chronic Mental Stress | No Chronic Mental Stress | p-Value | |
|---|---|---|---|
| PHE/TYR (µmol/µmol) | 1.06 (1.01–1.10) | 1.16 (1.12–1.20) | p < 0.001 * |
| KYN/TRP (µmol/mmol) | 28.5 (27.0–29.9) | 27.5 (26.0–29.0) | p = 0.171 2 |
| QA (ng/ml) | 48.5 (39.3–57.7) | 63.6 (54.5–72.8) | p = 0.002 *,2 |
| KA (ng/ml) | 20.5 (18.7–22.3) | 19.3 (17.5–21.1) | p = 0.148 |
| Neopterin (nmol/L) | 5.50 (5.16–5.83) | 5.16 (4.83–5.50) | p = 0.015 *,1,3 |
| Nitrite (µmol/L) | 23.0 (18.2–27.8) | 25.6 (20.8–30.4) | p = 0.017 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hüfner, K.; Galffy, M.; Egeter, J.; Giesinger, J.M.; Arnhard, K.; Oberacher, H.; Gostner, J.M.; Fuchs, D.; Sperner-Unterweger, B. Acute and Chronic Mental Stress both Influence Levels of Neurotransmitter Precursor Amino Acids and Derived Biogenic Amines. Brain Sci. 2020, 10, 322. https://doi.org/10.3390/brainsci10060322
Hüfner K, Galffy M, Egeter J, Giesinger JM, Arnhard K, Oberacher H, Gostner JM, Fuchs D, Sperner-Unterweger B. Acute and Chronic Mental Stress both Influence Levels of Neurotransmitter Precursor Amino Acids and Derived Biogenic Amines. Brain Sciences. 2020; 10(6):322. https://doi.org/10.3390/brainsci10060322
Chicago/Turabian StyleHüfner, Katharina, Matyas Galffy, Jonas Egeter, Johannes M. Giesinger, Kathrin Arnhard, Herbert Oberacher, Johanna M. Gostner, Dietmar Fuchs, and Barbara Sperner-Unterweger. 2020. "Acute and Chronic Mental Stress both Influence Levels of Neurotransmitter Precursor Amino Acids and Derived Biogenic Amines" Brain Sciences 10, no. 6: 322. https://doi.org/10.3390/brainsci10060322
APA StyleHüfner, K., Galffy, M., Egeter, J., Giesinger, J. M., Arnhard, K., Oberacher, H., Gostner, J. M., Fuchs, D., & Sperner-Unterweger, B. (2020). Acute and Chronic Mental Stress both Influence Levels of Neurotransmitter Precursor Amino Acids and Derived Biogenic Amines. Brain Sciences, 10(6), 322. https://doi.org/10.3390/brainsci10060322