Activation of the Supplementary Motor Areas Enhances Spinal Reciprocal Inhibition in Healthy Individuals
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
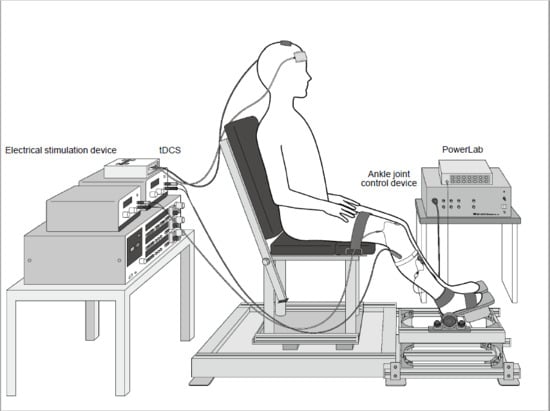
2.2. Measurement of Limb Position
2.3. Electromyography (EMG)
2.4. tDCS
2.5. Electrical Stimulation
2.6. Reciprocal Inhibition
2.7. Experimental Protocol
2.8. Statistical Analyses
3. Results
4. Discussion
Clinical Application
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hayashi, A.; Kagamihara, Y.; Nakajima, Y.; Narabayashi, H.; Okuma, Y.; Tanaka, R. Disorder in reciprocal innervation upon initiation of voluntary movement in patients with Parkinson’s disease. Exp. Brain Res. 1988, 70, 437–440. [Google Scholar] [CrossRef] [PubMed]
- Kagamihara, Y.; Ohi, K.; Tanaka, R. Disorder in reciprocal inhibition upon initiation of voluntary ankle dorsiflexion movement in cerebellar motor disorders. In Role of the Cerebellum and Basal Ganglia in Voluntary Movement; Elsevier Science: Amsterdam, The Netherlands, 1993; pp. 33–40. [Google Scholar]
- Kagamihara, Y.; Tanaka, R. Disorder of the reciprocal Ia inhibitory pathway in spasticity. In Recent Advances in Clinical Neurophysiology; Elsevier Science: Amsterdam, The Netherlands, 1996; pp. 859–862. [Google Scholar]
- Calancie, B.; Broton, J.G.; Klose, K.J.; Traad, M.; Difini, J.; Ayyar, D.R. Evidence that alterations in presynaptic inhibition contribute to segmental hypo- and hyperexcitability after spinal cord injury in man. Electroencephalogr. Clin. Neurophysiol. 1993, 89, 177–186. [Google Scholar] [CrossRef]
- Nagai, K.; Yamada, M.; Uemura, K.; Yamada, Y.; Ichihashi, N.; Tsuboyama, T. Differences in muscle coactivation during postural control between healthy older and young adults. Arch. Gerontol. Geriatr. 2011, 53, 338–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hortobagyi, T.; Solnik, S.; Gruber, A.; Rider, P.; Steinweg, K.; Helseth, J.; DeVita, P. Interaction between age and gait velocity in the amplitude and timing of antagonist muscle coactivation. Gait Posture 2009, 29, 558–564. [Google Scholar] [CrossRef] [PubMed]
- Hortobagyi, T.; Devita, P. Mechanisms responsible for the age-associated increase in coactivation of antagonist muscles. Exerc. Sport Sci. Rev. 2006, 34, 29–35. [Google Scholar] [CrossRef]
- Morita, H.; Shindo, M.; Yanagawa, S.; Yoshida, T.; Momoi, H.; Yanagisawa, N. Progressive decrease in heteronymous monosynaptic Ia facilitation with human ageing. Exp. Brain Res. 1995, 104, 167–170. [Google Scholar] [CrossRef]
- Morita, H.; Shindo, M.; Ikeda, S.; Yanagisawa, N. Decrease in presynaptic inhibition on heteronymous monosynaptic Ia terminals in patients with Parkinson’s disease. Movement disorders Off. J. Mov. Dis. Soc. 2000, 15, 830–834. [Google Scholar] [CrossRef]
- Baudry, S.; Maerz, A.H.; Enoka, R.M. Presynaptic modulation of Ia afferents in young and old adults when performing force and position control. J. Neurophysiol. 2010, 103, 623–631. [Google Scholar] [CrossRef] [Green Version]
- Bhagchandani, N.; Schindler-Ivens, S. Reciprocal inhibition post-stroke is related to reflex excitability and movement ability. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2012, 123, 2239–2246. [Google Scholar] [CrossRef] [Green Version]
- Fung, J.; Barbeau, H. A dynamic EMG profile index to quantify muscular activation disorder in spastic paretic gait. Electroencephalogr. Clin. Neurophysiol. 1989, 73, 233–244. [Google Scholar] [CrossRef]
- Blackwell, J.R.; Cole, K.J. Wrist kinematics differ in expert and novice tennis players performing the backhand stroke: Implications for tennis elbow. J. Biomech. 1994, 27, 509–516. [Google Scholar] [CrossRef]
- Mizuno, Y.; Tanaka, R.; Yanagisawa, N. Reciprocal group I inhibition on triceps surae motoneurons in man. J. Neurophysiol. 1971, 34, 1010–1017. [Google Scholar] [CrossRef] [PubMed]
- Okuma, Y.; Lee, R.G. Reciprocal inhibition in hemiplegia: Correlation with clinical features and recovery. Can. J. Neurol. Sci. J. Can. Sci. Neurol. 1996, 23, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, R.; Edama, M.; Kojima, S.; Miyaguchi, S.; Onishi, H. Effects of repetitive passive movement on ankle joint on spinal reciprocal inhibition. Exp. Brain Res. 2019, 237, 3409–3417. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, R.; Edama, M.; Kojima, S.; Miyaguchi, S.; Onishi, H. Enhancement of spinal reciprocal inhibition depends on the movement speed and range of repetitive passive movement. Eur. J. Neurosci. 2020. [Google Scholar] [CrossRef]
- Perez, M.A.; Field-Fote, E.C.; Floeter, M.K. Patterned sensory stimulation induces plasticity in reciprocal ia inhibition in humans. J. Neurosci. Off. J. Soc. Neurosci. 2003, 23, 2014–2018. [Google Scholar] [CrossRef] [Green Version]
- Roche, N.; Lackmy, A.; Achache, V.; Bussel, B.; Katz, R. Impact of transcranial direct current stimulation on spinal network excitability in humans. J. Physiol. 2009, 587, 5653–5664. [Google Scholar] [CrossRef]
- Fujiwara, T.; Tsuji, T.; Honaga, K.; Hase, K.; Ushiba, J.; Liu, M. Transcranial direct current stimulation modulates the spinal plasticity induced with patterned electrical stimulation. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2011, 122, 1834–1837. [Google Scholar] [CrossRef]
- Roche, N.; Lackmy, A.; Achache, V.; Bussel, B.; Katz, R. Effects of anodal transcranial direct current stimulation over the leg motor area on lumbar spinal network excitability in healthy subjects. J. Physiol. 2011, 589, 2813–2826. [Google Scholar] [CrossRef]
- Jessop, T.; DePaola, A.; Casaletto, L.; Englard, C.; Knikou, M. Short-term plasticity of human spinal inhibitory circuits after isometric and isotonic ankle training. Eur. J. Appl. Physiol. 2013, 113, 273–284. [Google Scholar] [CrossRef]
- Kubota, S.; Hirano, M.; Morishita, T.; Uehara, K.; Funase, K. Patterned sensory nerve stimulation enhances the reactivity of spinal Ia inhibitory interneurons. Neuroreport 2015, 26, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Fujiwara, T.; Tsai, Y.A.; Tang, S.C.; Kawakami, M.; Mizuno, K.; Kodama, M.; Masakado, Y.; Liu, M. The effects of anodal transcranial direct current stimulation and patterned electrical stimulation on spinal inhibitory interneurons and motor function in patients with spinal cord injury. Exp. Brain Res. 2016, 234, 1469–1478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritzmann, R.; Krause, A.; Freyler, K.; Gollhofer, A. Acute whole-body vibration increases reciprocal inhibition. Hum. Mov. Sci. 2018, 60, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Fujiwara, T.; Lin, S.C.; Takahashi, Y.; Hatori, K.; Liu, M.; Huang, Y.Z. Priming with intermittent theta burst transcranial magnetic stimulation promotes spinal plasticity induced by peripheral patterned electrical stimulation. Front. Neurosci. 2018, 12, 508. [Google Scholar] [CrossRef]
- Takahashi, Y.; Fujiwara, T.; Yamaguchi, T.; Kawakami, M.; Mizuno, K.; Liu, M. The effects of patterned electrical stimulation combined with voluntary contraction on spinal reciprocal inhibition in healthy individuals. Neuroreport 2017, 28, 434–438. [Google Scholar] [CrossRef]
- Vollmann, H.; Conde, V.; Sewerin, S.; Taubert, M.; Sehm, B.; Witte, O.W.; Villringer, A.; Ragert, P. Anodal transcranial direct current stimulation (tDCS) over supplementary motor area (SMA) but not pre-SMA promotes short-term visuomotor learning. Brain Stimul. 2013, 6, 101–107. [Google Scholar] [CrossRef]
- Ullman, M.T. Is Broca’s area part of a basal ganglia thalamocortical circuit? Cortex J. Devoted Study Nerv. Syst. Behav. 2006, 42, 480–485. [Google Scholar] [CrossRef]
- Nguyen, V.T.; Breakspear, M.; Cunnington, R. Reciprocal interactions of the SMA and cingulate cortex sustain premovement activity for voluntary actions. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 16397–16407. [Google Scholar] [CrossRef] [Green Version]
- Nachev, P.; Kennard, C.; Husain, M. Functional role of the supplementary and pre-supplementary motor areas. Nat. Rev. Neurosci. 2008, 9, 856–869. [Google Scholar] [CrossRef]
- Lu, C.; Amundsen Huffmaster, S.L.; Tuite, P.J.; MacKinnon, C.D. The effects of anodal tDCS over the supplementary motor area on gait initiation in Parkinson’s disease with freezing of gait: A pilot study. J. Neurol. 2018, 265, 2023–2032. [Google Scholar] [CrossRef]
- Manji, A.; Amimoto, K.; Matsuda, T.; Wada, Y.; Inaba, A.; Ko, S. Effects of transcranial direct current stimulation over the supplementary motor area body weight-supported treadmill gait training in hemiparetic patients after stroke. Neurosci. Lett. 2018, 662, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Hupfeld, K.E.; Ketcham, C.J.; Schneider, H.D. Transcranial direct current stimulation (tDCS) to the supplementary motor area (SMA) influences performance on motor tasks. Exp. Brain Res. 2017, 235, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Keizer, K.; Kuypers, H.G. Distribution of corticospinal neurons with collaterals to the lower brain stem reticular formation in monkey (Macaca fascicularis). Exp. Brain Res. 1989, 74, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, K.; Drew, T. Organization of the projections from the pericruciate cortex to the pontomedullary brainstem of the cat: A study using the anterograde tracer Phaseolus vulgaris-leucoagglutinin. J. Comp. Neurol. 1997, 389, 617–641. [Google Scholar] [CrossRef]
- Matsuyama, K.; Takakusaki, K. Organizing principles of axonal projections of the long descending reticulospinal pathway and its target spinal lamina VIII commissural neurons: With special reference to the locomotor function. In Handbook on White Matter: Structure, Function and Changes; Nova Science Publishing: New York, NY, USA, 2009; pp. 335–356. [Google Scholar]
- Zwergal, A.; Linn, J.; Xiong, G.; Brandt, T.; Strupp, M.; Jahn, K. Aging of human supraspinal locomotor and postural control in fMRI. Neurobiol. Aging 2012, 33, 1073–1084. [Google Scholar] [CrossRef] [PubMed]
- Takakusaki, K. Neurophysiology of gait: From the spinal cord to the frontal lobe. Mov. Disord. Off. J. Mov. Disord. Soc. 2013, 28, 1483–1491. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. Off. J. Int. Soc. Electrophysiol. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Crone, C.; Hultborn, H.; Jespersen, B.; Nielsen, J. Reciprocal Ia inhibition between ankle flexors and extensors in man. J. Physiol. 1987, 389, 163–185. [Google Scholar] [CrossRef]
- Takada, Y.; Miyahara, T.; Tanaka, T.; Ohyama, T.; Nakamura, Y. Modulation of H reflex of pretibial muscles and reciprocal Ia inhibition of soleus muscle during voluntary teeth clenching in humans. J. Neurophysiol. 2000, 83, 2063–2070. [Google Scholar] [CrossRef] [Green Version]
- Hikosaka, O.; Sakai, K.; Miyauchi, S.; Takino, R.; Sasaki, Y.; Putz, B. Activation of human presupplementary motor area in learning of sequential procedures: A functional MRI study. J. Neurophysiol. 1996, 76, 617–621. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.-M.; Chang, K.-H.; Roh, J.-K. Subregions within the supplementary motor area activated at different stages of movement preparation and execution. NeuroImage 1999, 9, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Cona, G.; Marino, G.; Semenza, C. TMS of supplementary motor area (SMA) facilitates mental rotation performance: Evidence for sequence processing in SMA. NeuroImage 2017, 146, 770–777. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Wright, D.L. Transcranial direct current stimulation of supplementary motor region impacts the effectiveness of interleaved and repetitive practice schedules for retention of motor skills. Neuroscience 2020, 435, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, R.; Edama, M.; Kojima, S.; Ito, W.; Nakamura, E.; Kikumoto, T.; Onishi, H. Spinal reciprocal inhibition in the co-contraction of the lower leg depends on muscle activity ratio. Exp. Brain Res. 2019. [Google Scholar] [CrossRef]
- Hirabayashi, R.; Edama, M.; Kojima, S.; Nakamura, M.; Ito, W.; Nakamura, E.; Kikumoto, T.; Onishi, H. Effects of Reciprocal Ia Inhibition on Contraction Intensity of Co-contraction. Front. Hum. Neurosci. 2018, 12, 527. [Google Scholar] [CrossRef] [Green Version]
- Crone, C.; Hultborn, H.; Mazieres, L.; Morin, C.; Nielsen, J.; Pierrot-Deseilligny, E. Sensitivity of monosynaptic test reflexes to facilitation and inhibition as a function of the test reflex size: A study in man and the cat. Exp. Brain Res. 1990, 81, 35–45. [Google Scholar] [CrossRef]
- Nielsen, J.; Kagamihara, Y. The regulation of disynaptic reciprocal Ia inhibition during co-contraction of antagonistic muscles in man. J. Physiol. 1992, 456, 373–391. [Google Scholar] [CrossRef]
- Floeter, M.K.; Kohn, A.F. H-reflexes of different sizes exhibit differential sensitivity to low frequency depression. Electroencephalogr. Clin. Neurophysiol. 1997, 105, 470–475. [Google Scholar] [CrossRef]




| a. Background electromyography (EMG) (Sol) | ||||||||
| Pre | Int 5 | Int 10 | Post 0 | Post 5 | Post 10 | Post 15 | Post 20 | |
| Sham-tDCS | 2.9 ± 0.1 | 2.9 ± 0.1 | 2.9 ± 0.1 | 2.9 ± 0.1 | 2.9 ± 0.1 | 3.0 ± 0.1 | 2.9 ± 0.1 | 2.8 ± 0.1 |
| Anodal-tDCS | 3.1 ± 0.3 | 3.1 ± 0.2 | 3.4 ± 0.3 | 3.4 ± 0.3 | 3.2 ± 0.2 | 3.1 ± 0.3 | 3.1 ± 0.2 | 3.3 ± 0.3 |
| b. Mmax amplitude values (Sol) | ||||||||
| Sham-tDCS | Anodal-tDCS | |||||||
| 10.96 ± 0.81 | 11.58 ± 0.92 | |||||||
| c. M-wave amplitude values (TA) | ||||||||
| Pre | Int 5 | Int 10 | Post 0 | Post 5 | Post 10 | Post 15 | Post 20 | |
| Sham-tDCS | 89.0 ± 1.6 | 90.1 ± 1.9 | 88.6 ± 1.3 | 87.7 ± 2.0 | 88.6 ± 1.3 | 91.0 ± 1.8 | 87.5 ± 1.8 | 87.9 ± 1.9 |
| Anodal-tDCS | 88.1 ± 3.0 | 88.4 ± 3.6 | 89.4 ± 3.1 | 90.2 ± 2.1 | 88.0 ± 3.0 | 88.2 ± 3.5 | 85.7 ± 5.2 | 86.9 ± 3.6 |
| Pre | Int 5 | Int 10 | Post 0 | Post 5 | Post 10 | Post 15 | Post 20 | ||
|---|---|---|---|---|---|---|---|---|---|
| Sham-tDCS | Single | 20.0 ± 0.3 | 19.7 ± 0.3 | 20.4 ± 0.3 | 20.3 ± 0.2 | 20.2 ± 0.3 | 20.7 ± 0.3 | 20.5 ± 0.3 | 20.4 ± 0.4 |
| CTI 2 ms | 17.1 ± 0.4 ‡ | 17.0 ± 0.4 ‡ | 17.8 ± 0.4 ‡ | 17.6 ± 0.4 ‡ | 17.5 ± 0.4 ‡ | 17.6 ± 0.4 ‡ | 17.5 ± 0.4 ‡ | 17.4 ± 0.5 ‡ | |
| CTI 20 ms | 15.2 ± 0.4 ‡ | 14.8 ± 0.4 ‡ | 15.3 ± 0.4 ‡ | 15.4 ± 0.4 ‡ | 15.3 ± 0.4 ‡ | 15.9 ± 0.4 ‡ | 15.7 ± 0.4 ‡ | 15.3 ± 0.4 ‡ | |
| Anodal-tDCS | Single | 20.4 ± 0.4 | 20.6 ± 0.4 | 20.1 ± 0.3 | 20.1 ± 0.3 | 20.2 ± 0.4 | 20.5 ± 0.3 | 20.4 ± 0.3 | 19.6 ± 0.3 |
| CTI 2 ms | 17.1 ± 0.4 ‡ | 16.2 ± 0.4 ‡ | 16.0 ± 0.5 ‡ | 16.0 ± 0.6 ‡ | 16.6 ± 0.5 ‡ | 17.0 ± 0.5 ‡ | 17.1 ± 0.4 ‡ | 16.7 ± 0.5 ‡ | |
| CTI 20 ms | 15.4 ± 0.4 ‡ | 14.5 ± 0.5 ‡ | 13.8 ± 0.4 ‡ | 14.4 ± 0.6 ‡ | 14.3 ± 0.5 ‡ | 14.8 ± 0.5 ‡ | 15.2 ± 0.5 ‡ | 14.6 ± 0.5 ‡ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirabayashi, R.; Kojima, S.; Edama, M.; Onishi, H. Activation of the Supplementary Motor Areas Enhances Spinal Reciprocal Inhibition in Healthy Individuals. Brain Sci. 2020, 10, 587. https://doi.org/10.3390/brainsci10090587
Hirabayashi R, Kojima S, Edama M, Onishi H. Activation of the Supplementary Motor Areas Enhances Spinal Reciprocal Inhibition in Healthy Individuals. Brain Sciences. 2020; 10(9):587. https://doi.org/10.3390/brainsci10090587
Chicago/Turabian StyleHirabayashi, Ryo, Sho Kojima, Mutsuaki Edama, and Hideaki Onishi. 2020. "Activation of the Supplementary Motor Areas Enhances Spinal Reciprocal Inhibition in Healthy Individuals" Brain Sciences 10, no. 9: 587. https://doi.org/10.3390/brainsci10090587
APA StyleHirabayashi, R., Kojima, S., Edama, M., & Onishi, H. (2020). Activation of the Supplementary Motor Areas Enhances Spinal Reciprocal Inhibition in Healthy Individuals. Brain Sciences, 10(9), 587. https://doi.org/10.3390/brainsci10090587