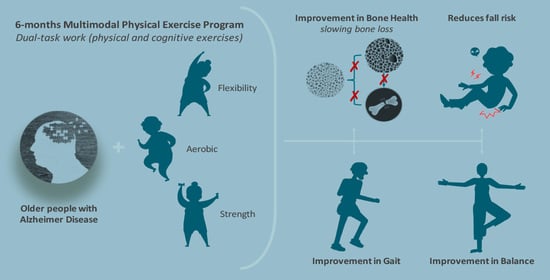
Effects of 6-Month Multimodal Physical Exercise Program on Bone Mineral Density, Fall Risk, Balance, and Gait in Patients with Alzheimer’s Disease: A Controlled Clinical Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Sample Size Calculation
2.3. Participants and Recruitment
2.4. Masking and Allocation
2.5. Outcome Measures
2.5.1. Balance and Gait Assessment
Performance-Oriented Mobility Assessment (POMA)
Timed Up and Go Test (TUG)
One-Leg Balance Test (OLB)
Functional Reach Test (FR)
2.5.2. Bone Health Status
Calcaneal Quantitative Ultrasound (QUS)
2.5.3. Falls
2.6. Interventions
Multimodal Physical Exercise Program (MPEP)
2.7. Statistical Analysis
3. Results
3.1. Descriptive Analysis
3.2. Primary and Secondary Outcomes
3.3. Falls
3.4. Correlations between Variables
4. Discussion
4.1. Intervention
4.2. Effects on Falls
4.3. Effects on Gait and Balance
4.4. Effects on Bone Health Status
4.5. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olmo, J.G. Epidemiología de la enfermedad de Alzheimer y otras demencias. Rev. Neurol. 2018, 66, 377–386. [Google Scholar] [CrossRef]
- Santana-Sosa, E.; Barriopedro, M.I.; López-Mojares, L.M.; Pérez, M.; Lucia, A. Exercise Training Is Beneficial for Alzheimer’s Patients. Int. J. Sports Med. 2008, 29, 845–850. [Google Scholar]
- Prince, M.; Wimo, A.; Guerchet, M.; Ali, G.C.; Wu, Y.T.; Prima, M. World Alzheimer Report 2015. The Global Impact of Dementia an Analysis of Prevalence, Incidence, Cost and Trends. Available online: https://www.alz.co.uk/research/WorldAlzheimerReport2015.pdf (accessed on 2 March 2020).
- Wimo, A.; Guerchet, M.; Ali, G.-C.; Wu, Y.-T.; Prina, A.M.; Winblad, B.; Jönsson, L.; Liu, Z.; Prince, M. The worldwide costs of dementia 2015 and comparisons with 2010. Alzheimer’s Dement. 2017, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Mañas, L.; Féart, C.; Mann, G.; Viña, J.; Chatterji, S.; Chodzko-Zajko, W.; Harmand, M.G.-C.; Bergman, H.; Carcaillon, L.; Nicholson, C.; et al. Searching for an Operational Definition of Frailty: A Delphi Method Based Consensus Statement. The Frailty Operative Definition-Consensus Conference Project. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2013, 68, 62–67. [Google Scholar] [CrossRef] [Green Version]
- Lang, P.-O.; Michel, J.P.; Zekry, D. Frailty Syndrome: A Transitional State in a Dynamic Process. Gerontology 2009, 55, 539–549. [Google Scholar] [CrossRef]
- Fried, L.P.; Ferrucci, L.; Darer, J.; Williamson, J.D.; Anderson, G. Untangling the Concepts of Disability, Frailty, and Comorbidity: Implications for Improved Targeting and Care. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2004, 59, M255–M263. [Google Scholar] [CrossRef] [Green Version]
- Apóstolo, J.; Cooke, R.; Bobrowicz-Campos, E.; Santana, S.; Marcucci, M.; Cano, A.; Vollenbroek-Hutten, M.; Germini, F.; Holland, C. Predicting risk and outcomes for frail older adults: An umbrella review of frailty screening tools. JBI Database Syst. Rev. Implement Rep. 2017, 15, 1154–1208. [Google Scholar] [CrossRef] [Green Version]
- Robertson, D.A.; Savva, G.M.; Coen, R.F.; Kenny, R.-A. Cognitive Function in the Prefrailty and Frailty Syndrome. J. Am. Geriatr. Soc. 2014, 62, 2118–2124. [Google Scholar] [CrossRef]
- Casas-Herrero, Á.; Anton-Rodrigo, I.; Zambom-Ferraresi, F.; De Asteasu, M.L.-S.; Velilla, N.M.; Elexpuru-Estomba, J.; Marin-Epelde, I.; Ramon-Espinoza, F.; Petidier-Torregrosa, R.; Sanchez-Sanchez, J.L. Effect of a multicomponent exercise programme (VIVIFRAIL) on functional capacity in frail community elders with cognitive decline: Study protocol for a randomized multicentre control trial. Trials 2019, 20, 362. [Google Scholar] [CrossRef]
- Castrillo, A.; Olmos, L.M.G.; Rodríguez, F.; Duarte, J. Gait Disorder in a Cohort of Patients with Mild and Moderate Alzheimer’s Disease. Am. J. Alzheimer’s Dis. Other Dement. 2015, 31, 257–262. [Google Scholar] [CrossRef]
- Muñoz, V.M.; van Kan, G.A.; Cantet, C.; Cortes, F.; Ousset, P.J.; Rolland, Y.; Vellas, B. Gait and balance impairments in Alzheimer disease patients. Alzheimer Dis. Assoc. Disord. 2010, 24, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Yoon, B.; Choi, S.H.; Jeong, J.H.; Park, K.W.; Kim, E.-J.; Hwang, J.; Jang, J.-W.; Kim, H.J.; Hong, J.Y.; Lee, J.-M.; et al. Balance and Mobility Performance Along the Alzheimer’s Disease Spectrum. J. Alzheimer’s Dis. 2020, 73, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Allan, L.M.; Ballard, C.G.; Burn, D.J.; Kenny, R.A. Prevalence and severity of gait disorders in Alzheimer’s and non-Alzheimer’s dementias. J. Am. Geriatr. Soc. 2005, 53, 1681–1687. [Google Scholar] [CrossRef] [PubMed]
- Padala, K.P.; Padala, P.R.; Lensing, S.Y.; Dennis, R.A.; Bopp, M.M.; Roberson, P.K.; Sullivan, D.H. Home-Based Exercise Program Improves Balance and Fear of Falling in Community-Dwelling Older Adults with Mild Alzheimer’s Disease: A Pilot Study. J. Alzheimer’s Dis. 2017, 59, 565–574. [Google Scholar] [CrossRef]
- Park, H.; Na, H.R.; Hiroyuki, S.; Kim, W.K.; Jung, M.K. Combined Intervention of Physical Activity, Aerobic Exercise, and Cognitive Exercise Intervention to Prevent Cognitive Decline for Patients with Mild Cognitive Impairment: A Randomized Controlled Clinical Study. J. Clin. Med. 2019, 8, 940. [Google Scholar] [CrossRef] [Green Version]
- Martin, K.; Thomson, R.; Blizzard, L.; Wood, A.; Garry, M.; Srikanth, V. Visuospatial ability and memory are associated with falls risk in older people: A population-based study. Dement. Geriatr. Cogn. Disord. 2009, 27, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Tangen, G.G.; Engedal, K.; Bergland, A.; Moger, T.A.; Mengshoel, A.M. Relationships between Balance and Cognition in Patients With Subjective Cognitive Impairment, Mild Cognitive Impairment, and Alzheimer Disease. Phys. Ther. 2014, 94, 1123–1134. [Google Scholar] [CrossRef] [Green Version]
- Cedervall, Y.; Halvorsen, K.; Aberg, A.C. A longitudinal study of gait function and characteristics of gait disturbance in individuals with Alzheimer’s disease. Gait Posture 2014, 39, 1022–1027. [Google Scholar] [PubMed]
- Wolfson, L. Gait and Balance Dysfunction: A Model of the Interaction of Age and Disease. Neuroscientist 2001, 7, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Shaw, F.E.; Bond, J.; Richardson, D.A.; Dawson, P.; Steen, I.N.; McKeith, I.G.; Kenny, R.A. Multifactorial intervention after a fall in older people with cognitive impairment and dementia presenting to the accident and emergency department: Randomised controlled trial. BMJ 2003, 326, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isik, A.T.; Soysal, P.; Usarel, C. Effects of Acetylcholinesterase Inhibitors on Balance and Gait Functions and Orthostatic Hypotension in Elderly Patients with Alzheimer Disease. Am. J. Alzheimer’s Dis. Other Dement. 2016, 31, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Amouzougan, A.; Lafaie, L.; Marotte, H.; Dẻnariẻ, D.; Collet, P.; Pallot-Prades, B.; Thomas, T. High prevalence of dementia in women with osteoporosis. Jt. Bone Spine 2017, 84, 611–614. [Google Scholar] [CrossRef]
- Bonafede, M.; Shi, N.; Barron, R.; Li, X.; Crittenden, D.; Chandler, D. Predicting imminent risk for fracture in patients aged 50 or older with osteoporosis using US claims data. Arch. Osteoporos. 2016, 11, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailly, S.; Haesebaert, J.; Decullier, E.; Dargentmolina, P.; Annweiler, C.; Beauchet, O.; Schott, A.-M.; Rabilloud, M. Mortality and profiles of community-dwelling fallers. Results from the EPIDOS cohort. Maturitas 2014, 79, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Pu, Z.; Tang, X.; Fei, Y.; Hou, Q.; Lin, Y.; Zha, X. Bone metabolic biomarkers and bone mineral density in male patients with early-stage Alzheimer’s disease. Eur. Geriatr. Med. 2020, 11, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Laudisio, A.; Fontana, D.O.; Rivera, C.; Ruggiero, C.; Bandinelli, S.; Gemma, A.; Ferrucci, L.; Incalzi, R.A. Bone Mineral Density and Cognitive Decline in Elderly Women: Results from the InCHIANTI Study. Calcif. Tissue Int. 2016, 98, 479–488. [Google Scholar] [CrossRef]
- Looker, A.C.; Borrud, L.G.; Dawson-Hughes, B.; Shepherd, J.A.; Wright, N.C. Osteoporosis or low bone mass at the femur neck or lumbar spine in older adults: United States, 2005–2008. NCHS Data Brief 2012, 93, 1–8. [Google Scholar]
- Frame, G.; Bretland, K.A.; Dengler-Crish, C.M. Mechanistic complexities of bone loss in Alzheimer’s disease: A review. Connect. Tissue Res. 2020, 61, 4–18. [Google Scholar] [CrossRef]
- Chang, K.-H.; Chung, C.-J.; Lin, C.-L.; Sung, F.-C.; Wu, T.-N.; Kao, C. Increased risk of dementia in patients with osteoporosis: A population-based retrospective cohort analysis. AGE 2014, 36, 967–975. [Google Scholar] [CrossRef] [Green Version]
- Manckoundia, P.; Taroux, M.; Kubicki, A.; Mourey, F. Impact of ambulatory physiotherapy on motor abilities of elderly subjects with Alzheimer’s disease. Geriatr. Gerontol. Int. 2014, 14, 167–175. [Google Scholar]
- Rolland, Y.; Pillard, F.; Klapouszczak, A.; Reynish, E.; Thomas, D.; Andrieu, S.; Rivière, D.; Vellas, B. Exercise program for nursing home residents with Alzheimer’s disease: A 1-year randomized, controlled trial. J. Am. Geriatr. Soc. 2007, 55, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Rolland, Y.; van Kan, G.A.; Nourhashemi, F.; Andrieu, S.; Cantet, C.; Guyonnet-Gillette, S.; Vellas, B. An abnormal “one-leg balance” test predicts cognitive decline during Alzheimer’s disease. J. Alzheimer’s Dis. 2009, 16, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.; Thiel, C.; Schulz, R.-J.; Grüneberg, C. Reliability of mobility measures in older medical patients with cognitive impairment. BMC Geriatr. 2019, 19, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, P.W.; Weiner, D.K.; Chandler, J.; Studenski, S. Functional Reach: A New Clinical Measure of Balance. J. Gerontol. 1990, 45, M192–M197. [Google Scholar] [CrossRef] [PubMed]
- McGurran, H.; Glenn, J.M.; Madero, E.N.; Bott, N. Prevention and Treatment of Alzheimer’s Disease: Biological Mechanisms of Exercise. J. Alzheimer’s Dis. 2019, 69, 311–338. [Google Scholar] [CrossRef]
- Zhang, T.; Liu, N.; Cao, H.; Wei, W.; Ma, L.; Li, H. Different Doses of Pharmacological Treatments for Mild to Moderate Alzheimer’s Disease: A Bayesian Network Meta-Analysis. Front. Pharmacol. 2020, 11, 778. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, N.; Chau, S.A.; Kircanski, I.; Lanctôt, K.L. Current and emerging drug treatment options for Alzheimer’s disease: A systematic review. Drugs 2011, 71, 2031–2065. [Google Scholar] [CrossRef]
- National Institute on Aging. Alzheimer’s Disease Medications Fact Sheet. NIH Publication No. 18-AG-3431. 2018. Available online: https://order.nia.nih.gov/sites/default/files/2018-03/alzheimers-disease-medications-fact-sheet.pdf (accessed on 18 March 2020).
- Haeger, A.; Costa, A.S.; Schulz, J.B.; Reetz, K. Cerebral changes improved by physical activity during cognitive decline: A systematic review on MRI studies. NeuroImage Clin. 2019, 23, 101933. [Google Scholar] [CrossRef]
- Nelson, M.E.; Rejeski, W.J.; Blair, S.N.; Duncan, P.W.; Judge, J.O. Physical activity and public health in older adults: Recommendation from the American College of Sports Medicine and the American Heart Association. Circulation 2007, 116, 1094–1105. [Google Scholar] [CrossRef] [Green Version]
- Jia, R.-X.; Liang, J.-H.; Xu, Y.; Wang, Y.-Q. Effects of physical activity and exercise on the cognitive function of patients with Alzheimer disease: A meta-analysis. BMC Geriatr. 2019, 19, 181. [Google Scholar] [CrossRef]
- Tobeiha, M.; Moghadasian, M.H.; Amin, N.; Jafarnejad, S. RANKL/RANK/OPG Pathway: A Mechanism Involved in Exercise-Induced Bone Remodeling. BioMed Res. Int. 2020, 2020, 6910312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biazus-Sehn, L.F.; Schuch, F.B.; Firth, J.; Stigger, F.D.S. Effects of physical exercise on cognitive function of older adults with mild cognitive impairment: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2020, 89, 104048. [Google Scholar] [CrossRef]
- Thompson, P.D.; Arena, R.; Riebe, D.; Pescatello, L.S.; American College of Sports Medicine. ACSM’s new preparticipation health screening recommendations from ACSM’s guidelines for exercise testing and prescription, ninth edition. Curr. Sports Med. Rep. 2013, 12, 215–217. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Strategy on Diet, Physical Activity and Health. Physical Activity and Older Adults. Recommended Levels of Physical Activity for Adults Aged 65 and Above. 2010. Available online: https://https://www.who.int/dietphysicalactivity/global-PA-recs-2010.pdf (accessed on 11 August 2020).
- Cadore, E.L.; Rodríguez-Mañas, L.; Sinclair, A.; Izquierdo, M. Effects of Different Exercise Interventions on Risk of Falls, Gait Ability, and Balance in Physically Frail Older Adults: A Systematic Review. Rejuvenation Res. 2013, 16, 105–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnett, A.; Smith, B.; Lord, S.R.; Williams, M.; Baumand, A. Community-based group exercise improves balance and reduces falls in at-risk older people: A randomised controlled trial. Age Ageing 2003, 32, 407–414. [Google Scholar] [CrossRef] [Green Version]
- Villareal, D.T.; Smith, G.I.; Sinacore, D.R.; Shah, K.; Mittendorfer, B. Regular Multicomponent Exercise Increases Physical Fitness and Muscle Protein Anabolism in Frail, Obese, Older Adults. Obesity (Silver Spring) 2011, 19, 312–318. [Google Scholar] [CrossRef] [Green Version]
- Chodzko-Zajko, W.J.; Proctor, D.N.; Fiatarone Singh, M.A.; Minson, C.T.; Nigg, C.R.; Salem, G.J.; Skinner, J.S. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med. Sci. Sports Exerc. 2009, 41, 1510–1530. [Google Scholar] [CrossRef]
- Galan-Martin, M.A.; Montero-Cuadrado, F.; Lluch-Girbes, E.; Coca-López, M.C.; Mayo-Iscar, A.; Cuesta-Vargas, A. Pain Neuroscience Education and Physical Exercise therapy for Patients with Chronic Spinal Pain in Spanish Physiotherapy Primary Care: A Pragmatic Randomized Controlled Trial. J. Clin. Med. 2020, 9, 1201. [Google Scholar] [CrossRef]
- Gardner, T.; Refshauge, K.; McAuley, J.; Hübscher, M.; Goodall, S.; Smith, L. Combined education and patient-led goal setting intervention reduced chronic low back pain disability and intensity at 12 months: A randomised controlled trial. Br. J. Sports Med. 2019, 53, 1424–1431. [Google Scholar] [CrossRef]
- Fritz, N.E.; Cheek, F.M.; Nichols-Larsen, D.S. Motor-Cognitive Dual-Task Training in Persons with Neurologic Disorders: A Systematic Review. J. Neurol. Phys. Ther. 2015, 39, 142–153. [Google Scholar] [CrossRef] [Green Version]
- Ghai, S.; Ghai, I.; Effenberg, A.O. Effects of dual tasks and dual-task training on postural stability: A systematic review and meta-analysis. Clin. Interv. Aging 2017, 12, 557–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazarus, N.R.; Izquierdo, M.; Higginson, I.J.; Harridge, S.D.R. Exercise Deficiency Diseases of Ageing: The Primacy of Exercise and Muscle Strengthening as First-Line Therapeutic Agents to Combat Frailty. J. Am. Med. Dir. Assoc. 2018, 19, 741–743. [Google Scholar] [CrossRef] [PubMed]
- Liu-Ambrose, T.; Nagamatsu, L.S.; Graf, P.; Beattie, B.L.; Ashe, M.C.; Handy, T.C. Resistance training and executive functions: A 12-month randomized controlled trial. Arch. Intern. Med. 2010, 170, 170–178. [Google Scholar] [CrossRef] [Green Version]
- Cadore, E.L.; Moneo, A.B.B.; Mensat, M.M.; Muñoz, A.R.; Casas-Herrero, A.; Rodriguez-Mañas, L.; Izquierdo, M. Positive effects of resistance training in frail elderly patients with dementia after long-term physical restraint. AGE 2013, 36, 801–811. [Google Scholar] [CrossRef]
- Sherrington, C.; Tiedemann, A.; Fairhall, N.; Close, J.C.; Lord, S.R. Exercise to prevent falls in older adults: An updated meta-analysis and best practice recommendations. N. S. W. Public Health Bull. 2011, 22, 78–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burton, E.; Cavalheri, V.; Adams, R.; Browne, C.O.; Bovery-Spencer, P.; Fenton, A.M.; Campbell, B.W.; Hill, K.D. Effectiveness of exercise programs to reduce falls in older people with dementia living in the community: A systematic review and meta-analysis. Clin. Intervig. Aging 2015, 10, 421–434. [Google Scholar] [CrossRef] [Green Version]
- Gonzalo-Encabo, P.; McNeil, J.; Boyne, D.J.; Courneya, K.S.; Friedenreich, C.M. Dose-response effects of exercise on bone mineral density and content in post-menopausal women. Scand. J. Med. Sci. Sports 2019, 29, 1121–1129. [Google Scholar] [CrossRef]
- Russo, C.R. The effects of exercise on bone. Basic concepts and implications for the prevention of fractures. Clin. Cases Miner. Bone Metab. 2009, 6, 223–228. [Google Scholar]
- Stanghelle, B.; Bentzen, H.; Giangregorio, L.; Pripp, A.H.; Bergland, A. Effect of a resistance and balance exercise programme for women with osteoporosis and vertebral fracture: Study protocol for a randomized controlled trial. BMC Musculoskelet. Disord. 2018, 19, 100. [Google Scholar] [CrossRef]
- Giangregorio, L.; Papaioannou, A.; MacIntyre, N.J.; Ashe, M.C.; Heinonen, A.; Shipp, K.; Wark, J.D.; McGill, S.; Keller, H.H.; Jain, R.; et al. Too Fit To Fracture: Exercise recommendations for individuals with osteoporosis or osteoporotic vertebral fracture. Osteoporos. Int. 2014, 25, 821–835. [Google Scholar] [CrossRef] [Green Version]
- Palombaro, K.M.; Black, J.D.; Buchbinder, R.; Jette, D.U. Effectiveness of Exercise for Managing Osteoporosis in Women Postmenopause. Phys. Ther. 2013, 93, 1021–1025. [Google Scholar] [CrossRef] [Green Version]
- Hong, A.R.; Kim, S.W. Effects of Resistance Exercise on Bone Health. Endocrinol. Metab. 2018, 33, 435–444. [Google Scholar] [CrossRef]
- Qi, Z.; Liu, W.; Lu, J. The mechanisms underlying the beneficial effects of exercise on bone remodeling: Roles of bone-derived cytokines and microRNAs. Prog. Biophys. Mol. Biol. 2016, 122, 131–139. [Google Scholar] [CrossRef]
- Burke, S.M.; Carron, A.V.; Eys, M.A.; Ntoumanis, N.; Estabrooks, P.A. Group versus individual approach? A meta-analysis of the effectiveness of interventions to promote physical activity. Sport Exerc. Psychol. Rev. 2006, 2, 1–13. [Google Scholar]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 Explanation and Elaboration: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c869. [Google Scholar] [CrossRef] [Green Version]
- Llamas Velasco, S.; Llorente Ayuso, L.; Contador, I.; Bermejo Pareja, F. Versiones en español del Minimental State Examination (MMSE). Cuestiones para su uso en la práctica clínica. Rev. Neurol. 2015, 61, 363–371. [Google Scholar] [CrossRef]
- Reisberg, B.; Ferris, S.H.; De Leon, M.J.; Crook, T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am. J. Psychiatry 1982, 139, 1136–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faber, M.J.; Bosscher, R.J.; Van Wieringen, P.C.W. Clinimetric Properties of the Performance-Oriented Mobility Assessment. Phys. Ther. 2006, 86, 944–954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tinetti, M.E. Performance-Oriented Assessment of Mobility Problems in Elderly Patients. J. Am. Geriatr. Soc. 1986, 34, 119–126. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The Timed “Up & Go”: A Test of Basic Functional Mobility for Frail Elderly Persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef]
- Bischoff, H.A.; Stahelin, H.B.; Monsch, A.U.; Iversen, M.D.; Weyh, A.; von Dechend, M.; Akos, R.; Conzelmann, M.; Dick, W.; Theiler, R. Identifying a cut-off point for normal mobility: A comparison of the timed “up and go” test in community-dwelling and institutionalised elderly women. Age Ageing 2003, 32, 315–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vellas, B.J.; Ms, S.J.W.; Romero, L.; Baumgartner, R.N.; Rubenstein, L.Z.; Garry, P.J. One-Leg Balance Is an Important Predictor of Injurious Falls in Older Persons. J. Am. Geriatr. Soc. 1997, 45, 735–738. [Google Scholar] [CrossRef] [PubMed]
- Weiner, D.K.; Duncan, P.W.; Chandler, J.; Studenski, S.A. Functional Reach: A Marker of Physical Frailty. J. Am. Geriatr. Soc. 1992, 40, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Rothenberg, R.J.; Boyd, J.L.; Holcomb, J.P. Quantitative ultrasound of the calcaneus as a screening tool to detect osteoporosis: Different reference ranges for caucasian women, african american women, and caucasian men. J. Clin. Densitom. 2004, 7, 101–110. [Google Scholar] [CrossRef]
- Chin, K.-Y.; Ima-Nirwana, S. Calcaneal Quantitative Ultrasound as a Determinant of Bone Health Status: What Properties of Bone Does It Reflect? Int. J. Med. Sci. 2013, 10, 1778–1783. [Google Scholar] [CrossRef] [Green Version]
- Iseme, R.A.; McEvoy, M.; Kelly, B.; Agnew, L.; Walker, F.R.; Boyle, M.; Attia, J. A Cross-Sectional Study of the Association between Autoantibodies and Qualitative Ultrasound Index of Bone in an Elderly Sample without Clinical Autoimmune Disease. J. Immunol. Res. 2018, 2018, 9407971. [Google Scholar] [CrossRef] [Green Version]
- Lusardi, M.M.; Fritz, S.; Middleton, A.; Allison, L.; Wingood, M.; Phillips, E.; Criss, M.; Verma, S.; Osborne, J.; Chui, K.K. Determining Risk of Falls in Community Dwelling Older Adults: A Systematic Review and Meta-analysis Using Posttest Probability. J. Geriatr. Phys. Ther. 2017, 40, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Shimada, H.; Makizako, H.; Doi, T.; Park, H.; Tsutsumimoto, K.; Verghese, J.; Suzuki, T. Effects of Combined Physical and Cognitive Exercises on Cognition and Mobility in Patients with Mild Cognitive Impairment: A Randomized Clinical Trial. J. Am. Med. Dir. Assoc. 2018, 19, 584–591. [Google Scholar] [CrossRef]
- Gray, C.D.; Kinnear, P.R. IBM SPSS Statistics 19 Made Simple; Psychology Press: Hove, UK, 2012. [Google Scholar]
- Luan, X.; Tian, X.; Zhang, H.; Huang, R.; Li, N.; Chen, P.; Wang, R. Exercise as a prescription for patients with various diseases. J. Sport Heal. Sci. 2019, 8, 422–441. [Google Scholar] [CrossRef]
- Li, X.; Guo, R.; Wei, Z.; Jia, J.; Wei, C. Effectiveness of Exercise Programs on Patients with Dementia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. BioMed Res. Int. 2019, 2019, 2308475. [Google Scholar] [CrossRef] [Green Version]
- Hopewell, S.; Adedire, O.; Copsey, B.; Boniface, G.J.; Sherrington, C.; Clemson, L.; Close, J.C.; Lamb, S.E. Multifactorial and multiple component interventions for preventing falls in older people living in the community. Cochrane Database Syst. Rev. 2018, 7, CD012221. [Google Scholar] [CrossRef] [PubMed]
- Daly, R.M.; Gianoudis, J.; Kersh, M.E.; Bailey, C.A.; Ebeling, P.R.; Krug, R.; Nowson, C.A.; Hill, K.; Sanders, K.M. Effects of a 12-Month Supervised, Community-Based, Multimodal Exercise Program Followed by a 6-Month Research-to-Practice Transition on Bone Mineral Density, Trabecular Microarchitecture, and Physical Function in Older Adults: A Randomized Controlled Trial. J. Bone Miner Res. 2020, 35, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Lipardo, D.S.; Aseron, A.M.C.; Kwan, M.M.-S.; Tsang, W.W. Effect of Exercise and Cognitive Training on Falls and Fall-Related Factors in Older Adults With Mild Cognitive Impairment: A Systematic Review. Arch. Phys. Med. Rehabil. 2017, 98, 2079–2096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teri, L.; Gibbons, L.E.; McCurry, S.M.; Logsdon, R.G.; Buchner, D.M.; Barlow, W.E.; Kukull, W.A.; LaCroix, A.Z.; McCormick, W.; Larson, E.B. Exercise plus behavioral management in patients with Alzheimer disease: A randomized controlled trial. JAMA 2003, 290, 2015–2022. [Google Scholar] [CrossRef] [PubMed]
- Stephen, R.; Hongisto, K.; Solomon, A.; Lönnroos, E. Physical Activity and Alzheimer’s Disease: A Systematic Review. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2017, 72, 733–739. [Google Scholar] [CrossRef] [Green Version]
- Booth, V.; Hood, V.; Kearney, F. Interventions incorporating physical and cognitive elements to reduce falls risk in cognitively impaired older adults: A systematic review. JBI Database Syst. Rev. Implement Rep. 2016, 14, 110–135. [Google Scholar]
- Jensen, L.E.; Padilla, R. Effectiveness of interventions to prevent falls in people with Alzheimer’s disease and related dementias. Am. J. Occup. Ther. 2011, 65, 532–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suttanon, P.; Hill, K.D.; Said, C.M.; Williams, S.B.; Byrne, K.N.; Logiudice, D.; Lautenschlager, N.T.; Dodd, K.J. Feasibility, safety and preliminary evidence of the effectiveness of a home-based exercise programme for older people with Alzheimer’s disease: A pilot randomized controlled trial. Clin. Rehabil. 2012, 27, 427–438. [Google Scholar] [CrossRef]
- Hill, K.D.; Hunter, S.W.; Batchelor, F.A.; Cavalheri, V.; Burton, E. Individualized home-based exercise programs for older people to reduce falls and improve physical performance: A systematic review and meta-analysis. Maturitas 2015, 82, 72–84. [Google Scholar] [CrossRef]
- Burge, E.; Kuhne, N.; Berchtold, A.; Maupetit, C.; Von Gunten, A. Impact of physical activity on activity of daily living in moderate to severe dementia: A critical review. Eur. Rev. Aging Phys. Act. 2011, 9, 27–39. [Google Scholar] [CrossRef] [Green Version]
- Netz, Y.; Axelrad, S.; Argov, E. Group physical activity for demented older adults—Feasibility and effectiveness. Clin. Rehabil. 2007, 21, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Ambrose, A.F.; Cruz, L.; Paul, G. Falls and Fractures: A systematic approach to screening and prevention. Maturitas 2015, 82, 85–93. [Google Scholar] [CrossRef]
- Vogel, T.; Brechat, P.-H.; Leprêtre, P.-M.; Kaltenbach, G.; Berthel, M.; Lonsdorfer, J. Health benefits of physical activity in older patients: A review. Int. J. Clin. Pr. 2009, 63, 303–320. [Google Scholar] [CrossRef] [PubMed]
- Puisieux, F.; Pardessus, V.; Bombois, S. Dementia and falls: Two related syndromes in old age. Psychol. Neuropsychiatr. Vieil. 2005, 3, 271–279. [Google Scholar] [PubMed]
- Coelho, F.G.; Stella, F.; de Andrade, L.P.; Barbieri, F.A.; Santos-Galduróz, R.F.; Gobbi, S.; Costa, J.L.; Gobbi, L.T. Gait and risk of falls associated with frontal cognitive functions at different stages of Alzheimer’s disease. Neuropsychol. Dev. Cogn. B Aging Neuropsychol. Cogn. 2012, 19, 644–656. [Google Scholar] [CrossRef]
- Muir, S.W.; Speechley, M.; Wells, J.; Borrie, M.; Gopaul, K.; Montero-Odasso, M. Gait assessment in mild cognitive impairment and Alzheimer’s disease: The effect of dual-task challenges across the cognitive spectrum. Gait Posture 2012, 35, 96–100. [Google Scholar] [CrossRef]
- Allan, L.M.; Ballard, C.G.; Rowan, E.N.; Kenny, R.A. Incidence and Prediction of Falls in Dementia: A Prospective Study in Older People. PLoS ONE 2009, 4, e5521. [Google Scholar] [CrossRef]
- Hernandez, S.S.; Coelho, F.G.; Gobbi, S.; Stella, F. Effects of physical activity on cognitive functions, balance and risk of falls in elderly patients with Alzheimer’s dementia. Rev. Bras. Fisioter. 2010, 14, 68–74. [Google Scholar] [CrossRef] [Green Version]
- Lord, S.R.; Ward, J.A.; Williams, P.; Anstey, K.J. An epidemiological study of falls in older community-dwelling women: The Randwick falls and fractures study. Aust. J. Public Health 1993, 17, 240–245. [Google Scholar] [CrossRef]
- Stalenhoef, P.A.; Diederiks, J.P.; Knottnerus, J.A.; Kester, A.D.; Crebolder, H.F. A risk model for the prediction of recurrent falls in community-dwelling elderly: A prospective cohort study. J. Clin. Epidemiol. 2002, 55, 1088–1094. [Google Scholar] [CrossRef]
- Gillespie, L.D.; Robertson, M.C.; Gillespie, W.J.; Sherrington, C.; Gates, S.; Clemson, L.M.; Lamb, S.E. Interventions for preventing falls in older people living in the community. Cochrane Database Syst. Rev. 2012, 9, CD007146. [Google Scholar] [CrossRef] [PubMed]
- Souza, A.Q.; Pegorari, M.S.; Nascimento, J.S.; Oliveira, P.B.; Tavares, D.M.D.S. Incidence and predictive factors of falls in community-dwelling elderly: A longitudinal study. Cien. Saude Colet. 2019, 24, 3507–3516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, A.D.M.; Coutinho, E.D.S.F. Demência como fator de risco para fraturas graves em idosos. Rev. Saúde Pública 2002, 36, 448–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eshkoor, S.A.; Hamid, T.A.; Nudin, S.S.H.; Mun, C.Y. A Research on Functional Status, Environmental Conditions, and Risk of Falls in Dementia. Int. J. Alzheimer’s Dis. 2014, 2014, 769062. [Google Scholar] [CrossRef] [Green Version]
- Lundin-Olsson, L.; Nyberg, L.; Gustafson, Y. Attention, frailty, and falls: The effect of a manual task on basic mobility. J. Am. Geriatr. Soc. 1998, 46, 758–761. [Google Scholar] [CrossRef]
- Yoon, J.E.; Lee, S.M.; Lim, H.S.; Kim, T.H.; Jeon, J.K.; Mun, M.H. The Effects of Cognitive Activity Combined with Active Extremity Exercise on Balance, Walking Activity, Memory Level and Quality of Life of an Older Adult Sample with Dementia. J. Phys. Ther. Sci. 2013, 25, 1601–1604. [Google Scholar] [CrossRef] [Green Version]
- Tseng, B.Y.; Cullum, C.M.; Zhang, R. Older Adults with Amnestic Mild Cognitive Impairment Exhibit Exacerbated Gait Slowing under Dual-Task Challenges. Curr. Alzheimer Res. 2014, 11, 494–500. [Google Scholar] [CrossRef] [Green Version]
- Yamada, M.; Ichihashi, N. Predicting the probability of falls in community-dwelling elderly individuals using the trail-walking test. Environ. Health Prev. Med. 2010, 15, 386–391. [Google Scholar] [CrossRef] [Green Version]
- Mirolsky-Scala, G.; Kraemer, T. Fall management in Alzheimer-related dementia: A case study. J. Geriatr. Phys. Ther. 2009, 32, 181–189. [Google Scholar] [CrossRef]
- Sterke, C.S.; Huisman, S.L.; van Beeck, E.F.; Looman, C.W.N.; van der Cammen, T. Is the Tinetti Performance Oriented Mobility Assessment (POMA) a feasible and valid predictor of short-term fall risk in nursing home residents with dementia? Int. Psychogeriatr. 2010, 22, 254–263. [Google Scholar] [CrossRef]
- Bossers, W.J.R.; Van Der Woude, L.H.; Boersma, F.; Scherder, E.J.; Van Heuvelen, M.J. Recommended Measures for the Assessment of Cognitive and Physical Performance in Older Patients with Dementia: A Systematic Review. Dement. Geriatr. Cogn. Disord. Extra 2012, 2, 589–609. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, L.P.; Gobbi, L.T.; Coelho, F.G.; Christofoletti, G.; Costa, J.L.; Stella, F. Benefits of multimodal exercise intervention for postural control and frontal cognitive functions in individuals with Alzheimer’s disease: A controlled trial. J. Am. Geriatr. Soc. 2013, 61, 1919–1926. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.; Giordani, B.J.; Algase, D.L.; You, M.; Alexander, N.B. Fall Risk-Relevant Functional Mobility Outcomes in Dementia Following Dyadic Tai Chi Exercise. West. J. Nurs. Res. 2012, 35, 281–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duncan, P.W.; Studenski, S.; Chandler, J.; Prescott, B. Functional Reach: Predictive Validity in a Sample of Elderly Male Veterans. J. Gerontol. 1992, 47, M93–M98. [Google Scholar] [CrossRef] [PubMed]
- Brauer, S.G.; Burns, Y.R.; Galley, P. A Prospective Study of Laboratory and Clinical Measures of Postural Stability to Predict Community-Dwelling Fallers. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2000, 55, M469–M476. [Google Scholar] [CrossRef] [Green Version]
- Newton, R.A. Validity of the Multi-Directional Reach Test: A Practical Measure for Limits of Stability in Older Adults. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2001, 56, M248–M252. [Google Scholar] [CrossRef] [Green Version]
- Dawson, J.D.; Anderson, S.W.; Uc, E.Y.; Dastrup, E.; Rizzo, M. Predictors of driving safety in early Alzheimer disease. Neurology 2009, 72, 521–527. [Google Scholar] [CrossRef] [Green Version]
- Miu, D.K.; Szeto, S.L.; Mak, Y.F. A randomised controlled trial on the effect of exercise on physical, cognitive and affective function in dementia subjects. Asian J. Gerontol. Geriatr. 2008, 3, 8–16. [Google Scholar]
- Vreugdenhil, A.; Cannell, J.; Davies, A.; Razay, G. A community-based exercise programme to improve functional ability in people with Alzheimer’s disease: A randomized controlled trial. Scand. J. Caring Sci. 2012, 26, 12–19. [Google Scholar] [CrossRef]
- Briggs, R.; Gossman, M.; Birch, R.; Drews, J.; Shaddeau, S. Balance performance among non-institutionalized elderly women. Phys. Ther. 1989, 69, 748–756. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Larkin, P.A.; Cook, A.C.; Gear, J.; Singer, J. Decrease in Timed Balance Test Scores with Aging. Phys. Ther. 1984, 64, 1067–1070. [Google Scholar] [CrossRef] [PubMed]
- Pedroso, R.V.; Coelho, F.G.; Santos-Galduróz, R.F.; Costa, J.L.; Gobbi, S.; Stella, F. Balance, executive functions and falls in elderly with Alzheimer’s disease (AD): A longitudinal study. Arch. Gerontol. Geriatr. 2012, 54, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Barer, D. Fracture risk in Alzheimer’s disease patients. J. Am. Geriatr. Soc. 1995, 43, 454. [Google Scholar] [CrossRef] [PubMed]
- Loskutova, N.; Honea, R.A.; Vidoni, E.D.; Brooks, W.M.; Burns, J.M. Bone density and brain atrophy in early Alzheimer’s disease. J. Alzheimer’s Dis. 2009, 18, 777–785. [Google Scholar] [CrossRef] [Green Version]
- Cornelius, C.; Koverech, G.; Crupi, R.; Di Paola, R.; Koverech, A.; Lodato, F.; Scuto, M.; Salinaro, A.T.; Cuzzocrea, S.; Calabrese, E.J.; et al. Osteoporosis and alzheimer pathology: Role of cellular stress response and hormetic redox signaling in aging and bone remodeling. Front. Pharmacol. 2014, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gianoudis, J.; Bailey, C.A.; Ebeling, P.R.; Nowson, C.A.; Sanders, K.M.; Hill, K.; Daly, R.M. Effects of a Targeted Multimodal Exercise Program Incorporating High-Speed Power Training on Falls and Fracture Risk Factors in Older Adults: A Community-Based Randomized Controlled Trial. J. Bone Miner. Res. 2014, 29, 182–191. [Google Scholar] [CrossRef]
- Stanghelle, B.; Bentzen, H.; Giangregorio, L.; Pripp, A.H.; Skelton, D.A.; Bergland, A. Effects of a resistance and balance exercise programme on physical fitness, health-related quality of life and fear of falling in older women with osteoporosis and vertebral fracture: A randomized controlled trial. Osteoporos. Int. 2020, 31, 1069–1078. [Google Scholar] [CrossRef]
- Turcotte, A.-F.; Kukuljan, S.; Via, J.D.; Gagnon, C.; Abbott, G.; Daly, R.M. Changes in spinal bone density, back muscle size, and visceral adipose tissue and their interaction following a multi-component exercise program in older men: Secondary analysis of an 18-month randomized controlled trial. Osteoporos. Int. 2020. [Google Scholar] [CrossRef]
- Rahimi, G.R.M.; Smart, N.A.; Liang, M.T.C.; Bijeh, N.; Albanaqi, A.L.; Fathi, M.; Niyazi, A.; Rahimi, N.M. The Impact of Different Modes of Exercise Training on Bone Mineral Density in Older Postmenopausal Women: A Systematic Review and Meta-analysis Research. Calcif. Tissue Int. 2020, 106, 577–590. [Google Scholar] [CrossRef]
- Cheng, L.; Ba, H. Effect of Tai Chi exercise with the same frequency and different exercise duration on the bone mineral density of older women. J. Sports Med. Phys. Fit. 2020, 60. [Google Scholar] [CrossRef]
- Loskutova, N.; Honea, R.A.; Brooks, W.M.; Burns, J.M. Reduced limbic and hypothalamic volumes correlate with bone density in early Alzheimer’s disease. J. Alzheimer’s Dis. 2010, 20, 313–322. [Google Scholar] [CrossRef] [Green Version]
- Sato, Y.; Asoh, T.; Oizumi, K. High prevalence of vitamin D deficiency and reduced bone mass in elderly women with Alzheimer’s disease. Bone 1998, 23, 555–557. [Google Scholar] [CrossRef]
- Sato, Y.; Kanoko, T.; Satoh, K.; Iwamoto, J. Risk factors for hip fracture among elderly patients with Alzheimer’s disease. J. Neurol. Sci. 2004, 223, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Honda, Y.; Hayashida, N.; Iwamoto, J.; Kanoko, T.; Satoh, K. Vitamin K deficiency and osteopenia in elderly women with Alzheimer’s disease. Arch. Phys. Med. Rehabil. 2005, 86, 576–581. [Google Scholar] [CrossRef] [PubMed]
- Berkemeyer, S.; Schumacher, J.; Thiem, U.; Pientka, L. Bone T-Scores and Functional Status: A Cross-Sectional Study on German Elderly. PLoS ONE 2009, 4, e8216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perez-Castrillon, J.L.; Martin-Escudero, J.C.; del Pino-Montes, J.; Blanco, F.S.; Martin, F.J.; Paredes, M.G.; Fernández, F.P.; Arés, T.A. Prevalence of osteoporosis using DXA bone mineral density measurements at the calcaneus: Cut-off points of diagnosis and exclusion of osteoporosis. J. Clin. Densitom. 2005, 8, 404–408. [Google Scholar] [CrossRef]
- Sosa, M.; Saavedra, P.; Alegre, J.; Gómez, C.; González, J.; Guañabens, N. Prevalencia de osteoporosis en la población española por ultrasonografía de calcáneo en función del criterio diagnóstico utilizado. Datos del estudio GIUMO. Rev. Clin. Esp. 2003, 203, 329–333. [Google Scholar] [CrossRef]
- Iwamoto, J.; Sato, Y.; Tanaka, K.; Takeda, T.; Matsumoto, H. Prevention of hip fractures by exposure to sunlight and pharmacotherapy in patients with Alzheimer’s disease. Aging Clin. Exp. Res. 2009, 21, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Moayyeri, A. The Association between Physical Activity and Osteoporotic Fractures: A Review of the Evidence and Implications for Future Research. Ann. Epidemiol. 2008, 18, 827–835. [Google Scholar] [CrossRef]





| Variable | Intervention Group | Control Group | p-Value |
|---|---|---|---|
| Gender, women % (n) | 64.15 (34) | 78.95 (15) | 0.235 |
| Age (year) | 78.19 ± 9.87 | 72.79 ± 8.42 | 0.037 * |
| Weight (kg) | 65.69 ± 13.81 | 70.42 ± 9.71 | 0.516 |
| Height (m) | 1.56 ± 0.87 | 1.58 ± 0.96 | 0.777 |
| BMI (kg/m2) | 26.97 ± 4.26 | 28.66 ± 4.98 | 0.160 |
| MMSE (points) | 15.49 ± 5.12 | 21.42 ± 3.06 | <0.001 * |
| GDS (points) | 4.81 ± 0.81 | 3.89 ± 0567 | <0.001 * |
| Falls (n) | 0.21 ± 0.57 | 0.58 ± 0.77 | 0.065 ** |
| Variable | Gr. | Intervention Group (Estimated Means ± SD) | Between-Group Difference (IG–CG) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 1 Month | 3 Months | 6 Months | Mean | SEM | p Value | 95% CI (Lower–Upper) | |||
| POMA-T (points) | IG | 25.21 ± 3.01 | 26.38 ± 2.20 | 26.74 ± 2.02 | 26.29 ± 2.21 | 1.372 | 0.769 | 0.082 | −0.180 | 2.923 |
| CG | 26.14 ± 1.56 | 25.57 ± 1.45 | 25.07 ± 1.00 | 24.79 ± 1.58 | ||||||
| POMA-B (points) | IG | 15.06 ± 1.10 | 15.21 ± 0.91 | 15.44 ± 0.82 | 15.12 ± 0.88 | 0.661 | 0.296 | 0.031 * | 0.064 | 1.258 |
| CG | 15.14 ± 0.66 | 14.93 ± 0.73 | 14.71 ± 0.47 | 14.57 ± 0.51 | ||||||
| POMA-G (points) | IG | 10.15 ± 2.12 | 11.18 ± 1.59 | 11.29 ± 1.40 | 11.18 ± 1.47 | 0.710 | 0.535 | 0.191 | −0.368 | 1.789 |
| CG | 11.00 ± 1.18 | 10.64 ± 1.08 | 10.36 ± 0.63 | 10.21 ± 1.12 | ||||||
| TUG (s) | IG | 13.58 ± 4.85 | 11.33 ± 2.42 | 11.56 ± 2.87 | 11.57 ± 2.97 | −1.968 | 0.854 | 0.026 * | −3.687 | −0.248 |
| CG | 13.09 ± 2.74 | 12.91 ± 2.85 | 13.18 ± 2.95 | 14.18 ± 3.23 | ||||||
| OLB-r (s) | IG | 4.58 ± 5.93 | 5.96 ± 6.87 | 5.83 ± 7.49 | 5.13 ± 5.43 | 4.437 | 2.064 | 0.037 * | 0.275 | 8.598 |
| CG | 6.23 ± 10.15 | 6.01 ± 8.14 | 5.82 ± 7.45 | 5.36 ± 7.38 | ||||||
| OLB-l (s) | IG | 4.45 ± 5.83 | 4.84 ± 6.75 | 5.03 ± 6.66 | 5.18 ± 5.89 | 4.099 | 2.046 | 0.051 | −0.027 | 8.225 |
| CG | 6.72 ± 9.22 | 5.85 ± 7.73 | 4.88 ± 6.13 | 4.60 ± 5.66 | ||||||
| FR-r (cm) | IG | 19.42 ± 9.17 | 21.80 ± 8.77 | 23.52 ± 8.03 | 24.69 ± 7.17 | 1.857 | 2.635 | 0.485 | −3.458 | 7.172 |
| CG | 24.20 ± 7.52 | 24.06 ± 7.01 | 24.06 ± 6.73 | 23.63 ± 6.51 | ||||||
| FR-l (cm) | IG | 20.86 ± 8.44 | 24.31 ± 7.47 | 25.47 ± 7.61 | 25.77 ± 7.72 | 4.481 | 2.672 | 0.101 | −0.908 | 9.869 |
| CG | 23.60 ± 9.94 | 23.87 ± 8.89 | 24.39 ± 7.83 | 22.94 ± 7.39 | ||||||
| T-Score | IG | −1.06 ± 1.09 | −0.90 ± 1.13 | −0.77 ± 1.18 | −0.80 ± 1.26 | 0.333 | 0.395 | 0.404 | −0.463 | 1.130 |
| CG | −1.06 ± 0.97 | −1.16 ± 0.93 | −1.16 ± 0.87 | −1.31 ± 0.92 | ||||||
| BMD (g/cm2) | IG | 0.49 ± 0.13 | 0.48 ± 0.13 | 0.49 ± 0.13 | 0.49 ± 0.14 | 0.041 | 0.046 | 0.372 | −0.051 | 0.134 |
| CG | 0.49 ± 0.11 | 0.45 ± 0.10 | 0.45 ± 0.10 | 0.43 ± 0.10 | ||||||
| BUA (dB/MHz) | IG | 73.29 ± 20.29 | 73.84 ± 19.34 | 74.89 ± 20.69 | 74.61 ± 23.68 | 8.230 | 6.600 | 0.219 | −5.072 | 21.532 |
| CG | 74.72 ± 14.84 | 65.59 ± 13.01 | 65.08 ± 12.60 | 67.69 ± 13.50 | ||||||
| SOS (dB/MHz) | IG | 1537.09 ± 31.90 | 1533.47 ± 31.54 | 1537.77 ± 31.90 | 1534.75 ± 36.26 | 8.321 | 11.42 | 0.470 | −14.695 | 31.337 |
| CG | 1534.53 ± 27.17 | 1529.29 ± 25.63 | 1530.24 ± 26.85 | 1521.19 ± 27.01 | ||||||
| QUI | IG | 88.91 ± 20.61 | 87.95 ± 20.05 | 90.18 ± 20.95 | 89.57 ± 22.29 | 6.563 | 7.249 | 0.370 | −8.047 | 21.172 |
| CG | 89.01 ± 16.95 | 83.32 ± 16.20 | 83.02 ± 15.49 | 80.43 ± 16.10 | ||||||
| Variable | Covariate | Grouping Variables | Significant Model | |||||
|---|---|---|---|---|---|---|---|---|
| Age | Time | Group | Sex | Interaction | MANOVA (F) | p-Value * | (ƞ2p) | |
| POMA-T | ✓ | ✓ | ✓ | ✓ | Time * group | 3.105 | 0.037 | 0.185 |
| POMA-B | ✓ | ✓ | ✓ | ✓ | group | 4.980 | 0.031 | 0.104 |
| POMA-G | ✓ | ✓ | ✓ | Time * group | 3.526 | 0.023 | 0.197 | |
| TUG | ✓ | ✓ | ✓ | Time * group | 3.606 | 0.021 | 0.201 | |
| OLB-r | ✓ | ✓ | ✓ | ✓ | group | 4.622 | 0.037 | 0.097 |
| OLB-l | ✓ | ✓ | ✓ | ✓ | Group * sex | 4.289 | 0.044 | 0.091 |
| FR-r | ✓ | ✓ | ✓ | ✓ | no significant model | - | - | - |
| FR-l | ✓ | ✓ | ✓ | ✓ | no significant model | - | - | - |
| T-Score | ✓ | ✓ | ✓ | no significant model | - | - | - | |
| BMD | ✓ | ✓ | ✓ | no significant model | - | - | - | |
| BUA | ✓ | ✓ | ✓ | no significant model | - | - | - | |
| SOS | ✓ | ✓ | ✓ | no significant model | - | - | - | |
| QUI | ✓ | ✓ | ✓ | no significant model | - | - | - | |
| Variables and Significant Model (MANOVA) | ||||
|---|---|---|---|---|
| Grouping Variable | (I) | (J) | Between-Factor Difference (I–J) (95% CI) | p-Value |
| POMA-T (time * group with age) | ||||
| IG | Baseline | 1 month | −1.233 (−2.108 to −0.358) | 0.002 |
| 3 months | −1.522 (−2.553 to −0.491) | 0.001 | ||
| 6 months | −1.235 (−2.271 to −0.198) | 0.012 | ||
| 1 month | IG | CG | 1.700 (0.034 to 3.366) | 0.046 |
| 3 months | IG | CG | 2.160 (0.629 to 3.692) | 0.007 |
| 6 months | IG | CG | 2.127 (0.468 to 3.785) | 0.013 |
| POMA-B (group with age) | ||||
| Group | IG | CG | 0.661 (0.064 to 1.258) | 0.031 |
| POMA-G (time * group with age) | ||||
| IG | Baseline | 1 month | −0.989 (−1.633 to −0.345) | 0.001 |
| 3 months | −1.101 (−1.827 to −0.376) | 0.001 | ||
| 6 months | −0.992 (−1.782 to −0.202) | 0.007 | ||
| 3 months | IG | CG | 1.130 (0.352 to 1.907) | 0.005 |
| 6 months | IG | CG | 1.186 (0.323 to 2.048) | 0.008 |
| TUG (time * group with age) | ||||
| IG | Baseline | 1 month | 2.202 (0.758 to 3.646) | 0.001 |
| 3 months | 2.012 (0.494 to 3.531) | 0.004 | ||
| 6 months | 1.960 (0.491 to 3.429) | 0.004 | ||
| 1 month | IG | CG | −2.137 (−3.652 to −0.623) | 0.007 |
| 3 months | IG | CG | −2.321 (−3.997 to −0.646) | 0.008 |
| 6 months | IG | CG | −3.172 (−5.048 to −1.295) | 0.001 |
| OLB-r (group with age) | ||||
| Group | IG | CG | 4.437 (0.275 to 8.598) | 0.037 |
| OLB-l (group * sex with age) | ||||
| CG | Men | Women | −7.525 (−14.840 to −0.210) | 0.044 |
| Men | IG | CG | 8.225 (0.860 to 15.589) | 0.029 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puente-González, A.S.; Sánchez-Sánchez, M.C.; Fernández-Rodríguez, E.J.; Hernández-Xumet, J.E.; Barbero-Iglesias, F.J.; Méndez-Sánchez, R. Effects of 6-Month Multimodal Physical Exercise Program on Bone Mineral Density, Fall Risk, Balance, and Gait in Patients with Alzheimer’s Disease: A Controlled Clinical Trial. Brain Sci. 2021, 11, 63. https://doi.org/10.3390/brainsci11010063
Puente-González AS, Sánchez-Sánchez MC, Fernández-Rodríguez EJ, Hernández-Xumet JE, Barbero-Iglesias FJ, Méndez-Sánchez R. Effects of 6-Month Multimodal Physical Exercise Program on Bone Mineral Density, Fall Risk, Balance, and Gait in Patients with Alzheimer’s Disease: A Controlled Clinical Trial. Brain Sciences. 2021; 11(1):63. https://doi.org/10.3390/brainsci11010063
Chicago/Turabian StylePuente-González, A. Silvia, M. Carmen Sánchez-Sánchez, Eduardo J. Fernández-Rodríguez, J. Elicio Hernández-Xumet, Fausto J. Barbero-Iglesias, and Roberto Méndez-Sánchez. 2021. "Effects of 6-Month Multimodal Physical Exercise Program on Bone Mineral Density, Fall Risk, Balance, and Gait in Patients with Alzheimer’s Disease: A Controlled Clinical Trial" Brain Sciences 11, no. 1: 63. https://doi.org/10.3390/brainsci11010063
APA StylePuente-González, A. S., Sánchez-Sánchez, M. C., Fernández-Rodríguez, E. J., Hernández-Xumet, J. E., Barbero-Iglesias, F. J., & Méndez-Sánchez, R. (2021). Effects of 6-Month Multimodal Physical Exercise Program on Bone Mineral Density, Fall Risk, Balance, and Gait in Patients with Alzheimer’s Disease: A Controlled Clinical Trial. Brain Sciences, 11(1), 63. https://doi.org/10.3390/brainsci11010063