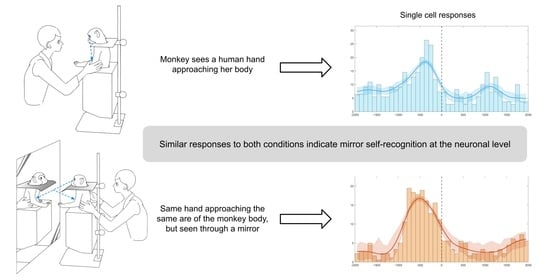
Neural Evidence of Mirror Self-Recognition in the Secondary Somatosensory Cortex of Macaque: Observations from a Single-Cell Recording Experiment and Implications for Consciousness
Abstract
:1. Introduction
2. Materials and Methods
2.1. Training and Behavioral Indicators of Mirror Self-Recognition
2.2. Surgery
2.3. Data Acquisition and Recording Sites
2.4. Identification of Visual and Somatosensory Responses
3. Results
3.1. Mirror Image Responses
3.2. Self-Related/Non-Self-Related Touch Responses
4. Discussion
4.1. Study Limitations
4.2. Single-Cell Activity as An Indicator of Mirror Self-Recognition in the Brain
4.3. Role of SII in Self-Body Consciousness
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gallup, G.G. Chimpanzees: Self-Recognition. Science 1970, 167, 86–87. [Google Scholar] [CrossRef]
- De Veer, M.W.; van den Bos, R. A critical review of methodology and interpretation of mirror self-recognition research in nonhuman primates. Anim. Behav. 1999, 58, 459–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Epstein, R.; Lanza, R.P.; Skinner, B.F. “Self-Awareness” in the Pigeon. Science 1981, 212, 695–696. [Google Scholar] [CrossRef] [PubMed]
- Huttunen, A.W.; Adams, G.K.; Platt, M.L. Can self-awareness be taught? Monkeys pass the mirror test—again. Proc. Natl. Acad. Sci. USA 2017, 114, 3281–3283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asendorpf, J.B. Self-Awareness, Other-Awareness, and Secondary Representation. In The Imitative Mind: Development, Evolution, and Brain Bases; Cambridge University Press: Cambridge, UK, 2002; p. 66. [Google Scholar]
- Gallup, G.G. Absence of self-recognition in a monkey (Macaca fascicularis) following prolonged exposure to a mirror. Dev. Psychobiol. 1977, 10, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Zhang, S.; Poo, M.M.; Gong, N. Spontaneous expression of mirror self-recognition in monkeys after learning precise visual-proprioceptive association for mirror images. Proc. Natl. Acad. Sci. USA 2017, 114, 3258–3263. [Google Scholar] [CrossRef] [Green Version]
- Chang, L.; Fang, Q.; Zhang, S.; Poo, M.M.; Gong, N. Mirror-Induced Self-Directed Behaviors in Rhesus Monkeys after Visual-Somatosensory Training. Curr. Biol. 2015, 25, 212–217. [Google Scholar] [CrossRef] [Green Version]
- Rajala, A.Z.; Reininger, K.R.; Lancaster, K.M.; Populin, L.C. Rhesus Monkeys (Macaca mulatta) Do Recognize Themselves in the Mirror: Implications for the Evolution of Self-Recognition. PLoS ONE 2010, 5, e12865. [Google Scholar] [CrossRef] [Green Version]
- Asendorpf, J.; Warkentin, V.; Baudonniere, P.M. Self-awareness and other-awareness II: Mirror self-recognition, social contingency awareness, and synchronic imitation. Dev. Psychobiol. 1996, 32, 313–321. [Google Scholar] [CrossRef]
- Bard, K.A.; Todd, B.K.; Bernier, C.; Love, J.; Leavens, D.A. Self-Awareness in Human and Chimpanzee Infants: What Is Measured and What Is Meant by the Mark and Mirror Test? Infancy 2006, 9, 191–219. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.R. The Development of Self-Recognition: A Review. Dev. Psychobiol. 1984, 17, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Plotnik, J.M.; De Waal, F.B.; Reiss, D. Self-recognition in an Asian elephant. Proc. Natl. Acad. Sci. USA 2006, 103, 17053–17057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reiss, D.; Marino, L. Mirror self-recognition in the bottlenose dolphin: A case of cognitive convergence. Proc. Natl. Acad. Sci. USA 2001, 98, 5937–5942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suarez, S.D.; Gallup, G.G., Jr. Self-recognition in chimpanzees and orangutans, but not gorillas. J. Hum. Evol. 1981, 10, 175–188. [Google Scholar] [CrossRef]
- Morin, A. Levels of consciousness and self-awareness: A comparison and integration of various neurocognitive views q. Conscious. Cogn. 2006, 15, 358–371. [Google Scholar] [CrossRef]
- Longo, M.R.; Azañón, E.; Haggard, P. More than skin deep: Body representation beyond primary somatosensory cortex. Neuropsychologia 2010, 48, 655–668. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.L.; Babiloni, C.; Ferretti, A.; Perrucci, M.G.; Romani, G.L.; Rossini, P.M.; Tartaro, A.; Del Gratta, C. Human secondary somatosensory cortex is involved in the processing of somatosensory rare stimuli: An fMRI study. Neuroimage 2008, 40, 1765–1771. [Google Scholar] [CrossRef]
- Romo, R.; Hernández, A.; Zainos, A.; Lemus, L.; Brody, C.D. Neuronal correlates of decision-making in secondary somatosensory cortex. Nat. Neurosci. 2002, 5, 1217–1225. [Google Scholar] [CrossRef]
- Bremmer, F.; Schlack, A.; Shah, N.J.; Zafiris, O.; Kubischik, M.; Hoffmann, K.P.; Zilles, K.; Fink, G.R. Polymodal Motion Processing in Posterior Parietal and Premotor Cortex: A Human fMRI Study Strongly Implies Equivalencies between Humans and Monkeys. Neuron 2001, 29, 287–296. [Google Scholar] [CrossRef]
- Hihara, S.; Taoka, M.; Tanaka, M.; Iriki, A. Visual Responsiveness of Neurons in the Secondary Somatosensory Area and its Surrounding Parietal Operculum Regions in Awake Macaque Monkeys. Cereb. Cortex 2015, 25, 4535–4550. [Google Scholar] [CrossRef]
- Keysers, C.; Wicker, B.; Gazzola, V.; Anton, J.L.; Fogassi, L.; Gallese, V. A Touching Sight: SII/PV Activation during the Observation and Experience of Touch. Neuron 2004, 42, 335–346. [Google Scholar] [CrossRef] [Green Version]
- Burton, H.; Fabri, M.; Alloway, K. Cortical areas within the lateral sulcus connected to cutaneous representations in areas 3b and 1: A revised interpretation of the second somatosensory area in macaque monkeys. J. Comp. Neurol. 1995, 355, 539–562. [Google Scholar] [CrossRef] [PubMed]
- Robinson, C.J.; Burton, H. Somatotopographic organization in the second somatosensory area of M. fascicularis. J. Comp. Neurol. 1980, 192, 43–67. [Google Scholar] [CrossRef]
- Taoka, M.; Tanaka, M.; Hihara, S.; Ojima, H.; Iriki, A. Neural response to movement of the hand and mouth in the secondary somatosensory cortex of Japanese monkeys during a simple feeding task. Somatosens. Mot. Res. 2013, 30, 140–152. [Google Scholar] [CrossRef]
- Taoka, M.; Toda, T.; Hihara, S.; Tanaka, M.; Iriki, A.; Iwamura, Y. A systematic analysis of neurons with large somatosensory receptive fields covering multiple body regions in the secondary somatosensory area of macaque monkeys. J. Neurophysiol. 2016, 116, 2152–2162. [Google Scholar] [CrossRef] [Green Version]
- Whitsel, B.L.; Petrucelli, L.M.; Werner, G. Symmetry and connectivity in the map of the body surface in somatosensory area II of primates. J. Neurophysiol. 1969, 32, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Burton, H.; MacLeod, A.M.; Videen, T.O.; Raichle, M.E. Multiple foci in parietal and frontal cortex activated by rubbing embossed grating patterns across fingerpads: A positron emission tomography study in humans. Cereb. Cortex 1997, 7, 3–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsiao, S. Central mechanisms of tactile shape perception. Curr. Opin. Neurobiol. 2008, 18, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Hinkley, L.B.; Krubitzer, L.A.; Nagarajan, S.S.; Disbrow, E.A. Sensorimotor Integration in S2, PV, and Parietal Rostroventral Areas of the Human Sylvian Fissure. J. Neurophysiol. 2007, 97, 1288–1297. [Google Scholar] [CrossRef]
- Wasaka, T.; Nakata, H.; Akatsuka, K.; Kida, T.; Inui, K.; Kakigi, R. Differential modulation in human primary and secondary somatosensory cortices during the preparatory period of self-initiated finger movement. Eur. J. Neurosci. 2005, 22, 1239–1247. [Google Scholar] [CrossRef]
- Ghazanfar, A.A.; Schroeder, C.E. Is neocortex essentially multisensory? Trends Cogn. Sci. 2006, 10, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Meredith, M.A.; Stein, B.E. Visual, auditory, and somatosensory convergence on cells in superior colliculus results in multisensory integration. J. Neurophysiol. 1986, 56, 640–662. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, S.S.; O’shaughnessy, D.M.; Johnson, K.O. Effects of selective attention on spatial form processing in monkey primary and secondary somatosensory cortex. J. Neurophysiol. 1993, 70, 444–447. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, P.N.; Roy, A.; Fitzgerald, P.J.; Hsiao, S.S.; Johnson, K.O.; Niebur, E. Attention modulates synchronized neuronal firing in primate somatosensory cortex. Nature 2000, 404, 187–190. [Google Scholar] [CrossRef]
- Lewis, J.W.; van Essen, D.C. Corticocortical connections of visual, sensorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. J. Comp. Neurol. 2000, 428, 112–137. [Google Scholar] [CrossRef]
- Seger, C.A.; Stone, M.; Keenan, J.P. Cortical Activations during judgments about the self and an other person. Neuropsychologia 2004, 42, 1168–1177. [Google Scholar] [CrossRef]
- Blanke, O.; Slater, M.; Serino, A. Behavioral, Neural, and Computational Principles of Bodily Self-Consciousness. Neuron 2015, 88, 145–166. [Google Scholar] [CrossRef] [Green Version]
- Critchley, H.D.; Mathias, C.J.; Dolan, R.J. Neuroanatomical basis for first- and second-order representations of bodily states. Nat. Neurosci. 2001, 4, 207–212. [Google Scholar] [CrossRef] [Green Version]
- Eickhoff, S.B.; Schleicher, A.; Zilles, K.; Amunts, K. The Human Parietal Operculum. I. Cytoarchitectonic Mapping of Subdivisions. Cereb. Cortex 2006, 16, 254–267. [Google Scholar] [CrossRef]
- Fitzgerald, P.J.; Lane, J.W.; Thakur, P.H.; Hsiao, S.S. Receptive Field Properties of the Macaque Second Somatosensory Cortex: Evidence for Multiple Functional Representations. J. Neurosci. 2004, 24, 11193–11204. [Google Scholar] [CrossRef]
- Krubitzer, L. The organization of neocortex in mammals: Are species differences really so different? Trends Neurosci. 1995, 18, 408–417. [Google Scholar] [CrossRef]
- Beauchamp, M.S.; Argall, B.D.; Bodurka, J.; Duyn, J.H.; Martin, A. Unraveling multisensory integration: Patchy organization within human STS multisensory cortex. Nat. Neurosci. 2004, 7, 1190–1192. [Google Scholar] [CrossRef] [PubMed]
- Bottini, G.; Paulesu, E.; Sterzi, R.; Warburton, E.; Wise, R.J.S.; Vallar, G.; Frackowiak, R.S.J.; Frith, C.D. Modulation of conscious experience by peripheral sensory stimuli. Nature 1995, 376, 778–781. [Google Scholar] [CrossRef] [PubMed]
- Disbrow, E.; Litinas, E.; Recanzone, G.H.; Padberg, J.; Krubitzer, L. Cortical connections of the second somatosensory area and the parietal ventral area in macaque monkeys. J. Comp. Neurol. 2003, 462, 382–399. [Google Scholar] [CrossRef]
- Ferrè, E.R.; Bottini, G.; Haggard, P. Vestibular inputs modulate somatosensory cortical processing. Brain Struct. Funct. 2012, 217, 859–864. [Google Scholar] [CrossRef]
- Povinelli, D. Failure to find self-recognition in Asian elephants (Elephas maximus) in contrast to their use of mirror cues to discover hidden food. J. Comp. Psychol. 1989, 103, 122–131. [Google Scholar] [CrossRef]
- Robinson, J.A.; Connell, S.; McKenzie, B.E.; Day, R.H. Do Infants Use Their Own Images to Locate Objects Reflected in a Mirror? Child Dev. 1990, 61, 1558–1568. [Google Scholar] [CrossRef]
- Devue, C.; Brédart, S. The neural correlates of visual self-recognition. Conscious. Cogn. 2011, 20, 40–51. [Google Scholar] [CrossRef] [Green Version]
- Morin, A. Self-Awareness Part 1: Definition, Measures, Effects, Functions, and Antecedents: Self-Awareness. Soc. Personal. Psychol. Compass 2011, 5, 807–823. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, R.W. Mental models of mirror-self-recognition: Two theories. New Ideas Psychol. 1993, 11, 295–325. [Google Scholar] [CrossRef]
- Makin, T.R.; Holmes, N.P.; Zohary, E. Is That Near My Hand? Multisensory Representation of Peripersonal Space in Human Intraparietal Sulcus. J. Neurosci. 2007, 27, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Bahrick, L.E.; Watson, J.S. Detection of intermodal proprioceptive-visual contingency as a potential basis of self-perception in infancy. Dev. Psychol. 1985, 21, 963–973. [Google Scholar] [CrossRef]
- Cipolloni, P.B.; Pandya, D.N. Cortical connections of the frontoparietal opercular areas in the Rhesus monkey. J. Comp. Neurol. 1999, 403, 431–458. [Google Scholar] [CrossRef]
- Iriki, A.; Tanaka, M.; Obayashi, S.; Iwamura, Y. Self-images in the video monitor coded by monkey intraparietal neurons. Neurosci. Res. 2001, 40, 163–173. [Google Scholar] [CrossRef]
- Duhamel, J.-R.; Colby, C.L.; Goldberg, M.E. Ventral Intraparietal Area of the Macaque: Congruent Visual and Somatic Response Properties. J. Neurophysiol. 1998, 79, 126–136. [Google Scholar] [CrossRef]
- Snyder, L.H.; Grieve, K.L.; Brotchie, P.; Andersen, R.A. Separate body- and world-referenced representations of visual space in parietal cortex. Nature 1998, 394, 887–891. [Google Scholar] [CrossRef]
- Lenggenhager, B.; Tadi, T.; Metzinger, T.; Blanke, O. Video Ergo Sum: Manipulating Bodily Self-Consciousness. Science 2007, 317, 1096–1099. [Google Scholar] [CrossRef] [Green Version]
- Botvinick, M.; Cohen, J. Rubber hands ‘feel’ touch that eyes see. Nature 1998, 391, 756. [Google Scholar] [CrossRef]
- Bretas, R.V.; Taoka, M.; Suzuki, H.; Iriki, A. Secondary somatosensory cortex of primates: Beyond body maps, toward conscious self-in-the-world maps. Exp. Brain Res. 2020, 238, 259–272. [Google Scholar] [CrossRef] [Green Version]
- Borra, E.; Belmalih, A.; Calzavara, R.; Gerbella, M.; Murata, A.; Rozzi, S.; Luppino, G. Cortical Connections of the Macaque Anterior Intraparietal (AIP) Area. Cereb. Cortex 2008, 18, 1094–1111. [Google Scholar] [CrossRef] [Green Version]
- Gharbawie, O.A.; Stepniewska, I.; Qi, H.; Kaas, J.H. Multiple Parietal–Frontal Pathways Mediate Grasping in Macaque Monkeys. J. Neurosci. 2001, 31, 11660–11677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Vecchio, M.; Caruana, F.; Sartori, I.; Pelliccia, V.; Zauli, F.M.; Russo, G.L.; Rizzolatti, G.; Avanzini, P. Action execution and action observation elicit mirror responses with the same temporal profile in human SII. Commun. Biol. 2020, 3, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishida, H.; Nakajima, K.; Inase, M.; Murata, A. Shared Mapping of Own and Others’ Bodies in Visuotactile Bimodal Area of Monkey Parietal Cortex. J. Cogn. Neurosci. 2010, 22, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Desmedt, J.E.; Tomberg, C. Transient phase-locking of 40 Hz electrical oscillations in prefrontal and parietal human cortex reflects the process of conscious somatic perception. Neurosci. Lett. 1994, 168, 126–129. [Google Scholar] [CrossRef]
- Palva, S.; Linkenkaer-Hansen, K.; Näätänen, R.; Palva, J.M. Early Neural Correlates of Conscious Somatosensory Perception. J. Neurosci. 2005, 25, 5248–5258. [Google Scholar] [CrossRef] [Green Version]
- Boly, M.; Massimini, M.; Tsuchiya, N.; Postle, B.R.; Koch, C.; Tononi, G. Are the Neural Correlates of Consciousness in the Front or in the Back of the Cerebral Cortex? Clinical and Neuroimaging Evidence. J. Neurosci. 2017, 37, 9603–9613. [Google Scholar] [CrossRef] [Green Version]
- Nani, A.; Manuello, J.; Mancuso, L.; Liloia, D.; Costa, T.; Cauda, F. The Neural Correlates of Consciousness and Attention: Two Sister Processes of the Brain. Front. Neurosci. 2019, 13, 1169. [Google Scholar] [CrossRef] [Green Version]
- Crick, F.; Koch, C. Towards a neurobiological theory of consciousness. Semin. Neurosci. 1990, 2, 263–275. [Google Scholar]
- Dehaene, S.; Naccache, L. Towards a cognitive neuroscience of consciousness: Basic evidence and a workspace framework. Cognition 2001, 79, 1–37. [Google Scholar] [CrossRef]
- Naghavi, H.R.; Nyberg, L. Common fronto-parietal activity in attention, memory, and consciousness: Shared demands on integration? Conscious. Cogn. 2005, 14, 390–425. [Google Scholar] [CrossRef]
- Anderson, J.R.; Gallup, G.G. Mirror self-recognition: A review and critique of attempts to promote and engineer self-recognition in primates. Primates 2015, 56, 317–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bretas, R.V.; Matsumoto, J.; Nishimaru, H.; Takamura, Y.; Hori, E.; Ono, T.; Nishijo, H. Neural Representation of Overlapping Path Segments and Reward Acquisitions in the Monkey Hippocampus. Front. Syst. Neurosci. 2019, 13, 48. [Google Scholar] [CrossRef] [PubMed]
- Gulli, R.A.; Duong, L.R.; Corrigan, B.W.; Doucet, G.; Williams, S.; Fusi, S.; Martinez-Trujillo, J.C. Context-dependent representations of objects and space in the primate hippocampus during virtual navigation. Nat. Neurosci. 2020, 23, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Lavenex, P.B.; Lavenex, P. Spatial memory and the monkey hippocampus: Not all space is created equal. Hippocampus 2009, 19, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Krubitzer, L.A.; Kaas, J.H. The organization and connections of somatosensory cortex in marmosets. J. Neurosci. 1990, 10, 952–974. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bretas, R.; Taoka, M.; Hihara, S.; Cleeremans, A.; Iriki, A. Neural Evidence of Mirror Self-Recognition in the Secondary Somatosensory Cortex of Macaque: Observations from a Single-Cell Recording Experiment and Implications for Consciousness. Brain Sci. 2021, 11, 157. https://doi.org/10.3390/brainsci11020157
Bretas R, Taoka M, Hihara S, Cleeremans A, Iriki A. Neural Evidence of Mirror Self-Recognition in the Secondary Somatosensory Cortex of Macaque: Observations from a Single-Cell Recording Experiment and Implications for Consciousness. Brain Sciences. 2021; 11(2):157. https://doi.org/10.3390/brainsci11020157
Chicago/Turabian StyleBretas, Rafael, Miki Taoka, Sayaka Hihara, Axel Cleeremans, and Atsushi Iriki. 2021. "Neural Evidence of Mirror Self-Recognition in the Secondary Somatosensory Cortex of Macaque: Observations from a Single-Cell Recording Experiment and Implications for Consciousness" Brain Sciences 11, no. 2: 157. https://doi.org/10.3390/brainsci11020157
APA StyleBretas, R., Taoka, M., Hihara, S., Cleeremans, A., & Iriki, A. (2021). Neural Evidence of Mirror Self-Recognition in the Secondary Somatosensory Cortex of Macaque: Observations from a Single-Cell Recording Experiment and Implications for Consciousness. Brain Sciences, 11(2), 157. https://doi.org/10.3390/brainsci11020157