Neurophysiological Changes Induced by Music-Supported Therapy for Recovering Upper Extremity Function after Stroke: A Case Series
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.2.1. MEG Acquisition and Protocol
2.2.2. MEG Acquisition
2.2.3. MEG Data Analysis
2.2.4. Regions of Interest
3. Results
3.1. Clinical Assessment
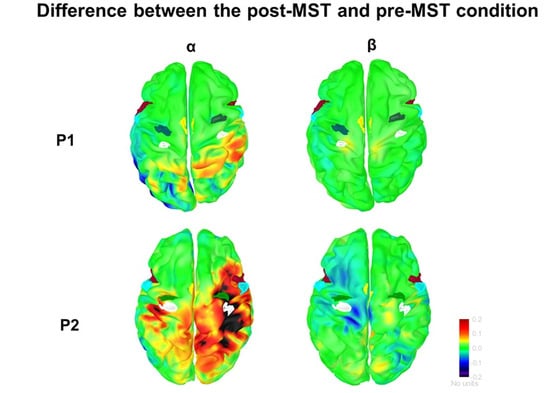
3.2. Functional Connectivity
3.2.1. α-Band
3.2.2. β-Band
4. Discussion
4.1. Behavioral Observations
4.2. Neurophysiological Observations
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duncan, P.W.; Goldstein, L.B.; Horner, R.D.; Landsman, P.B.; Samsa, G.P.; Matchar, D.B. Similar motor recovery of upper and lower extremities after stroke. Stroke 1994, 25, 1181–1188. [Google Scholar] [CrossRef] [Green Version]
- Winstein Carolee, J.; Stein, J.; Arena, R.; Bates, B.; Cherney Leora, R.; Cramer Steven, C.; Deruyter, F.; Eng Janice, J.; Fisher, B.; Harvey Richard, L.; et al. Guidelines for Adult Stroke Rehabilitation and Recovery. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, H.; Jørgensen, H.S.; Raaschou, H.O.; Olsen, T.S. Recovery of upper extremity function in stroke patients: The Copenhagen Stroke Study. Arch. Phys. Med. Rehabil. 1994, 75, 394–398. [Google Scholar] [CrossRef]
- Kwakkel, G.; Kollen, B.J. Predicting Activities after Stroke: What is Clinically Relevant? Int. J. Stroke 2012, 8, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Van Peppen, R.P.; Kwakkel, G.; Wood-Dauphinee, S.; Hendriks, H.J.; Van der Wees, P.J.; Dekker, J. The impact of physical therapy on functional outcomes after stroke: What’s the evidence? Clin. Rehabil. 2004, 18, 833–862. [Google Scholar] [CrossRef]
- Langhorne, P.; Bernhardt, J.; Kwakkel, G. Stroke rehabilitation. Lancet 2011, 377, 1693–1702. [Google Scholar] [CrossRef]
- Pollock, A.; Farmer, S.E.; Brady, M.C.; Langhorne, P.; Mead, G.E.; Mehrholz, J.; van Wijck, F. Interventions for improving upper limb function after stroke. Cochrane Database Syst. Rev. 2014, 11. [Google Scholar] [CrossRef] [PubMed]
- Carey, L.M.; Matyas, T.A.; Baum, C. Effects of Somatosensory Impairment on Participation After Stroke. Am. J. Occup. Ther. 2018, 72, 7203205100–7203205101. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Shi, Y.-Z.; Zhang, N.; Wang, S.; Ungvari, G.S.; Ng, C.H.; Wang, Y.-L.; Zhao, X.-Q.; Wang, Y.-J.; Wang, C.-X.; et al. The Disability Rate of 5-Year Post-Stroke and Its Correlation Factors: A National Survey in China. PLoS ONE 2016, 11, e0165341. [Google Scholar] [CrossRef] [Green Version]
- Opheim, A.; Danielsson, A.; Alt Murphy, M.; Persson, H.C.; Sunnerhagen, K.S. Upper-Limb Spasticity During the First Year After Stroke: Stroke Arm Longitudinal Study at the University of Gothenburg. Am. J. Phys. Med. Rehabil. 2014, 93, 884–896. [Google Scholar] [CrossRef]
- Buvarp, D.; Rafsten, L.; Sunnerhagen Katharina, S. Predicting Longitudinal Progression in Functional Mobility After Stroke. Stroke 2020, 51, 2179–2187. [Google Scholar] [CrossRef]
- Hebert, D.; Lindsay, M.P.; McIntyre, A.; Kirton, A.; Rumney, P.G.; Bagg, S.; Bayley, M.; Dowlatshahi, D.; Dukelow, S.; Garnhum, M. Canadian stroke best practice recommendations: Stroke rehabilitation practice guidelines, update 2015. Int. J. Stroke 2016, 11, 459–484. [Google Scholar] [CrossRef] [Green Version]
- Hubbard, I.J.; Parsons, M.W.; Neilson, C.; Carey, L.M. Task-specific training: Evidence for and translation to clinical practice. Occup. Ther. Int. 2009, 16, 175–189. [Google Scholar] [CrossRef]
- Higgins, J.; Salbach, N.M.; Wood-Dauphinee, S.; Richards, C.L.; Côté, R.; Mayo, N.E. The effect of a task-oriented intervention on arm function in people with stroke: A randomized controlled trial. Clin. Rehabil. 2006, 20, 296–310. [Google Scholar] [CrossRef]
- Ghai, S.; Schmitz, G.; Hwang, T.-H.; Effenberg, A.O. Training proprioception with sound: Effects of real-time auditory feedback on intermodal learning. Ann. N. Y. Acad. Sci. 2018, 1438, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Villeneuve, M.; Lamontagne, A. Playing Piano Can Improve Upper Extremity Function after Stroke: Case Studies. Stroke Res. Treat. 2013, 2013, 159105. [Google Scholar] [CrossRef] [Green Version]
- Villeneuve, M.; Penhune, V.; Lamontagne, A. A piano training program to improve manual dexterity and upper extremity function in chronic stroke survivors. Front. Hum. Neurosci. 2014, 8, 662. [Google Scholar] [CrossRef] [Green Version]
- Ghai, S. Effects of real-time (sonification) and rhythmic auditory stimuli on recovering arm function post stroke: A systematic review and meta-analysis. Front. Neurol. 2018, 9, 488. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Cai, J.; Zhang, Y.; Ren, T.; Zhao, M.; Zhao, Q. Improvement in stroke-induced motor dysfunction by music-supported therapy: A systematic review and meta-analysis. Sci. Rep. 2016, 6, 38521. [Google Scholar] [CrossRef] [Green Version]
- Ogourtsova, T.; Sitcoff, E.; Landry, S.; Bissonnette, V.; Laforest, A.-J.; Lavoi, J.; Parenteau, V.; McDermott, A.; Korner-Bitensky, N. Music Therapy. Available online: https://strokengine.ca/en/interventions/music-therapy/ (accessed on 2 November 2020).
- Grau-Sánchez, J.; Münte, T.F.; Altenmüller, E.; Duarte, E.; Rodríguez-Fornells, A. Potential benefits of music playing in stroke upper limb motor rehabilitation. Neurosci. Biobehav. Rev. 2020, 112, 585–599. [Google Scholar] [CrossRef]
- Fluet, G.G.; Merians, A.S.; Qiu, Q.; Davidow, A.; Adamovich, S.V. Comparing integrated training of the hand and arm with isolated training of the same effectors in persons with stroke using haptically rendered virtual environments, a randomized clinical trial. J. Neuroeng. Rehabil. 2014, 11, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thielbar, K.O.; Lord, T.J.; Fischer, H.C.; Lazzaro, E.C.; Barth, K.C.; Stoykov, M.E.; Triandafilou, K.M.; Kamper, D.G. Training finger individuation with a mechatronic-virtual reality system leads to improved fine motor control post-stroke. J. Neuroeng. Rehabil. 2014, 11, 171. [Google Scholar] [CrossRef] [Green Version]
- Friedman, N.; Chan, V.; Reinkensmeyer, A.N.; Beroukhim, A.; Zambrano, G.J.; Bachman, M.; Reinkensmeyer, D.J. Retraining and assessing hand movement after stroke using the MusicGlove: Comparison with conventional hand therapy and isometric grip training. J. Neuroeng. Rehabil. 2014, 11, 76. [Google Scholar] [CrossRef] [Green Version]
- Ghai, S.; Schmitz, G.; Hwang, T.-H.; Effenberg, A.O. Auditory proprioceptive integration: Effects of real-time kinematic auditory feedback on knee proprioception. Front. Neurosci. 2018, 12, 142. [Google Scholar] [CrossRef] [Green Version]
- Ghai, S.; Ghai, I.; Schmitz, G.; Effenberg, A.O. Effect of rhythmic auditory cueing on parkinsonian gait: A systematic review and meta-analysis. Sci. Rep. 2018, 8, 506. [Google Scholar] [CrossRef] [PubMed]
- Ghai, S.; Ghai, I. Effects of (music-based) rhythmic auditory cueing training on gait and posture post-stroke: A systematic review & dose-response meta-analysis. Sci. Rep. 2019, 9, 2183. [Google Scholar] [PubMed]
- Paszkiel, S.; Dobrakowski, P.; Łysiak, A. The impact of different sounds on stress level in the context of EEG, Cardiac Measures and Subjective Stress Level: A Pilot Study. Brain Sci. 2020, 10, 728. [Google Scholar] [CrossRef] [PubMed]
- Zatorre, R.J.; Chen, J.L.; Penhune, V.B. When the brain plays music: Auditory–motor interactions in music perception and production. Nat. Rev. Neurosci. 2007, 8, 547–558. [Google Scholar] [CrossRef] [PubMed]
- Altenmüller, E.; Schlaug, G. Apollo’s gift: New aspects of neurologic music therapy. In Progress in Brain Research; Elsevier: Amsterdam, The Netherlands, 2015; Volume 217, pp. 237–252. [Google Scholar]
- Pascual-Leone, A. The Brain That Plays Music and Is Changed by It. Ann. N. Y. Acad. Sci. 2001, 930, 315–329. [Google Scholar] [CrossRef]
- Münte, T.F.; Altenmüller, E.; Jäncke, L. The musician’s brain as a model of neuroplasticity. Nat. Rev. Neurosci. 2002, 3, 473–478. [Google Scholar] [CrossRef]
- Altenmuller, E.; Marco-Pallares, J.; Munte, T.F.; Schneider, S. Neural reorganization underlies improvement in stroke-induced motor dysfunction by music-supported therapy. Ann. N. Y. Acad. Sci. 2009, 1169, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Magee, W.L.; Clark, I.; Tamplin, J.; Bradt, J. Music interventions for acquired brain injury. Cochrane Database Syst. Rev. 2017. [Google Scholar] [CrossRef] [PubMed]
- Amengual, J.L.; Rojo, N.; Veciana de las Heras, M.; Marco-Pallarés, J.; Grau-Sánchez, J.; Schneider, S.; Vaquero, L.; Juncadella, M.; Montero, J.; Mohammadi, B.; et al. Sensorimotor Plasticity after Music-Supported Therapy in Chronic Stroke Patients Revealed by Transcranial Magnetic Stimulation. PLoS ONE 2013, 8, e61883. [Google Scholar] [CrossRef] [PubMed]
- Fujioka, T.; Ween, J.E.; Jamali, S.; Stuss, D.T.; Ross, B. Changes in neuromagnetic beta-band oscillation after music-supported stroke rehabilitation. Ann. N. Y. Acad. Sci. 2012, 1252, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Rojo, N.; Amengual, J.; Juncadella, M.; Rubio, F.; Camara, E.; Marco-Pallares, J.; Schneider, S.; Veciana, M.; Montero, J.; Mohammadi, B. Music-supported therapy induces plasticity in the sensorimotor cortex in chronic stroke: A single-case study using multimodal imaging (fMRI-TMS). Brain Inj. 2011, 25, 787–793. [Google Scholar] [CrossRef]
- Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef]
- Kohlmetz, C.; Kopiez, R.; Altenmüller, E. Stability of Motor Programs During a State of Meditation: Electrocortical Activity in a Pianist Playing ‘Vexations’ by Erik Satie Continuously for 28 Hours. Psychol. Music 2003, 31, 173–186. [Google Scholar] [CrossRef] [Green Version]
- Bolognini, N.; Russo, C.; Edwards, D.J. The sensory side of post-stroke motor rehabilitation. Restor. Neurol. Neurosci. 2016, 34, 571–586. [Google Scholar] [CrossRef] [Green Version]
- Ward, N. Assessment of cortical reorganisation for hand function after stroke. J. Physiol. 2011, 589, 5625–5632. [Google Scholar] [CrossRef] [Green Version]
- Jones, T.A.; Adkins, D.L. Motor System Reorganization After Stroke: Stimulating and Training Toward Perfection. Physiology 2015, 30, 358–370. [Google Scholar] [CrossRef] [Green Version]
- Larivière, S.; Ward, N.S.; Boudrias, M.-H. Disrupted functional network integrity and flexibility after stroke: Relation to motor impairments. NeuroImage Clin. 2018, 19, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Gowland, C.; Stratford, P.; Ward, M.; Moreland, J.; Torresin, W.; Van Hullenaar, S.; Sanford, J.; Barreca, S.; Vanspall, B.; Plews, N. Measuring physical impairment and disability with the Chedoke-McMaster Stroke Assessment. Stroke 1993, 24, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Gauthier, L.; Dehaut, F.; Joanette, Y. The bells test: A quantitative and qualitative test for visual neglect. Int. J. Clin. Neuropsychol. 1989, 11, 49–54. [Google Scholar]
- Platz, T.; Pinkowski, C.; van Wijck, F.; Kim, I.-H.; di Bella, P.; Johnson, G. Reliability and validity of arm function assessment with standardized guidelines for the Fugl-Meyer Test, Action Research Arm Test and Box and Block Test: A multicentre study. Clin. Rehabil. 2005, 19, 404–411. [Google Scholar] [CrossRef]
- Chen, H.-M.; Chen, C.C.; Hsueh, I.P.; Huang, S.-L.; Hsieh, C.-L. Test-Retest Reproducibility and Smallest Real Difference of 5 Hand Function Tests in Patients with Stroke. Neurorehabil. Neural Repair 2009, 23, 435–440. [Google Scholar] [CrossRef]
- Rodrigues, M.R.M.; Slimovitch, M.; Chilingaryan, G.; Levin, M.F. Does the Finger-to-Nose Test measure upper limb coordination in chronic stroke? J. Neuroeng. Rehabil. 2017, 14, 6. [Google Scholar] [CrossRef] [Green Version]
- Sano, Y.; Kandori, A.; Shima, K.; Tamura, Y.; Takagi, H.; Tsuji, T.; Noda, M.; Higashikawa, F.; Yokoe, M.; Sakoda, S. Reliability of Finger Tapping Test Used in Diagnosis of Movement Disorders. In Proceedings of the 2011 5th International Conference on Bioinformatics and Biomedical Engineering, Wuhan, China, 10–12 May 2011; pp. 1–4. [Google Scholar]
- Berardi, A.; Saffioti, M.; Tofani, M.; Nobilia, M.; Culicchia, G.; Valente, D.; Servadio, A.; Galeoto, G. Internal consistency and validity of the Jebsen–Taylor hand function test in an Italian population with hemiparesis. NeuroRehabilitation 2019, 45, 331–339. [Google Scholar] [CrossRef]
- Bertrand, A.M.; Fournier, K.; Brasey, M.-G.W.; Kaiser, M.-L.; Frischknecht, R.; Diserens, K. Reliability of maximal grip strength measurements and grip strength recovery following a stroke. J. Hand Ther. 2015, 28, 356–363. [Google Scholar] [CrossRef]
- Hollinger, A.; Steele, C.; Penhune, V.; Zatorre, R.; Wanderley, M. fMRI-compatible electronic controllers. In Proceedings of the 7th International Conference on New Interfaces for Musical Expression, New York, NY, USA, 31 May–10 June 2007; pp. 246–249. [Google Scholar]
- Tadel, F.; Baillet, S.; Mosher, J.C.; Pantazis, D.; Leahy, R.M. Brainstorm: A User-Friendly Application for MEG/EEG Analysis. Comput. Intell. Neurosci. 2011, 2011, 879716. [Google Scholar] [CrossRef]
- Huang, M.X.; Mosher, J.C.; Leahy, R.M. A sensor-weighted overlapping-sphere head model and exhaustive head model comparison for MEG. Phys. Med. Biol. 1999, 44, 423. [Google Scholar] [CrossRef] [PubMed]
- Attal, Y.; Schwartz, D. Assessment of subcortical source localization using deep brain activity imaging model with minimum norm operators: A MEG study. PLoS ONE 2013, 8, e59856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J.L.; Penhune, V.B.; Zatorre, R.J. Listening to Musical Rhythms Recruits Motor Regions of the Brain. Cereb. Cortex 2008, 18, 2844–2854. [Google Scholar] [CrossRef] [PubMed]
- Boudrias, M.-H.; Gonçalves, C.S.; Penny, W.D.; Park, C.-H.; Rossiter, H.E.; Talelli, P.; Ward, N.S. Age-related changes in causal interactions between cortical motor regions during hand grip. NeuroImage 2012, 59, 3398–3405. [Google Scholar] [CrossRef]
- Fischl, B.; Rajendran, N.; Busa, E.; Augustinack, J.; Hinds, O.; Yeo, B.T.T.; Mohlberg, H.; Amunts, K.; Zilles, K. Cortical folding patterns and predicting cytoarchitecture. Cereb. Cortex 2008, 18, 1973–1980. [Google Scholar] [CrossRef]
- Larivière, S.; Xifra-Porxas, A.; Kassinopoulos, M.; Niso, G.; Baillet, S.; Mitsis, G.D.; Boudrias, M.-H. Functional and effective reorganization of the aging brain during unimanual and bimanual hand movements. Hum. Brain Mapp. 2019, 40, 3027–3040. [Google Scholar] [CrossRef]
- Eggermont, J.J.; Ponton, C.W. The neurophysiology of auditory perception: From single units to evoked potentials. Audiol. Neurootol. 2002, 7, 71–99. [Google Scholar] [CrossRef]
- Tadel, F.; Bock, E.; Niso, G.; Mosher, J.C.; Cousineau, M.; Pantazis, D.; Leahy, R.M.; Baillet, S. MEG/EEG group analysis with Brainstorm. Front. Neurosci. 2019, 13, 76. [Google Scholar] [CrossRef] [Green Version]
- Grice, K.O.; Vogel, K.A.; Le, V.; Mitchell, A.; Muniz, S.; Vollmer, M.A. Adult norms for a commercially available Nine Hole Peg Test for finger dexterity. Am. J. Occup. Ther. 2003, 57, 570–573. [Google Scholar] [CrossRef] [Green Version]
- Mathiowetz, V.; Volland, G.; Kashman, N.; Weber, K. Adult norms for the Box and Block Test of manual dexterity. Am. J. Occup. Ther. 1985, 39, 386–391. [Google Scholar] [CrossRef] [Green Version]
- Hackel, M.E.; Wolfe, G.A.; Bang, S.M.; Canfield, J.S. Changes in hand function in the aging adult as determined by the Jebsen Test of Hand Function. Phys. Ther. 1992, 72, 373–377. [Google Scholar] [CrossRef] [Green Version]
- Ruff, R.M.; Parker, S.B. Gender-and age-specific changes in motor speed and eye-hand coordination in adults: Normative values for the Finger Tapping and Grooved Pegboard Tests. Percept. Mot. Ski. 1993, 76, 1219–1230. [Google Scholar] [CrossRef]
- Massy-Westropp, N.M.; Gill, T.K.; Taylor, A.W.; Bohannon, R.W.; Hill, C.L. Hand Grip Strength: Age and gender stratified normative data in a population-based study. BMC Res. Notes 2011, 4, 127. [Google Scholar] [CrossRef] [Green Version]
- Lang, C.E.; DeJong, S.L.; Beebe, J.A. Recovery of Thumb and Finger Extension and Its Relation to Grasp Performance after Stroke. J. Neurophysiol. 2009, 102, 451–459. [Google Scholar] [CrossRef]
- Ranganathan, V.K.; Siemionow, V.; Sahgal, V.; Liu, J.Z.; Yue, G.H. Skilled Finger Movement Exercise Improves Hand Function. J. Gerontol. A 2001, 56, 518–522. [Google Scholar] [CrossRef]
- Meirovitch, Y.; Harris, H.; Dayan, E.; Arieli, A.; Flash, T. Alpha and Beta Band Event-Related Desynchronization Reflects Kinematic Regularities. J. Neurosci. 2015, 35, 1627. [Google Scholar] [CrossRef] [Green Version]
- Nicolo, P.; Rizk, S.; Magnin, C.; Pietro, M.D.; Schnider, A.; Guggisberg, A.G. Coherent neural oscillations predict future motor and language improvement after stroke. Brain 2015, 138, 3048–3060. [Google Scholar] [CrossRef] [Green Version]
- Ray, A.M.; Figueiredo, T.D.C.; López-Larraz, E.; Birbaumer, N.; Ramos-Murguialday, A. Brain oscillatory activity as a biomarker of motor recovery in chronic stroke. Hum. Brain Mapp. 2020, 41, 1296–1308. [Google Scholar] [CrossRef]
- Arce-McShane, F.I.; Ross, C.F.; Takahashi, K.; Sessle, B.J.; Hatsopoulos, N.G. Primary motor and sensory cortical areas communicate via spatiotemporally coordinated networks at multiple frequencies. Proc. Natl. Acad. Sci. USA 2016, 113, 5083–5088. [Google Scholar] [CrossRef] [Green Version]
- Dubovik, S.; Pignat, J.-M.; Ptak, R.; Aboulafia, T.; Allet, L.; Gillabert, N.; Magnin, C.; Albert, F.; Momjian-Mayor, I.; Nahum, L. The behavioral significance of coherent resting-state oscillations after stroke. NeuroImage 2012, 61, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Doelling, K.B.; Poeppel, D. Cortical entrainment to music and its modulation by expertise. Proc. Natl. Acad. Sci. USA 2015, 112, 6233–6242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haueisen, J.; Knösche, T.R. Involuntary motor activity in pianists evoked by music perception. J. Cogn. Neurosci. 2001, 13, 786–792. [Google Scholar] [CrossRef] [Green Version]
- Gordon, C.L.; Cobb, P.R.; Balasubramaniam, R. Recruitment of the motor system during music listening: An ALE meta-analysis of fMRI data. PLoS ONE 2018, 13, e0207213. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.-C.; Liu, H.-Q.; Hua, X.-Y.; Shen, Y.-D.; Xu, W.-D.; Xu, J.-G.; Gu, Y.-D. Supplementary motor area deactivation impacts the recovery of hand function from severe peripheral nerve injury. Neural Regen. Res. 2016, 11, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.D.; Meguerditchian, A.; Coulon, O.; Misiura, M.; Pope, S.; Mareno, M.C.; Schapiro, S.J. Motor skill for tool-use is associated with asymmetries in Broca’s area and the motor hand area of the precentral gyrus in chimpanzees (Pan troglodytes). Behav. Brain Res. 2017, 318, 71–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davare, M.; Andres, M.; Cosnard, G.; Thonnard, J.-L.; Olivier, E. Dissociating the role of ventral and dorsal premotor cortex in precision grasping. J. Neurosci. 2006, 26, 2260–2268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witt, S.T.; Laird, A.R.; Meyerand, M.E. Functional neuroimaging correlates of finger-tapping task variations: An ALE meta-analysis. NeuroImage 2008, 42, 343–356. [Google Scholar] [CrossRef] [Green Version]
- Riecker, A.; Gröschel, K.; Ackermann, H.; Schnaudigel, S.; Kassubek, J.; Kastrup, A. The role of the unaffected hemisphere in motor recovery after stroke. Hum. Brain Mapp. 2010, 31, 1017–1029. [Google Scholar] [CrossRef]
- Buetefisch, C.M. Role of the Contralesional Hemisphere in Post-Stroke Recovery of Upper Extremity Motor Function. Front. Neurol. 2015, 6, 214. [Google Scholar] [CrossRef]
- Schaechter, J.D.; Perdue, K.L. Enhanced Cortical Activation in the Contralesional Hemisphere of Chronic Stroke Patients in Response to Motor Skill Challenge. Cereb. Cortex 2007, 18, 638–647. [Google Scholar] [CrossRef] [Green Version]
- Mohapatra, S.; Harrington, R.; Chan, E.; Dromerick, A.W.; Breceda, E.Y.; Harris-Love, M. Role of contralesional hemisphere in paretic arm reaching in patients with severe arm paresis due to stroke: A preliminary report. Neurosci. Lett. 2016, 617, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Touvykine, B.; Mansoori, B.K.; Jean-Charles, L.; Deffeyes, J.; Quessy, S.; Dancause, N. The Effect of Lesion Size on the Organization of the Ipsilesional and Contralesional Motor Cortex. Neurorehabil. Neural Repair 2016, 30, 280–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Participant 1 | Participant 2 | |
|---|---|---|
| Age (years) | 66 | 67 |
| Gender (M/F) | M | M |
| Affected hemisphere | Right | Right |
| Nature of CVA | Ischemic | Ischemic |
| Time after stroke (months) | 15 | 15 |
| Site of lesion | Subcortical | Lacunar internal capsule |
| CMSA arm/hand score | 5/5 | 3/3 |
| Piano experience (years) | 0 | 0 |
| Handedness | Right | Right |
| P1 | P2 | Age Norms ** | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Base | Pre | Post | % | F-up | % | Base | Pre | Post | % | F-up | % | ||
| Nine-Hole Peg Test (s) | 44 | 47.2 | 43.7 | (−)4 | 45.9 | 1 | 120 + | 120 + | 109.6 | (−)9 | 102.3 | (−)15 | 21.6 ± 2.9 [63] |
| Box and Block Test (n) | 41 | 42.6 | 46.3 | 11 | 45.6 | 9 | 19.6 | 23.6 | 24.6 | 14 | 29.3 | 35 | 67.4 ± 7.8 [64] |
| Jebsen Hand Function Test, subset 2–7 (s) | 9.2 | 8.4 | 7.8 | (−)28 | 7.1 | (−)35 | 16 | 14.6 | 12.7 | (−)15 | 12.6 | (−)23 | 5.9 ± 2.0 [65] |
| Finger-Tapping Test (n) | 35 | 32.3 | 36.6 | 9 | 42.3 | 26 | 21 | 19.6 | 33 | 62 | 34.6 | 70 | 48.3 ± 5.0 [66] |
| Finger-to-Nose Test (n) | 24.6 | 25.3 | 27.3 | 9 | 28.3 | 12.5 | * | * | * | - | * | - | - |
| Grip Strength (kg) | # | # | # | - | # | - | 4.3 | 4.6 | 6.3 | 17 | 7.6 | 18 | 38 ± 8.0 [67] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghai, S.; Maso, F.D.; Ogourtsova, T.; Porxas, A.-X.; Villeneuve, M.; Penhune, V.; Boudrias, M.-H.; Baillet, S.; Lamontagne, A. Neurophysiological Changes Induced by Music-Supported Therapy for Recovering Upper Extremity Function after Stroke: A Case Series. Brain Sci. 2021, 11, 666. https://doi.org/10.3390/brainsci11050666
Ghai S, Maso FD, Ogourtsova T, Porxas A-X, Villeneuve M, Penhune V, Boudrias M-H, Baillet S, Lamontagne A. Neurophysiological Changes Induced by Music-Supported Therapy for Recovering Upper Extremity Function after Stroke: A Case Series. Brain Sciences. 2021; 11(5):666. https://doi.org/10.3390/brainsci11050666
Chicago/Turabian StyleGhai, Shashank, Fabien Dal Maso, Tatiana Ogourtsova, Alba-Xifra Porxas, Myriam Villeneuve, Virginia Penhune, Marie-Hélène Boudrias, Sylvain Baillet, and Anouk Lamontagne. 2021. "Neurophysiological Changes Induced by Music-Supported Therapy for Recovering Upper Extremity Function after Stroke: A Case Series" Brain Sciences 11, no. 5: 666. https://doi.org/10.3390/brainsci11050666
APA StyleGhai, S., Maso, F. D., Ogourtsova, T., Porxas, A.-X., Villeneuve, M., Penhune, V., Boudrias, M.-H., Baillet, S., & Lamontagne, A. (2021). Neurophysiological Changes Induced by Music-Supported Therapy for Recovering Upper Extremity Function after Stroke: A Case Series. Brain Sciences, 11(5), 666. https://doi.org/10.3390/brainsci11050666