Cognitive Impairment in Anti-Phospholipid Syndrome and Anti-Phospholipid Antibody Carriers
Abstract
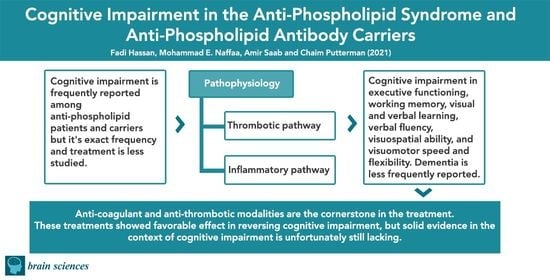
:1. Introduction
2. Epidemiology
2.1. Definitions
2.2. Carriers of aPL
2.3. APS Patients
3. Pathophysiology
4. Clinical Manifestations
4.1. Cognitive Impairment in aPL Carriers
4.2. Cognitive Impairment in APS
5. Treatment
6. Conclusions
7. Research Agenda
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lim, W. Anti-phospholipid syndrome. Hematol. Am. Soc. Hematol. Educ. Program. 2013, 2013, 675–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satta, R.; Biondi, G. Anti-phospholipid syndrome and pregnancy. G. Ital. Dermatol. Venereol. 2019, 154, 277–285. [Google Scholar] [CrossRef]
- Sanna, G.; Bertolaccini, M.L.; Cuadrado, M.J.; Khamashta, M.A. Central nervous system involvement in the anti-phospholipid (Hughes) syndrome. Rheumatology 2003, 42, 200–213. [Google Scholar] [CrossRef] [Green Version]
- Yelnik, C.M.; Kozora, E.; Appenzeller, S. Non-stroke central neurologic manifestations in anti-phospholipid syndrome. Curr. Rheumatol. Rep. 2016, 18, 11. [Google Scholar] [CrossRef] [PubMed]
- Yelnik, C.M.; Kozora, E.; Appenzeller, S. Cognitive disorders and anti-phospholipid antibodies. Autoimmun. Rev. 2016, 15, 1193–1198. [Google Scholar] [CrossRef] [PubMed]
- Colucci, A.T.; Di Lorenzo, G.; Ingrassia, A.; Crescimanno, G. Blood anti-phospholipid antibody levels are influenced by age, sex and HLA-B8, DR3 phenotype. Exp. Clin. Immunogenet. 1992, 9, 72–79. [Google Scholar] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Tsoi, K.K.; Chan, J.Y.; Hirai, H.W.; Wong, S.Y. Cognitive Tests to Detect Dementia: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2015, 175, 1450–1458. [Google Scholar] [CrossRef] [Green Version]
- Jacobson, M.W.; Rapport, L.J.; Keenan, P.A.; Coleman, R.D. Neuropsychological deficits associated with anti-phospholipid antibodies. J. Clin. Exp. Neuropsychol. 1999, 21, 251–264. [Google Scholar] [CrossRef]
- Erkan, D.; Barbhaiya, M.; George, D.; Sammaritano, L. Moderate versus high-titer persistently anticardiolipin antibody positive patients: Are they clinically different and does high-titer anti-beta 2-glycoprotein-I antibody positivity offer additional predictive information? Lupus 2010, 19, 613–619. [Google Scholar] [CrossRef]
- Kozora, E.; Erkan, D.; Zhang, L.; Zimmerman, R. Cognitive dysfunction in anti-phospholipid antibody (aPL)-negative systemic lupus erythematosus (SLE) versus aPL-positive non-SLE patients. Clin. Exp. Rheumatol. 2014, 32, 34–40. [Google Scholar]
- Bucci, T.; Menichelli, D.; Pignatelli, P.; Triggiani, M.; Violi, F.; Pastori, D. Relationship of Antiphospholipid Antibodies to Risk of Dementia: A Systematic Review. J. Alzheimer’s Dis. 2019, 69, 561–576. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, R.; Auer-Grumbach, P.; Fazekas, F.; Offenbacher, H. Anticardiolipin antibodies in normal subjects. Neuropsychological correlates and MRI findings. Stroke 1995, 26, 749–754. [Google Scholar] [CrossRef]
- Arvanitakis, Z.; Brey, R.L.; Rand, J.H.; Schneider, J.A. Anti-phospholipid antibodies, brain infarcts, and cognitive and motor decline in aging (ABICMA): Design of a community-based, longitudinal, clinical—Pathological study. Neuroepidemiology 2013, 40, 73–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosek, A.; Yust, I.; Treves, T.A.; Vardinon, N. Dementia and anti-phospholipid antibodies. Dement. Geriatr. Cogn. Disord. 2000, 11, 36–38. [Google Scholar] [CrossRef] [PubMed]
- Juby, A.; Davis, P.; Genge, T.; McElhaney, J. Anticardiolipin antibodies in two elderly subpopulations. Lupus 1995, 4, 482–485. [Google Scholar] [CrossRef]
- Afeltra, A.; Garzia, P.; Mitterhofer, A.P.; Vadacca, M. Neuropsychiatric lupus syndromes: Relationship with anti-phospholipid antibodies. Neurology 2003, 8, 108–110. [Google Scholar] [CrossRef]
- Mikdashi, J.; Handwerger, B. Predictors of neuropsychiatric damage in systemic lupus erythematosus: Data from the Maryland Lupus Cohort. Rheumatology 2004, 43, 1555–1560. [Google Scholar] [CrossRef] [Green Version]
- Conti, F.; Alessandri, C.; Perricone, C.; Scrivo, R. Neurocognitive dysfunction in systemic lupus erythematosus: Association with anti-phospholipid antibodies, disease activity and chronic damage. PLoS ONE 2012, 7, e33824. [Google Scholar]
- Borowoy, A.M.; Pope, J.E.; Silverman, E.; Fortin, P.R. Neuropsychiatric lupus: The prevalence and autoantibody associations depend on the definition: Results from the 1000 faces of lupus cohort. Semin. Arthritis Rheum. 2012, 42, 179–185. [Google Scholar] [CrossRef]
- Hanly, J.G.; Hong, C.; Smith, S.; Fisk, J.D. A prospective analysis of cognitive function and anticardiolipin antibodies in systemic lupus erythematosus. Arthritis Rheum. 1999, 42, 728–734. [Google Scholar] [CrossRef]
- Riancho-Zarrabeitia, L.; Martínez-Taboada, V.M.; Rúa-Figueroa, I.; Alonso, F. Do all anti-phospholipid antibodies confer the same risk for major organ involvement in systemic lupus erythematosus patients? Clin. Exp. Rheumatol. 2021, 39, 555–563. [Google Scholar] [PubMed]
- Coín, M.A.; Vilar-López, R.; Peralta-Ramírez, I.; Hidalgo-Ruzzante, N. The role of anti-phospholipid autoantibodies in the cognitive deficits of patients with systemic lupus erythematosus. Lupus 2015, 24, 875–879. [Google Scholar] [CrossRef] [PubMed]
- Tektonidou, M.G.; Varsou, N.; Kotoulas, G.; Antoniou, A. Cognitive deficits in patients with anti-phospholipid syndrome: Association with clinical, laboratory, and brain magnetic resonance imaging findings. Arch. Intern. Med. 2006, 13, 2278–2284. [Google Scholar] [CrossRef] [PubMed]
- Maeshima, E.; Yamada, Y.; Yukawa, S.; Nomoto, H. Higher cortical dysfunction, anti-phospholipid antibodies and neuroradiological examinations in systemic lupus erythematosus. Intern. Med. 1992, 31, 1169–1174. [Google Scholar] [CrossRef] [Green Version]
- Denburg, S.D.; Carbotte, R.M.; Ginsberg, J.S.; Denburg, J.A. The relationship of anti-phospholipid antibodies to cognitive function in patients with systemic lupus erythematosus. J. Int. Neuropsychol. Soc. 1997, 3, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Sanna, G.; Bertolaccini, M.L.; Cuadrado, M.J.; Laing, H. Neuropsychiatric manifestations in systemic lupus erythematosus: Prevalence and association with anti-phospholipid antibodies. J. Rheumatol. 2003, 30, 985–992. [Google Scholar]
- Tomietto, P.; Annese, V.; D’agostini, S.; Venturini, P. General and specific factors associated with severity of cognitive impairment in systemic lupus erythematosus. Arthritis Rheum. 2007, 15, 1461–1472. [Google Scholar] [CrossRef]
- Murray, S.G.; Yazdany, J.; Kaiser, R.; Criswell, L.A. Cardiovascular disease and cognitive dysfunction in systemic lupus erythematosus. Arthritis Care Res. 2012, 64, 1328–1333. [Google Scholar] [CrossRef] [Green Version]
- Cervera, R.; Piette, J.-C.; Font, J.; Khamashta, M.A. Anti-phospholipid syndrome: Clinical and immunologic manifestations and patterns of disease expression in a cohort of 1000 patients. Arthritis Rheum. 2002, 46, 1019–1027. [Google Scholar] [CrossRef]
- Etemadifar, M.; Dehghani, L.; Tahani, S.; Toghianifar, N. Neurological manifestations in patients with anti-phospholipid syndrome. Iran J. Neurol. 2013, 12, 172–175. [Google Scholar]
- Chapman, J.; Abu-Katash, M.; Inzelberg, R.; Yust, I. Prevalence and clinical features of dementia associated with the anti-phospholipid syndrome and circulating anticoagulants. J. Neurol. Sci. 2002, 15, 81–84. [Google Scholar] [CrossRef]
- McLaurin, E.Y.; Holliday, S.L.; Williams, P.; Brey, R.L. Predictors of cognitive dysfunction in patients with systemic lupus erythematosus. Neurology 2005, 25, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.; Jameson-Shortall, E.; Newman, S.P.; Hall-Craggs, M.R. A longitudinal study of anticardiolipin antibody levels and cognitive functioning in systemic lupus erythematosus. Arthritis Rheum. 1999, 42, 735–741. [Google Scholar] [CrossRef]
- Hanly, J.G.; McCurdy, G.; Fougere, L.; Douglas, J.-A. Neuropsychiatric events in systemic lupus erythematosus: Attribution and clinical significance. J. Rheumatol. 2004, 31, 2156–2162. [Google Scholar] [PubMed]
- Donnellan, C.; Cohen, H.; Werring, D.J. Cognitive dysfunction and associated neuroimaging biomarkers in antiphospholipid syndrome: A systematic review. Rheumatology 2021, 61, 24–41. [Google Scholar] [CrossRef]
- Man, Y.L.; Sanna, G. Neuropsychiatric Manifestations of Antiphospholipid Syndrome—A Narrative Review. Brain Sci. 2022, 12, 91. [Google Scholar] [CrossRef]
- Sevim, E.; Zisa, D.; Andrade, D.; Sciascia, S.; Pengo, V.; Tektonidou, M.G.; Ugarte, A.; Gerosa, M.; Belmont, H.M.; Zamorano, M.A.A.; et al. Characteristics of Patients with Antiphospholipid Antibody Positivity in the APS ACTION International Clinical Database and Repository. Arthritis Care Res. 2020, 74, 324–335. [Google Scholar] [CrossRef]
- Antovic, A.; Bruzelius, M. Impaired Fibrinolysis in the Antiphospholipid Syndrome. Semin. Thromb. Hemost. 2021, 47, 506–511. [Google Scholar] [CrossRef]
- Katzav, A.; Pick, C.G.; Korczyn, A.D.; Oest, E. Hyperactivity in a mouse model of the anti-phospholipid syndrome. Lupus 2001, 10, 496–499. [Google Scholar] [CrossRef]
- Ziporen, L.; Shoenfeld, Y.; Levy, Y.; Korczyn, A.D. Neurological dysfunction and hyperactive behavior associated with anti-phospholipid antibodies. A mouse model. J. Clin. Investig. 1997, 1, 613–619. [Google Scholar] [CrossRef]
- Katzav, A.; Ben-Ziv, T.; Blank, M.; Pick, C.G. Antibody-specific behavioral effects: Intracerebroventricular injection of anti-phospholipid antibodies induces hyperactive behavior while anti-ribosomal-P antibodies induces depression and smell deficits in mice. J. Neuroimmunol. 2014, 15, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Appenzeller, S.; Lapa, A.T.; de Carvalho, J.F.; Peres, F.A. Cognitive dysfunction and anti-phospholipid antibodies. Curr. Rheumatol. Rep. 2012, 14, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Shoenfeld, Y.; Nahum, A.; Korczyn, A.D.; Dano, M. Neuronal-binding antibodies from patients with anti-phospholipid syndrome induce cognitive deficits following intrathecal passive transfer. Lupus 2003, 12, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Frauenknecht, K.; Katzav, A.; Lavi, R.W.; Sabag, A. Mice with experimental anti-phospholipid syndrome display hippocampal dysfunction and a reduction of dendritic complexity in hippocampal CA1 neurones. Neuropathol. Appl. Neurobiol. 2015, 41, 657–671. [Google Scholar] [CrossRef] [PubMed]
- Van Horn, G.; Arnett, F.C.; Dimachkie, M.M. Reversible dementia and chorea in a young woman with the lupus anticoagulant. Neurology 1996, 46, 1599–1603. [Google Scholar] [CrossRef]
- Rosa, R.F.; Ugolini-Lopes, M.R.; Gandara, A.P.R.; Vendramini, M.B.G.; Campanholo, K.R.; Dutra, L.; de Andrade, D.C.O. Cognitive dysfunction and serum levels of brain-derived neurotrophic factor (BDNF) in primary anti-phospholipid syndrome (PAPS). Rheumatology 2021, 60, 179–187. [Google Scholar] [CrossRef]
- Howieson, D. Current limitations of neuropsychological tests and assessment procedures. Clin. Neuropsychol. 2019, 33, 200–208. [Google Scholar] [CrossRef]
- Gómez-Puerta, J.A.; Cervera, R.; Calvo, L.M.; Gómez-Ansón, B.; Espinosa, G.; Claver, G.; Bucciarelli, S.; Bové, A.; Ramos-Casals, M.; Ingelmo, M.; et al. Dementia associated with the antiphospholipid syndrome: Clinical and radiological characteristics of 30 patients. Rheumatology 2005, 44, 95–99. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Rocha, N.P.; Salem, H.; Diniz, B.S. The association between systemic lupus erythematosus and dementia A meta-analysis. Dement. Neuropsychol. 2018, 12, 143–151. [Google Scholar] [CrossRef]
- Kozora, E.; Uluğ, A.M.; Erkan, D.; Vo, A.; Filley, C.M. Functional Magnetic Resonance Imaging of Working Memory and Executive Dysfunction in Systemic Lupus Erythematosus and Antiphospholipid Antibody-Positive Patients. Arthritis Care Res. 2016, 68, 1655–1663. [Google Scholar] [CrossRef]
- İlgen, U.; Yayla, M.E.; Ateş, A.; Okatan, İ.E. Antiphospholipid antibodies and non-thrombotic manifestations of systemic lupus erythematosus. Lupus 2018, 27, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Erkan, D.; Yazici, Y.; Sobel, R.; Lockshin, M.D. Primary anti-phospholipid syndrome: Functional outcome after 10 years. J. Rheumatol. 2000, 27, 2817–2821. [Google Scholar] [PubMed]
- Ruiz-Irastorza, G.; Cuadrado, M.; Ruiz-Arruza, I.; Brey, R. Evidence-based recommendations for the prevention and long-term management of thrombosis in anti-phospholipid antibody-positive patients: Report of a Task Force at the13th International Congress on Anti-phospholipid Antibodies. Lupus 2011, 20, 206–218. [Google Scholar] [CrossRef]
- Hughes, G.R. Migraine, memory loss, and multiple sclerosis. Neurological features of the anti-phospholipid (Hughes’) syndrome. Postgrad. Med. J. 2003, 79, 81–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ClinicalTrials.gov. RIvaroxaban for Stroke Patients with AntiPhospholipid Syndrome (RISAPS). Identifier: NCT03684564. Available online: https://clinicaltrials.gov/ct2/show/NCT03684564 (accessed on 30 October 2021).
- Erkan, D.; Vega, J.; Ramón, G.; Kozora, E. A pilot open-label phase II trial of rituximab for non-criteria manifestations of anti-phospholipid syndrome. Arthritis Rheum. 2013, 65, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Tektonidou, M.G.; Andreoli, L.; Limper, M.; Amoura, Z. EULAR recommendations for the management of anti-phospholipid syndrome in adults. Ann. Rheum. Dis. 2019, 78, 1296–1304. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Wang, F.; Pan, W.; Liu, L.; Wu, M.; Hu, H.; Ding, X.; Wei, H.; Zou, Y.; Qian, X.; et al. Association of antimalarial drugs with decreased overall and cause specific mortality in systemic lupus erythematosus. Rheumatology 2021, 60, 1774–1783. [Google Scholar] [CrossRef]
- Groot, N.; Shaikhani, D.; Teng, Y.K.O.; de Leeuw, K.; Bijl, M.; Dolhain, R.J.E.M.; Zirkzee, E.; Fritsch-Stork, R.; Bultink, I.E.M.; Kamphuis, S. Long-Term Clinical Outcomes in a Cohort of Adults with Childhood-Onset Systemic Lupus Erythematosus. Arthritis Rheumatol. 2019, 71, 290–301. [Google Scholar] [CrossRef]
- Fessler, B.J.; Alarcón, G.S.; McGwin, G.; Roseman, J.; Bastian, H.M.; Friedman, A.W.; Baethge, B.A.; Vilá, L.; Reveille, J.D. LUMINA Study Group. Systemic lupus erythematosus in three ethnic groups: XVI. Association of hydroxychloroquine use with reduced risk of damage accrual. Arthritis Rheum. 2005, 52, 1473–1480. [Google Scholar] [CrossRef]
- Mimica, M.; Barra, I.; Ormeño, R.; Flores, P.; Calderón, J.; Padilla, O.; Bravo-Zehnder, M.; González, A.; Massardo, L. Predictors of damage accrual in systemic lupus erythematosus: A longitudinal observational study with focus on neuropsychological factors and anti-neuronal antibodies. Clin. Rheumatol. 2019, 38, 3129–3137. [Google Scholar] [CrossRef]
- Ceccarelli, F.; Perricone, C.; Pirone, C.; Massaro, L.; Alessandri, C.; Mina, C.; Marianetti, M.; Spinelli, F.R.; Valesini, G.; Conti, F. Cognitive dysfunction improves in systemic lupus erythematosus: Results of a 10 years prospective study. PLoS ONE 2018, 13, e0196103. [Google Scholar] [CrossRef] [PubMed]
- Crocker, T.F.; Brown, L.; Lam, N.; Wray, F.; Knapp, P.; Forster, A. Information provision for stroke survivors and their carers. Cochrane Database Syst. Rev. 2021, 2021. [Google Scholar] [CrossRef]
- Fardet, L.; Nazareth, I.; Petersen, I. Chronic hydroxychloroquine/chloroquine exposure for connective tissue diseases and risk of Alzheimer’s disease: A population-based cohort study. Ann. Rheum. Dis. 2019, 78, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Xourgia, E.; Tektonidou, M.G. Management of Non-criteria Manifestations in Antiphospholipid Syndrome. Curr. Rheumatol. Rep. 2020, 22, 51. [Google Scholar] [CrossRef]

|
|
|
|
|
| Study | Study Population | Control Group | Cognitive Tests | Main Results | Cognitive Impairment Frequency |
|---|---|---|---|---|---|
| aPL Carriers | |||||
| Schmidt et al., 1995 [13] | Elderly subjects (n = 53) | Age-matched non- aPL carriers | MWT-B, Janke and Debus, LGT-3, WCST, Alters Konzentrations of Gatterer, Purdue Pegboard | Impaired memory and visuopractical abilities. No brain abnormalities or differences in brain MRI. | Not reported |
| Jacobson et al., 1999 [9] | Asymptomatic, aPL-carriers, non-elderly adults (n = 27) | Age- and education-matched non-aPL carriers | Wechsler, CVLT, Benton line orientation, COWAT, finger oscillation, grooved pegboard, RCFT, trail making, WCST, Beck, state-trait anxiety inventory | Impaired executive functioning, verbal learning, memory, and visuospatial abilities. Attentional processes and fine motor skills appeared unaffected. | 33% in aPL carries vs. 4% in controls |
| Erkan et al., 2010 [10] | High titers of aPL antibodies (n = 85) | Moderate titers of aPL antibodies (n = 58) | Not specified | Increased prevalence of cognitive impairment in the higher-titer group in a linear pattern | 12% in high titers vs. 3% in moderate titers group |
| Kozora et al., 2014 [11] | Non SLE aPL-carriers (n = 20) | SLE patients with negative aPL | FSIQ, Wechsler digit symbol and block design, trail making, Stroop color and word, CVLT, Rey-O Immediate, Rey-O Recall, LNST, COWAT, PASAT, Dig Vig, category test, finger tapping test | High frequency of cognitive impairment in both groups with no significant difference between the groups | 40% in non-SLE aPL carriers vs. 60% in the SLE non aPL carriers |
| Primary APS | |||||
| Tektonidou et al., 2006 [24] | Primary APS (n= 39) and secondary APS (SLE related) (n = 21) | Healthy age-, sex-, and education-matched controls | Wechsler digit span, symbol and block design, Rey AVLT, RCFT, SCWT, TMT, COWAT. | Impairment of visual learning, memory, visuomotor and visuospatial speed and flexibility, verbal fluency, and rapid auditory information processing impaired. No difference between primary APS and secondary APS Predictors for cognitive impairment: Livedo reticularis and presence of white ma ter lesions on MRI | 42% in APS patients vs. 18% in the controls |
| Coin et al., 2015 [23] | Primary APS (n = 15), Secondary APS with SLE (n = 12) and SLE without aPLs (n = 27) | Healthy, age- and education-matched controls | TAVEC, RCFT, Stroop color and word test, verbal phonemic fluency and semantic fluency (Spanish version), Ruff 2&7 selective attention test. | Impaired executive functions and memory (verbal and visual) | 80% in primary APS, 75% in secondary APS with SLE, 48% in SLE without aPLs, and 16% in the controls |
| Secondary APS | |||||
| Maeshima et al., 1992 [25] | Secondary APS with SLE (n = 21) | Healthy controls | MMSE, “Kana” pick-up test, Miyake’s paired associated memory scale, word recall, digit span, Watamori method, line bisection test, line cancellation task, recognition of intricate pictures and perspective cube copying test. | Higher cortical impairment in study group | 76% vs. missing data |
| Afeltra et al., 2003 [17] | Secondary APS with SLE (n = 61) | Healthy controls | Not specified | High titers of aPL were associated with cognitive impairment No details on cognitive impairment patterns | 58% |
| Mikdashi et al., 2004 [18] | Secondary APS with SLE (n = 130) | MMSE with other tests not explicitly specified | No details on cognitive impairment patterns | 27% in study group | |
| McLaurin et al., 2005 [33] | Secondary APS with SLE (n = 123) | Mild impairment battery from the Automated Neuropsychological Assessment Metrics (ANAM). | No details on cognitive impairment patterns | 37.5% in study group | |
| Tomietto et al., 2007 [28] | Secondary APS with SLE (n = 52) | Rheumatoid arthritis | Raven’s progressive matrices, comprehension, similarities, block design, and digit symbol of Wechsler, Wechsler memory scale, Rey auditory-verbal learning, trail-making, Corsi block, number cancellation, reverse numerical sequence (MMSE), Stroop word and color test, semantic and phonemic verbal fluency, denomination of Aachener Aphasie and token test. | Executive functions and complex attention were more frequently impaired in APS patients. | 68.6% in study group vs. 41.2% in controls |
| Murray et al., 2012 [29] | Secondary APS with SLE (n = 694) | HVLT-R, COWAT | Verbal memory and verbal fluency | 15% in entire cohort | |
| Conti et al., 2012 [19] | Secondary APS with SLE (n = 58) | Standardized testing from ACR and the CSI standardized in an Italian population | Visuospatial domain mainly impaired | Missing data | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hassan, F.; Naffaa, M.E.; Saab, A.; Putterman, C. Cognitive Impairment in Anti-Phospholipid Syndrome and Anti-Phospholipid Antibody Carriers. Brain Sci. 2022, 12, 222. https://doi.org/10.3390/brainsci12020222
Hassan F, Naffaa ME, Saab A, Putterman C. Cognitive Impairment in Anti-Phospholipid Syndrome and Anti-Phospholipid Antibody Carriers. Brain Sciences. 2022; 12(2):222. https://doi.org/10.3390/brainsci12020222
Chicago/Turabian StyleHassan, Fadi, Mohammad E. Naffaa, Amir Saab, and Chaim Putterman. 2022. "Cognitive Impairment in Anti-Phospholipid Syndrome and Anti-Phospholipid Antibody Carriers" Brain Sciences 12, no. 2: 222. https://doi.org/10.3390/brainsci12020222
APA StyleHassan, F., Naffaa, M. E., Saab, A., & Putterman, C. (2022). Cognitive Impairment in Anti-Phospholipid Syndrome and Anti-Phospholipid Antibody Carriers. Brain Sciences, 12(2), 222. https://doi.org/10.3390/brainsci12020222