Experimental Induction of Intracranial Aneurysms in Rats: A New Model Utilizing a Genetic Modification within the EDNRA Gene
Abstract
:1. Introduction
2. Materials and Methods
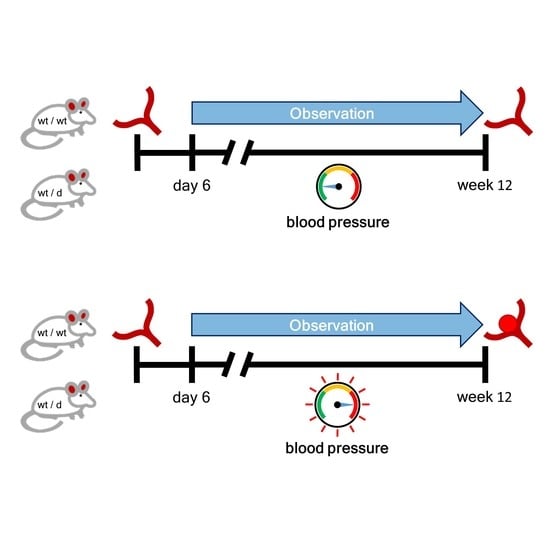
2.1. Animals
2.2. Genotyping
2.3. Noninvasive Blood Pressure Measurement
2.4. Induction of Arterial Hypertension (HT)
2.5. Brain Preparation
2.6. Immunofluorescence
2.7. Functional Investigations
2.8. Statistical Analysis
3. Results
3.1. Mortality
3.2. Noninvasive Blood Pressure Measurement
3.3. Morphological Changes in the Circle of Willis
3.4. Immunofluorescence
3.5. Functional Investigation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Macdonald, R.L.; Schweizer, T.A. Spontaneous subarachnoid haemorrhage. Lancet 2017, 389, 655–666. [Google Scholar] [CrossRef]
- Hop, J.W.; Rinkel, G.J.; Algra, A.; Van Gijn, J. Case-Fatality Rates and Functional Outcome after Subarachnoid Hemorrhage: A systematic review. Stroke 1997, 28, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Nieuwkamp, D.J.; Setz, L.E.; Algra, A.; Linn, F.H.; De Rooij, N.K.; Rinkel, G.J. Changes in case fatality of aneurysmal subarachnoid haemorrhage over time, according to age, sex, and region: A meta-analysis. Lancet Neurol. 2009, 8, 635–642. [Google Scholar] [CrossRef]
- Vlak, M.H.; Algra, A.; Brandenburg, R.; Rinkel, G.J. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: A systematic review and meta-analysis. Lancet Neurol. 2011, 10, 626–636. [Google Scholar] [CrossRef]
- Alg, V.S.; Sofat, R.; Houlden, H.; Werring, D.J. Genetic risk factors for intracranial aneurysms: A meta-analysis in more than 116,000 individuals. Neurology 2013, 80, 2154–2165. [Google Scholar] [CrossRef]
- Backes, D.; Rinkel, G.J.; Laban, K.G.; Algra, A.; Vergouwen, M.D. Patient- and Aneurysm-Specific Risk Factors for Intracranial Aneurysm Growth: A Systematic Review and Meta-Analysis. Stroke 2016, 47, 951–957. [Google Scholar] [CrossRef]
- Güresir, E.; Vatter, H.; Schuss, P.; Platz, J.; Konczalla, J.; Rochement, R.D.M.D.; Berkefeld, J.; Seifert, V. Natural History of Small Unruptured Anterior Circulation Aneurysms: A prospective cohort study. Stroke 2013, 44, 3027–3031. [Google Scholar] [CrossRef]
- Schievink, W.I. Intracranial Aneurysms. N. Engl. J. Med. 1997, 336, 28–40. [Google Scholar] [CrossRef]
- Zaremba, S.; Albus, L.; Vatter, H.; Klockgether, T.; Güresir, E. Poor blood pressure control following subarachnoid hemorrhage in patients with sleep apnea. Sleep Breath. 2021, 25, 777–785. [Google Scholar] [CrossRef]
- Bakker, M.K.; Stroke, H.A.-I.; Van der Spek, R.A.A.; Van Rheenen, W.; Morel, S.; Bourcier, R.; Hostettler, I.C.; Alg, V.S.; Van Eijk, K.R.; Koido, M.; et al. Genome-wide association study of intracranial aneurysms identifies 17 risk loci and genetic overlap with clinical risk factors. Nat. Genet. 2020, 52, 1303–1313. [Google Scholar] [CrossRef]
- Sheinberg, D.L.; McCarthy, D.J.; Elwardany, O.; Bryant, J.-P.; Luther, E.; Chen, S.H.; Thompson, J.W.; Starke, R.M. Endothelial dysfunction in cerebral aneurysms. Neurosurg. Focus 2019, 47, E3. [Google Scholar] [CrossRef] [PubMed]
- Frösen, J.; Cebral, J.; Robertson, A.M.; Aoki, T. Flow-induced, inflammation-mediated arterial wall remodeling in the formation and progression of intracranial aneurysms. Neurosurg. Focus 2019, 47, E21. [Google Scholar] [CrossRef] [PubMed]
- Chalouhi, N.; Hoh, B.L.; Hasan, D. Review of Cerebral Aneurysm Formation, Growth, and Rupture. Stroke 2013, 44, 3613–3622. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Wang, Z.; Hoi, Y.; Gao, L.; Metaxa, E.; Swartz, D.; Kolega, J. Complex Hemodynamics at the Apex of an Arterial Bifurcation Induces Vascular Remodeling Resembling Cerebral Aneurysm Initiation. Stroke 2007, 38, 1924–1931. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Nishimura, M. The Development and the Use of Experimental Animal Models to Study the Underlying Mechanisms of CA Formation. J. Biomed. Biotechnol. 2010, 2011, 53592. [Google Scholar] [CrossRef]
- Hashimoto, N.; Handa, H.; Nagata, I.; Hazama, F. Experimentally induced cerebral aneurysms in rats: Part, V. Relation of hemodynamics in the circle of Willis to formation of aneurysms. Surg. Neurol. 1980, 13, 41–45. [Google Scholar]
- Strange, F.; Grüter, B.E.; Fandino, J.; Marbacher, S. Preclinical Intracranial Aneurysm Models: A Systematic Review. Brain Sci. 2020, 10, 134. [Google Scholar] [CrossRef]
- Yasuno, K.; Bakırcıoğlu, M.; Low, S.-K.; Bilgüvar, K.; Gaál, E.; Ruigrok, Y.M.; Niemelä, M.; Hata, A.; Bijlenga, P.; Kasuya, H.; et al. Common variant near the endothelin receptor type A (EDNRA) gene is associated with intracranial aneurysm risk. Proc. Natl. Acad. Sci. USA 2011, 108, 19707–19712. [Google Scholar] [CrossRef]
- Hong, E.P.; Kim, B.J.; Jeon, J.P.; Yang, J.S.; Choi, H.J.; Kang, S.H.; Cho, Y.J. Association of Endothelin Receptor Type A with Intracranial Aneurysm in 20,609 East Asians: An Updated Meta-Analysis. World Neurosurg. 2019, 130, e804–e814. [Google Scholar] [CrossRef]
- Hussain, I.; Duffis, E.J.; Gandhi, C.D.; Prestigiacomo, C.J. Genome-Wide Association Studies of Intracranial Aneurysms: An update. Stroke 2013, 44, 2670–2675. [Google Scholar] [CrossRef]
- Houde, M.; Desbiens, L.; D’Orléans-Juste, P. Endothelin-1: Biosynthesis, Signaling and Vasoreactivity. Adv. Pharmacol. 2016, 77, 143–175. [Google Scholar] [CrossRef] [PubMed]
- Davenport, A.P.; Hyndman, K.A.; Dhaun, N.; Southan, C.; Kohan, D.E.; Pollock, J.S.; Pollock, D.M.; Webb, D.J.; Maguire, J.J. Endothelin. Pharmacol. Rev. 2016, 68, 357–418. [Google Scholar] [CrossRef] [PubMed]
- D’Uscio, L.V.; Barton, M.; Shaw, S.; Moreau, P.; Lüscher, T.F. Structure and Function of Small Arteries in Salt-Induced Hypertension: Effects of chronic endothelin-subtype-A-receptor blockade. Hypertension 1997, 30, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Moreau, P.; D’Uscio, L.V.; Shaw, S.; Takase, H.; Barton, M.; Lüscher, T.F. Angiotensin II Increases Tissue Endothelin and Induces Vascular Hypertrophy: Reversal by ET(A)-receptor antagonist. Circulation 1997, 96, 1593–1597. [Google Scholar] [CrossRef] [PubMed]
- Vatter, H.; Zimmermann, M.; Tesanovic, V.; Raabe, A.; Schilling, L.; Seifert, V. Cerebrovascular characterization of clazosentan, the first nonpeptide endothelin receptor antagonist clinically effective for the treatment of cerebral vasospasm. Part I: Inhibitory effect on endothelin(A) receptor—mediated contraction. J. Neurosurg. 2005, 102, 1101–1107. [Google Scholar] [CrossRef]
- Vatter, H.; Zimmermann, M.; Tesanovic, V.; Raabe, A.; Seifert, V.; Schilling, L. Cerebrovascular characterization of clazosentan, the first nonpeptide endothelin receptor antagonist shown to be clinically effective for the treatment of cerebral vasospasm. Part II: Effect on endothelin(B) receptor—mediated relaxation. J. Neurosurg. 2005, 102, 1108–1114. [Google Scholar] [CrossRef]
- Macdonald, R.L.; Higashida, R.T.; Keller, E.; Mayer, S.A.; Molyneux, A.; Raabe, A.; Vajkoczy, P.; Wanke, I.; Bach, D.; Frey, A.; et al. Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: A randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2). Lancet Neurol. 2011, 10, 618–625. [Google Scholar] [CrossRef]
- Du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Widdop, R.; Li, X.C. A Simple Versatile Method for Measuring Tail Cuff Systolic Blood Pressure in Conscious Rats. Clin. Sci. 1997, 93, 191–194. [Google Scholar] [CrossRef]
- Nagata, I.; Handa, H.; Hashimoto, N.; Hazama, F. Experimentally induced cerebral aneurysms in rats: Part VI. Hypertension. Surg. Neurol. 1980, 14, 477–479. [Google Scholar]
- Hashimoto, N.; Handa, H.; Hazama, F. Experimentally induced cerebral aneurysms in rats. Surg. Neurol. 1978, 10, 3–8. [Google Scholar]
- Brown, J.O. The morphology of circulus arteriosus cerebri in rats. Anat. Rec. 1966, 156, 99–106. [Google Scholar] [CrossRef]
- Ajiboye, N.; Chalouhi, N.; Starke, R.M.; Zanaty, M.; Bell, R. Unruptured Cerebral Aneurysms: Evaluation and Management. Sci. World J. 2015, 2015, 954954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, N.; Handa, H.; Hazama, F. Experimentally induced cerebral aneurysms in rats: Part III. Pathology. Surg. Neurol. 1979, 11, 299–304. [Google Scholar] [PubMed]
- Low, S.-K.; Takahashi, A.; Cha, P.-C.; Zembutsu, H.; Kamatani, N.; Kubo, M.; Nakamura, Y. Genome-wide association study for intracranial aneurysm in the Japanese population identifies three candidate susceptible loci and a functional genetic variant at EDNRA. Hum. Mol. Genet. 2012, 21, 2102–2110. [Google Scholar] [CrossRef] [PubMed]
- Clouthier, D.; Hosoda, K.; Richardson, J.; Williams, S.; Yanagisawa, H.; Kuwaki, T.; Kumada, M.; Hammer, R. Cranial and cardiac neural crest defects in endothelin-A receptor-deficient mice. Development 1998, 125, 813–824. [Google Scholar] [CrossRef]
- Alvarez, F.; Roda, J.M. Experimental model for induction of cerebral aneurysms in rats. J. Neurosurg. 1986, 65, 398–400. [Google Scholar] [CrossRef]
- Jamous, M.A.; Nagahiro, S.; Kitazato, K.T.; Tamura, T.; Aziz, H.A.; Shono, M.; Satoh, K. Endothelial injury and inflammatory response induced by hemodynamic changes preceding intracranial aneurysm formation: Experimental study in rats. J. Neurosurg. 2007, 107, 405–411. [Google Scholar] [CrossRef]
- Kojima, M.; Handa, H.; Hashimoto, N.; Kim, C.; Hazama, F. Early changes of experimentally induced cerebral aneurysms in rats: Scanning electron microscopic study. Stroke 1986, 17, 835–841. [Google Scholar] [CrossRef]
- Hazama, F.; Kataoka, H.; Yamada, E.; Kayembe, K.; Hashimoto, N.; Kojima, M.; Kim, C. Early changes of experimentally induced cerebral aneurysms in rats. Light-microscopic study. Am. J. Pathol. 1986, 124, 399–404. [Google Scholar]
- Sauvageau, S.; Thorin, E.; Villeneuve, L.; Dupuis, J. Change in pharmacological effect of endothelin receptor antagonists in rats with pulmonary hypertension: Role of ETB-receptor expression levels. Pulm. Pharmacol. Ther. 2009, 22, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, P.M.; Boulanger, C.M. Endothelium-Dependent Responses in Hypertension. Hypertens. Res. 1995, 18, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Touyz, R.M.; Alves-Lopes, R.; Rios, F.J.; Camargo, L.L.; Anagnostopoulou, A.; Arner, A.; Montezano, A.C. Vascular smooth muscle contraction in hypertension. Cardiovasc. Res. 2018, 114, 529–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Control Group (CTL) | Induction of HT | |
|---|---|---|
| WT | 14 | 13 |
| EDNRA | 14 | 18 |
| N | No Anomalies | Ectasia | Aneurysm | |
|---|---|---|---|---|
| WT_CTL | 14 | 14 (100%) | 0 (0%) | 0 (0%) |
| EDNRA_CTL | 14 | 14 (100%) | 0 (0%) | 0 (0%) |
| WT + HT | 10 | 7 (70%) | 2 (20%) | 1 (10%) |
| EDNRA + HT | 15 | 7 (47%) | 6 (40%) | 2 (13%) |
| p-value | 0.25 | 0.29 | 0.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lampmann, T.; Borger, V.; Konczalla, J.; Gispert, S.; Auburger, G.; Vatter, H.; Güresir, E. Experimental Induction of Intracranial Aneurysms in Rats: A New Model Utilizing a Genetic Modification within the EDNRA Gene. Brain Sci. 2022, 12, 1239. https://doi.org/10.3390/brainsci12091239
Lampmann T, Borger V, Konczalla J, Gispert S, Auburger G, Vatter H, Güresir E. Experimental Induction of Intracranial Aneurysms in Rats: A New Model Utilizing a Genetic Modification within the EDNRA Gene. Brain Sciences. 2022; 12(9):1239. https://doi.org/10.3390/brainsci12091239
Chicago/Turabian StyleLampmann, Tim, Valeri Borger, Jürgen Konczalla, Suzana Gispert, Georg Auburger, Hartmut Vatter, and Erdem Güresir. 2022. "Experimental Induction of Intracranial Aneurysms in Rats: A New Model Utilizing a Genetic Modification within the EDNRA Gene" Brain Sciences 12, no. 9: 1239. https://doi.org/10.3390/brainsci12091239
APA StyleLampmann, T., Borger, V., Konczalla, J., Gispert, S., Auburger, G., Vatter, H., & Güresir, E. (2022). Experimental Induction of Intracranial Aneurysms in Rats: A New Model Utilizing a Genetic Modification within the EDNRA Gene. Brain Sciences, 12(9), 1239. https://doi.org/10.3390/brainsci12091239