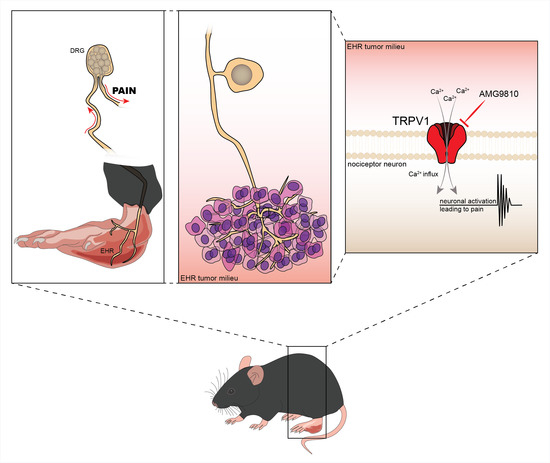
Ehrlich Tumor Induces TRPV1-Dependent Evoked and Non-Evoked Pain-like Behavior in Mice
Abstract
:1. Introduction
2. Material and Methods
2.1. Animals
2.2. Experimental Procedures
2.3. Calcium Imaging
2.4. Mechanical Hyperalgesia
2.5. Thermal Hyperalgesia
2.6. Tumor Growth: Paw Thickness and Paw Weight
2.7. Static Weight Bearing (SWB)
2.8. Statistical Analysis
3. Results
3.1. Ehrlich Tumor Cells Induce DRG Neuronal Activation and Enhance Capsaicin Response
3.2. TRPV1 Deficiency Reduces Ehrlich Tumor Cells Triggered Pain-like Behavior without Changing Parameters of Tumor Growth
3.3. The TRPV1 Antagonist AMG9810 Reduces Ongoing Hyperalgesia Induced by Ehrlich Tumor Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ehrlich, P.; Apolant, H. Beobachtungen Uber Maligne Mausetumoren; Berliner Klinische Wochenschrift: Berlin, Germany, 1905; Volume 42, pp. 871–874. [Google Scholar]
- Loewenthal, H.; Jahn, G. Ubertragungsversuche Mit Carcinomatoser Mause-Ascitesfliissigkeit Und Ihr Verhalten Gegen Physikalische Und Chemische Einwirkungen. Z. Krebsforsch. 1932, 37, 439–447. [Google Scholar] [CrossRef]
- Ahmed, H.; Chatterjee, B.P.; Debnath, A.K. Interaction and in vivo Growth Inhibition of Ehrlich Ascites Tumor Cells by Jacalin. J. Biosci. 1988, 13, 419–424. [Google Scholar] [CrossRef]
- Zhu, X.; Wu, H.; Xia, J.; Zhao, M.; Xianyu, Z. The relationship between 99mTc-MIBI uptakes and tumor cell death/proliferation state under irradiation. Cancer Lett. 2002, 182, 217–222. [Google Scholar] [CrossRef]
- Calixto-Campos, C.; Zarpelon, A.C.; Corrêa, M.; Cardoso, R.D.R.; Pinho-Ribeiro, F.A.; Cecchini, R.; Moreira, E.G.; Crespigio, J.; Bernardy, C.C.F.; Casagrande, R.; et al. The ehrlich tumor induces pain-like behavior in mice: A novel model of cancer pain for pathophysiological studies and pharmacological screening. BioMed Res. Int. 2013, 12, 624815. [Google Scholar] [CrossRef]
- Harris, W.G.; Benson, E.A.; Cartwright, R.; Cartwright, S.C.; Clayden, A.D.; Cowen, P.N.; Dossett, J.A.; Edwards, M.; Frank, H.G.; Gowland, P.G.; et al. Symptoms and signs of operable breast cancer report from the yorkshire breast cancer group Members of the Yorkshire Breast Cancer Group. J. R. Coll. Gen. Pract. 1983, 33, 473–476. [Google Scholar]
- Krøner, K.; Krebs, B.; Skov, J.; Jørgensen, H.S. Immediate and long-term phantom breast syndrome after mastectomy: Incidence, clinical characteristics and relationship to pre-mastectomy breast pain. Pain 1989, 36, 327–334. [Google Scholar] [CrossRef]
- Bol, M.; Leybaert, L.; Vanheel, B. Influence of methanandamide and CGRP on potassium currents in smooth muscle cells of small mesenteric arteries. Pflugers Arch. 2012, 463, 669–677. [Google Scholar] [CrossRef]
- Bevan, S.; Quallo, T.; Andersson, D.A. TRPV1. Handb. Exp. Pharmacol. 2014, 222, 207–245. [Google Scholar] [CrossRef]
- Ghilardi, J.R.; Röhrich, H.; Lindsay, T.H.; Sevcik, M.A.; Schwei, M.J.; Kubota, K.; Halvorson, K.G.; Poblete, J.; Chaplan, S.R.; Dubin, A.E.; et al. Selective Blockade of the Capsaicin Receptor TRPV1 Attenuates Bone Cancer Pain. J. Neurosci. 2005, 25, 3126–3131. [Google Scholar] [CrossRef]
- Menéndez, L.; Juárez, L.; García, E.; García-Suárez, O.; Hidalgo, A.; Baamonde, A. Analgesic effects of capsazepine and resiniferatoxin on bone cancer pain in mice. Neurosci. Lett. 2006, 393, 70–73. [Google Scholar] [CrossRef]
- Asai, H.; Ozaki, N.; Shinoda, M.; Nagamine, K.; Tohnai, I.; Ueda, M.; Sugiura, Y. Heat and mechanical hyperalgesia in mice model of cancer pain. Pain 2005, 117, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Hartel, M.; di Mola, F.F.; Selvaggi, F.; Mascetta, G.; Wente, M.N.; Felix, K.; Giese, N.A.; Hinz, U.; di Sebastiano, P.; Büchler, M.W.; et al. Vanilloids in pancreatic cancer: Potential for chemotherapy and pain management. Gut 2006, 55, 519–528. [Google Scholar] [CrossRef] [PubMed]
- TCunha, M.; Verri, W.A.; Vivancos, G.G.; Moreira, I.F.; Reis, S.; Parada, C.A.; Cunha, F.Q.; Ferreira, S.H. An electronic pressure-meter nociception paw test for mice. Braz. J. Med. Biol. Res. 2004, 37, 401–407. [Google Scholar] [CrossRef]
- Chiu, I.M.; Heesters, B.A.; Ghasemlou, N.; von Hehn, C.A.; Zhao, F.; Tran, J.; Wainger, B.; Strominger, A.; Muralidharan, S.; Horswill, A.R.; et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature 2013, 501, 52–57. [Google Scholar] [CrossRef]
- Almoughrabie, S.; Ngari, C.; Guillier, L.; Briandet, R.; Poulet, V.; Dubois-Brissonnet, F. Rapid assessment and prediction of the efficiency of two preservatives against S. aureus in cosmetic products using high content screening—Confocal laser scanning microscopy. PLoS ONE 2020, 15, e0236059. [Google Scholar] [CrossRef]
- Sang, W.; Zhong, Z.; Linghu, K.; Xiong, W.; Tse, A.K.W.; Cheang, W.S.; Yu, H.; Wang, Y. Siegesbeckia pubescens Makino inhibits Pam3CSK4-induced inflammation in RAW 264.7 macrophages through suppressing TLR1/TLR2-mediated NF-ΚB activation. Chin. Med. 2018, 13, 1–10. [Google Scholar] [CrossRef]
- Zaninelli, T.H.; Fattori, V.; Saraiva-Santos, T.; Badaro-Garcia, S.; Staurengo-Ferrari, L.; Andrade, K.C.; Artero, N.A.; Ferraz, C.R.; Bertozzi, M.M.; Rasquel-Oliveira, F.; et al. RvD1 disrupts nociceptor neuron and macrophage activation and neuroimmune communication, reducing pain and inflammation in gouty arthritis in mice. Br. J. Pharmacol. 2022, 179, 4500–4515. [Google Scholar] [CrossRef]
- Conte, F.P.; Menezes-De-Lima, O.J.; Verri, W.A.; Cunha, F.Q.; Penido, C.; Henriques, M.G.; Menezes-de-Lima, O., Jr.; Verri, W.A., Jr.; Cunha, F.Q.; Penido, C.; et al. Lipoxin A(4) attenuates zymosan-induced arthritis by modulating endothelin-1 and its effects. Br. J. Pharmacol. 2010, 161, 911–924. [Google Scholar] [CrossRef]
- Guazelli, C.F.S.; Staurengo-Ferrari, L.; Zarpelon, A.C.; Pinho-Ribeiro, F.A.; Ruiz-Miyazawa, K.W.; Vicentini, F.T.M.C.; Vignoli, J.A.; Camilios-Neto, D.; Georgetti, S.R.; Baracat, M.M.; et al. Quercetin attenuates zymosan-induced arthritis in mice. Biomed. Pharmacother. 2018, 102, 175–184. [Google Scholar] [CrossRef]
- Gavva, N.R.; Tamir, R.; Qu, Y.; Klionsky, L.; Zhang, T.J.; Immke, D.; Wang, J.; Zhu, D.; Vanderah, T.W.; Porreca, F.; et al. AMG 9810 [(E)-3-(4-t-Butylphenyl)-N-(2,3-dihydrobenzo[b][61,3] dioxin-6-yl)acrylamide], a Novel Vanilloid Receptor 1 (TRPV1) Antagonist with Antihyperalgesic Properties. J. Pharmacol. Exp. Ther. 2005, 313, 474–484. [Google Scholar] [CrossRef]
- Fattori, V.; Pinho-Ribeiro, F.A.; Staurengo-Ferrari, L.; Borghi, S.M.; Rossaneis, A.C.; Casagrande, R.; Verri, W.A. The specialised pro-resolving lipid mediator maresin 1 reduces inflammatory pain with a long-lasting analgesic effect. Br. J. Pharmacol. 2019, 176, 1728–1744. [Google Scholar] [CrossRef] [PubMed]
- Valerio, D.A.; Cunha, T.M.; Arakawa, N.S.; Lemos, H.P.; da Costa, F.B.; Parada, C.A.; Ferreira, S.H.; Cunha, F.Q.; Verri, W.A., Jr. Anti-inflammatory and analgesic effects of the sesquiterpene lactone budlein A in mice: Inhibition of cytokine production-dependent mechanism. Eur. J. Pharmacol. 2007, 562, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Amaral, J.G.; Cardoso, T.N.; de Carvalho, A.C.; de Coelho, C.; Waisse, S.; Perez, E.C.; Bonamin, L.V. High-diluted thymulin on Ehrlich tumor growth in mice and the importance of tumor microenvironment. Int. J. High Dilution Res. 2018, 17, 20–41. [Google Scholar] [CrossRef]
- Liu, S.; Bohl, D.; Blanchard, S.; Bacci, J.; Saïd, G.; Heard, J.M. Combination of microsurgery and gene therapy for spinal dorsal root injury repair. Mol. Ther. 2009, 17, 992–1002. [Google Scholar] [CrossRef]
- Marouane, E.; Resold, G.; el Mahmoudi, N.; Péricat, D.; Chabbert, C.; Artzner, V.; Tighilet, B. Identification of New Biomarkers of Posturo-Locomotor Instability in a Rodent Model of Vestibular Pathology. Front. Neurol. 2020, 11, 1–14. [Google Scholar] [CrossRef]
- Fattori, V.; Zaninelli, T.H.; Ferraz, C.R.; Brasil-Silva, L.; Borghi, S.M.; Cunha, J.M.; Chichorro, J.G.; Casagrande, R.; Verri, W.A. Maresin 2 is an analgesic specialized pro-resolution lipid mediator in mice by inhibiting neutrophil and monocyte recruitment, nociceptor neuron TRPV1 and TRPA1 activation, and CGRP release. Neuropharmacology 2022, 216, 109189. [Google Scholar] [CrossRef]
- Zhang, J.; Cavanaugh, D.J.; Nemenov, M.I.; Basbaum, A.I. The modality-specific contribution of peptidergic and non-peptidergic nociceptors is manifest at the level of dorsal horn nociresponsive neurons. J. Physiol. 2013, 591, 1097–1110. [Google Scholar] [CrossRef]
- Cavanaugh, D.J.; Lee, H.; Lo, L.; Shields, S.D.; Zylka, M.J.; Basbaum, A.I.; Anderson, D.J. Distinct subsets of unmyelinated primary sensory fibers mediate behavioral responses to noxious thermal and mechanical stimuli. Proc. Natl. Acad. Sci. USA 2009, 106, 9075–9080. [Google Scholar] [CrossRef]
- Caterina, M.J.; Leffler, A.; Malmberg, A.B.; Martin, W.J.; Trafton, J.; Petersen-Zeitz, K.R.; Koltzenburg, M.; Basbaum, A.I.; Julius, D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 2000, 288, 306–313. [Google Scholar] [CrossRef]
- Imamachi, N.; Goon, H.P.; Lee, H.; Anderson, D.J.; Simon, M.I.; Basbaum, A.I.; Han, S.K. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc. Natl. Acad. Sci. USA 2009, 106, 11330–11335. [Google Scholar] [CrossRef]
- Hu, Q.; Wang, Q.; Wang, C.; Tai, Y.; Liu, B.; Shao, X.; Fang, J.; Liu, B. TRPV1 Channel Contributes to the Behavioral Hypersensitivity in a Rat Model of Complex Regional Pain Syndrome Type 1. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinho-Ribeiro, F.A.; Verri, W.A.; Chiu, I.M. Nociceptor Sensory Neuron–Immune Interactions in Pain and Inflammation. Trends Immunol. 2017, 38, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Donnelly, C.R.; Jiang, C.; Liao, Y.; Luo, X.; Tao, X.; Bang, S.; McGinnis, A.; Lee, M.; Hilton, M.J.; et al. STING suppresses bone cancer pain via immune and neuronal modulation. Nat. Commun. 2021, 12, 1–21. [Google Scholar] [CrossRef]
- Prazeres, P.H.D.M.; Leonel, C.; Silva, W.N.; Rocha, B.G.S.; Santos, G.S.P.; Costa, A.C.; Picoli, C.C.; Sena, I.F.G.; Gonçalves, W.A.; Vieira, M.S.; et al. Ablation of sensory nerves favours melanoma progression. J. Cell. Mol. Med. 2020, 24, 9574–9589. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.A.C.; Silva, W.N.; Prazeres, P.H.D.M.; Picoli, C.C.; Guardia, G.D.A.; Costa, A.C.; Oliveira, M.A.; Guimarães, P.P.G.; Gonçalves, R.; Pinto, M.C.X.; et al. Chemogenetic modulation of sensory neurons reveals their regulating role in melanoma progression. Acta Neuropathol. Commun. 2021, 9, 1–41. [Google Scholar] [CrossRef]
- Fernandes, P.D.; Gomes, N.D.M.; Sirois, P. The bradykinin B1 receptor antagonist R-954 inhibits Ehrlich tumor growth in rodents. Peptides 2011, 32, 1849–1854. [Google Scholar] [CrossRef]
- Calixto-Campos, C.; Corrêa, M.P.; Carvalho, T.T.; Zarpelon, A.C.; Hohmann, M.S.N.; Rossaneis, A.C.; Coelho-Silva, L.; Pavanelli, W.R.; Pinge-Filho, P.; Crespigio, J.; et al. Quercetin reduces Ehrlich tumor-induced cancer pain in mice. Anal. Cell. Pathol. 2015, 2015, 285708. [Google Scholar] [CrossRef]
- McIntyre, A.; Harris, A.L. The role of ph regulation in cancer progression. Recent Results Cancer Res. 2016, 207, 93–134. [Google Scholar] [CrossRef]
- Kolosenko, I.; Avnet, S.; Baldini, N.; Viklund, J.; de Milito, A. Therapeutic implications of tumor interstitial acidification. Semin. Cancer Biol. 2017, 43, 119–133. [Google Scholar] [CrossRef]
- Dhaka, A.; Uzzell, V.; Dubin, A.E.; Mathur, J.; Petrus, M.; Bandell, M.; Patapoutian, A. TRPV1 Is Activated by Both Acidic and Basic pH. J. Neurosci. 2009, 29, 153. [Google Scholar] [CrossRef]
- Verri, W.A.; Cunha, T.M.; Parada, C.A.; Poole, S.; Cunha, F.Q.; Ferreira, S.H. Hypernociceptive role of cytokines and chemokines: Targets for analgesic drug development? Pharmacol. Ther. 2006, 112, 116–138. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.N.; Boorman, J.P.; Okuse, K.; Baker, M.D. Voltage-gated sodium channels and pain pathways. J. Neurobiol. 2004, 61, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Akbar, A.; Yiangou, Y.; Facer, P.; Brydon, W.G.; Walters, J.R.F.; Anand, P.; Ghosh, S. Expression of the TRPV1 receptor differs in quiescent inflammatory bowel disease with or without abdominal pain. Gut 2010, 59, 767–774. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.F.; Ching, L.C.; Kou, Y.R.; Lin, S.J.; Wei, J.; Shyue, S.K.; Lee, T.S. Activation of TRPV1 prevents OxLDL-induced lipid accumulation and TNF-α-induced inflammation in macrophages: Role of liver X receptor α. Mediat. Inflamm. 2013, 2013, 925171. [Google Scholar] [CrossRef] [PubMed]
- Xiao, T.; Sun, M.; Kang, J.; Zhao, C. Transient Receptor Potential Vanilloid1 (TRPV1) Channel Opens Sesame of T Cell Responses and T Cell-Mediated Inflammatory Diseases. Front Immunol. 2022, 13, 870952. [Google Scholar] [CrossRef]
- Joseph, J.; Qu, L.; Wang, S.; Kim, M.; Bennett, D.; Ro, J.; Caterina, M.J.; Chung, M.K. Phosphorylation of TRPV1 S801 Contributes to Modality-Specific Hyperalgesia in Mice. J. Neurosci. 2019, 39, 9954–9966. [Google Scholar] [CrossRef]
- Pinho-Ribeiro, F.A.; Hohmann, M.S.N.; Borghi, S.M.; Zarpelon, A.C.; Guazelli, C.F.S.; Manchope, M.F.; Casagrande, R.; Verri, W.A. Protective effects of the flavonoid hesperidin methyl chalcone in inflammation and pain in mice: Role of TRPV1, oxidative stress, cytokines and NF-κB. Chem. Biol. Interact. 2015, 228, 88–99. [Google Scholar] [CrossRef]
- Staurengo-Ferrari, L.; Mizokami, S.S.; Silva, J.J.; da Silva, F.O.N.; Sousa, E.H.S.; da França, L.G.; Matuoka, M.L.; Georgetti, S.R.; Baracat, M.M.; Casagrande, R.; et al. The ruthenium NO donor, [Ru(bpy)2(NO)SO3](PF6), inhibits inflammatory pain: Involvement of TRPV1 and cGMP/PKG/ATP-sensitive potassium channel signaling pathway. Pharmacol. Biochem. Behav. 2013, 105, 157–165. [Google Scholar] [CrossRef]
- Tsagareli, M.G.; Nozadze, I.; Tsiklauri, N.; Carstens, M.I.; Gurtskaia, G.; Carstens, E. Thermal Hyperalgesia and Mechanical Allodynia Elicited by Histamine and Non-histaminergic Itch Mediators: Respective Involvement of TRPV1 and TRPA1. Neuroscience 2020, 449, 35–45. [Google Scholar] [CrossRef]
- Planells-Cases, R.; Garcìa-Sanz, N.; Morenilla-Palao, C.; Ferrer-Montiel, A. Functional aspects and mechanisms of TRPV1 involvement in neurogenic inflammation that leads to thermal hyperalgesia. Pflugers Arch. 2005, 451, 151–159. [Google Scholar] [CrossRef]
- Roberts, L.A.; Connor, M. TRPV1 antagonists as a potential treatment for hyperalgesia. Recent Pat. CNS Drug Discov. 2006, 1, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Pitcher, M.H.; Price, T.J.; Entrena, J.M.; Cervero, F. Spinal NKCC1 blockade inhibits TRPV1-dependent referred allodynia. Mol. Pain 2007, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Tékus, V.; Bölcskei, K.; Kis-Varga, Á.; Dézsi, L.; Szentirmay, É.; Visegrády, A.; Horváth, C.; Szolcsányi, J.; Petho, G. Effect of transient receptor potential vanilloid 1 (TRPV1) receptor antagonist compounds SB705498, BCTC and AMG9810 in rat models of thermal hyperalgesia measured with an increasing-temperature water bath. Eur. J. Pharmacol. 2010, 641, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Uchytilova, E.; Spicarova, D.; Palecek, J. TRPV1 antagonist attenuates postoperative hypersensitivity by central and peripheral mechanisms. Mol. Pain 2014, 10, 67. [Google Scholar] [CrossRef] [PubMed]
- Warwick, C.A.; Shutov, L.P.; Shepherd, A.J.; Mohapatra, D.P.; Usachev, Y.M. Mechanisms underlying mechanical sensitization induced by complement C5a: The roles of macrophages, TRPV1, and calcitonin gene-related peptide receptors. Pain 2019, 160, 702–711. [Google Scholar] [CrossRef]
- Zylka, M.J.; Dong, X.; Southwell, A.L.; Anderson, D.J. Atypical expansion in mice of the sensory neuron-specific Mrg G protein-coupled receptor family. Proc. Natl. Acad. Sci. USA 2003, 100, 10043–10048. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Chen, S.R.; Chen, H.; Pan, H.L. The glutamatergic nature of TRPV1-expressing neurons in the spinal dorsal horn. J. Neurochem. 2009, 108, 305–318. [Google Scholar] [CrossRef]
- Ni, K.; Zhou, Y.; Sun, Y.E.; Liu, Y.; Gu, X.P.; Ma, Z.L. Intrathecal injection of selected peptide Myr-RC-13 attenuates bone cancer pain by inhibiting KIF17 and NR2B expression. Pharmacol. Biochem. Behav. 2014, 122, 228–233. [Google Scholar] [CrossRef]
- Xiaoping, G.; XiaoFang, Z.; YaGuo, Z.; Juan, Z.; JunHua, W.; ZhengLiang, M. Involvement of the spinal NMDA receptor/PKCγ signaling pathway in the development of bone cancer pain. Brain Res. 2010, 1335, 83–90. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bertozzi, M.M.; Saraiva-Santos, T.; Zaninelli, T.H.; Pinho-Ribeiro, F.A.; Fattori, V.; Staurengo-Ferrari, L.; Ferraz, C.R.; Domiciano, T.P.; Calixto-Campos, C.; Borghi, S.M.; et al. Ehrlich Tumor Induces TRPV1-Dependent Evoked and Non-Evoked Pain-like Behavior in Mice. Brain Sci. 2022, 12, 1247. https://doi.org/10.3390/brainsci12091247
Bertozzi MM, Saraiva-Santos T, Zaninelli TH, Pinho-Ribeiro FA, Fattori V, Staurengo-Ferrari L, Ferraz CR, Domiciano TP, Calixto-Campos C, Borghi SM, et al. Ehrlich Tumor Induces TRPV1-Dependent Evoked and Non-Evoked Pain-like Behavior in Mice. Brain Sciences. 2022; 12(9):1247. https://doi.org/10.3390/brainsci12091247
Chicago/Turabian StyleBertozzi, Mariana M., Telma Saraiva-Santos, Tiago H. Zaninelli, Felipe A. Pinho-Ribeiro, Victor Fattori, Larissa Staurengo-Ferrari, Camila R. Ferraz, Talita P. Domiciano, Cassia Calixto-Campos, Sergio M. Borghi, and et al. 2022. "Ehrlich Tumor Induces TRPV1-Dependent Evoked and Non-Evoked Pain-like Behavior in Mice" Brain Sciences 12, no. 9: 1247. https://doi.org/10.3390/brainsci12091247
APA StyleBertozzi, M. M., Saraiva-Santos, T., Zaninelli, T. H., Pinho-Ribeiro, F. A., Fattori, V., Staurengo-Ferrari, L., Ferraz, C. R., Domiciano, T. P., Calixto-Campos, C., Borghi, S. M., Zarpelon, A. C., Cunha, T. M., Casagrande, R., & Verri, W. A. (2022). Ehrlich Tumor Induces TRPV1-Dependent Evoked and Non-Evoked Pain-like Behavior in Mice. Brain Sciences, 12(9), 1247. https://doi.org/10.3390/brainsci12091247