Analytic Background in the Neuroscience of the Potential Project “Hippocrates”
Abstract
:1. A Need for International Cooperation for Analysis of Neurochemical Regulation of Behaviour
1.1. It’s Time to Start Sorting What We Know about Neurochemical “Soups” of Behavioural Regulation
1.2. Complexity of Neurochemical Systems Require Theories of Their Regulatory Principles beyond Excitation-Inhibition
- (1)
- “Where” the neurochemical biomarkers should be measured: the review expands the range of needed measurements to out-of-brain systems, including environmental factors, and explores the concept of Specialized Extended Phenotype.
- (2)
- “What” should be measured but is missing: the review points to the need of measurement of the “Throw & Catch” neurochemical relays; behavioural and neuronal events contributing to the consistency of the CBPs but not documented in measurements.
- (3)
- Structuring the setup: the review briefly describes a proposed earlier neurochemical framework that accommodates the neurochemical continuum between temperament and symptoms of psychiatric disorders. This framework is in line with documented “Throw & Catch” neurochemical relays and can also be used to organize data about the personal and professional history of an individual.
2. Introduction to Constructivism
2.1. Bernstein: “Repetition without Repetition” and Constructivism
2.2. Neurotransmission under a Magnifying Glass: Between Compositions and Decompositions
2.3. “Multiplicity of Candidates” Supports “Repetition without Repetition” at Many Levels of Behavioural Regulation but Leads to the Degrees of Freedom Problem
2.4. Anticipatory Neurodynamics Illustrates the Constructivism Paradigm
3. Where to Measure: Not Just in the Brain
3.1. Back to Hippocrates?
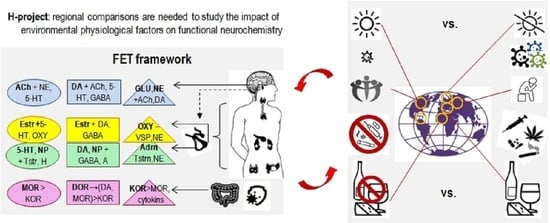
3.2. Back to Empedocles? Regional Contrasts in Environmental Factors Relevant to the H-Project
3.3. T&C Specialized Extended Phenotypes (Behavioural Bubbles) as a Useful Concept for CBP Research
4. What to Measure: The Chain of Construction or Its Isolated Spots?
4.1. Cycles, Run-Aways and Start-Ups Processes in Neurophysiology and Behaviour
- continuing generation of diverse run-aways most of which are not compatible with current cycles;
- continuing review of compatibility between generated and (ever-changing) needed DFs, tuned to the needs and capacities of the individual;
- continuing selection that supports existing cycles and protects them from being taken apart by the variance coming from run-aways.
4.2. “Throw & Catch” Relays Highlight Pro-Active and Constructive Nature of Neuronal Regulation of Behaviour
- -
- orientational, via cholinergic cortical-basal forebrain projections and interactions between ACh and NE at that level;
- -
- integrational, via cholinergic interneurons regulating striatal DA-GABA networks; habit-formation, via the PPN-dLTA, nuclei, and
- -
- action maintenance (i.e., energetic) aspects via collaboration between ACh and 5-HT systems;
- -
- coordination of automatic selection of DFs by the cerebellum to the pANS level, under the close supervision of the ACh forebrain and lateral hypothalamic systems.
- -
- at the Autonomic Nervous System (ANS) level, the NE-based sympathetic ANS provides a fast and non-specific massive arousal (Throw), whereas the ACh-basedparasympathetic pANS acts selectively and is much more structured in its action. The pANS trims the DFs at the ANS levelcontrols the sANS activation of specific somatic functions. Such trimming of DFs, even at the very low level of behavioural regulation (including selective muscle contraction) provides precise control over locomotion [139].
5. Structuring the Setup: A 12-Component Framework of Universal Aspects of Constructive Processes
5.1. A Neurochemical Framework Functional Ensemble of Temperament Uses a Constructivism-Based Classification
- -
- within-body cycles (“physical”), regulated by 5-HT, NPs and gut microbiota systems, with a prominent role of hypothalamic—anterior pituitary peptides and hormones such as Somatostatin, Growth Hormone and the status of the thyroid system. These cycles also include several levels of sub-cycles for the maintenance of neuronal activities, for example, in the form of neuropils [75,149]. Neuropils are known as glia-neuronal complexes taking spaces between cell bodies and including dendrites, axons, synapses, and microvasculature. Since we discuss here primarily the “managerial” neurochemical systems, which impact emerges at the behavioural level, there is no space here to discuss the numerous proteins and enzymes regulating the neuropils complexes at the cellular level;
- -
- cycles that include interactions with other bodies (peers, offspring, prey and predators) (“social”) regulated by other hypothalamic-pituitary systems (prolactin) and the “social” hormones oxytocin and vasopressin released from the posterior pituitary. Behavioural regulation at this level goes much beyond the communicative functions as involvement in these cycles allows sharing material (such as closing, housing, food sources, transport) and informational (knowledge, motivations, attitudes) infrastructures;
- -
- cycles that include the tuning of behaviour to more extensive infrastructures that might not be immediately present, including probabilistic features of reality (“probabilistic”). To ensure the maintenance of probabilistic activities, many structures and chemical systems of the brain get involved, with glial cells playing a major role.
- -
- immediate integration, triggered by environmental stimuli without plans of actions and mainly regulated by the autonomic nervous system with very limited involvement of orientation systems (known as impulsive, premature initiation of actions).
- -
- automatic integration of actions and cognition with a developed program that sequences behavioural elements and
- -
- novel integration of behaviour when a new program (choice and sequencing of actions) is required—common in complex and uncertain situations.
5.2. Possible Neurochemical and Genetic Investigations within the H-Project Should Target the T&C “Relays”
6. Conclusions
- (1)
- Where to measure: Investigations of neurochemical biomarkers of CBPs should be the main aim of the H-project. However, this review pointed out that the biomarkers of the CBPs are not just in the brain (that is the main focus of the Connectome-like projects) but also in endocrine and microbiome systems. There should be, therefore (complementary to neurochemical investigations of T&C relays), regional comparisons of the environmental factors [2] (such as stresses, diets, exposure to common psychostimulants and toxins) influencing these systems. Moreover, the review underlined the importance of tracing Specialized Extended Phenotypes associated with specific CBPs.SEP relates to the environmental establishments created or reinforced by people during their ontogenesis based on their bio-behavioural regulatory preferences and their CBPs involving these establishments. Then, in turn, these individualized social, physical and informational infrastructures (such as social relations including pets, people’s IDs, professional history, and outcomes of their physical actions) regulate people’s everyday life and, therefore, associated CBPs.
- (2)
- What to measure: many current projects inherit a reactivity paradigm known in behaviourism. In such a reactivity paradigm, experimental conditions are considered to be the leading factors of the differences in neuronal and behavioural variations: experimental events are induced, and the brain reacts as if there was no brain activity before these events. In contrast to that, constructivism points to the pro-active nature of CBP biomarkers seen in anticipatory neurodynamics described by Walter Freeman, neuroendocrinal regulation and in the Throw&Catch phenomena, largely unrelated to the current events. The T&C, seen at the multiple levels of neuronal and neurochemical regulation, self-generates an excessive variance in some neuronal subsystems, to be pruned by other subsystems in a relay manner. At each stage of the T&C, a selection of DFs becomes tighter, with specific Throw and Catch subsystems for each stage. Measurements, therefore, should trace key aspects and stages of behavioural construction:
- In the H-project, brain neurochemstry studies should measure not only NT relays but also endocrine variables indicative of the individual’s needs and capacities.
- The positioning of NT-releasing sites and their receptors is neither even nor random in the brain. Instead, it follows the constructivism trend, with the Throw&Catch relays aimed to highlight the most relevant and suppress irrelevant DFs in behaviour. The investigations of neurochemical systems, therefore, could be organized in a more systematic manner, following the relays between the NT release and binding sites according to the verifiable constructivist hypotheses about these relays. Such targeted measurements of these sites tracing several relays can be more informative than the current focus on one single site for the NT release or receptors density. These neurochemical investigations should mind regional comparisons of the environmental factors influencing the CBPs [2] (as noted above).
- This review suggested measuring behavioural and neuronal events that support CBPs but by themselves are not consistent: “start-ups” (initiated activities) and “run-aways” (incompleted activities). For example, current studies of CBP biomarkers identify CBPs mostly using self-reports, including clinical interviews and questionnaires asking about the most frequent and consistent events/experiences. This review pointed out the potential informational value of the structured analysis of the personal and professional, indicative of background and transient processes that led to the consistent CBPs. At the neuronal level, studies in neuroscience often focus on the most visible neuronal “hardware” (brain connectivity, activation of brain regions) within an individual’s nervous system and trace their associations with CBPs. Here, we suggest conducting not the “hardware” comparisons but the comparisons of the components of the neurochemical T&C relays involved in the construction of actions for individuals with different SEP and CBPs.
- (3)
- Structuring the setup: The outcomes of many current projects are often presented as connectivity maps listing excitatory-inhibitory associations. Since these associations are numerous, researchers often face a “big data problem”, not knowing how to make sense of it and counting on blind statistical software (such as factor analysis or data-mining [1]) to help with new useful insights. Theory-based hypotheses often help to increase the efficiency of data collection and analysis, and the principle of Functional Constructivism offers a set of such hypotheses. This principle points to the universality of dynamical features and stages of behavioural construction and suggests using these stages as the structural design for the outcomes of the H-project. Based on this principle, the neurochemical framework Functional Ensemble of Temperament highlights the correspondence between the functionality of families of neurochemical systems and 12 universal aspects of behavioural regulation. These aspects relate to orientation, integration of behaviour and maintenance of specific cycles of individual survival assessed separately for physical (body), social (other bodies) and probabilistic aspects of behaviour. Three other FET components relate to dispositional emotionality and the HPA-driven integration of behaviour(Figure 3). The FET is a conservative general summary of functional specialization within neurotransmitter systems; however, much more work is ahead to complement this summary with the details on receptor functionality within each of these systems. The FET offers verifiable hypotheses about the neurochemical T&C relays [8]). Moreover, the FET structure can be used in experimental studies of environmental “bubbles” (Specialized Extended Phenotypes) that support an individual’s CBPs. [7])
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trofimova, I.; Robbins, T.W.; Sulis, W.H.; Uher, J. Taxonomies of psychological individual differences: Biological perspectives on millennia-long challenges. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trofimova, I.; Bajaj, S.; Bashkatov, S.A.; Blair, J.; Brandt, A.; Chan, R.C.; Clemens, B.; Corr, P.J.; Cyniak-Cieciura, M.; Demidova, L.; et al. What is next for the neurobiology of temperament, personality and psychopathology? Curr. Opin. Behav. Sci. 2022, 45, 101143. [Google Scholar] [CrossRef]
- Trofimova, I.; Sulis, W. Benefits of Distinguishing between Physical and Social-Verbal Aspects of Behavior: An Example of Generalized Anxiety. Front. Psychol. 2016, 7, 338. [Google Scholar] [CrossRef] [Green Version]
- Trofimova, I.N.; Sulis, W. A Study of the Coupling of FET Temperament Traits with Major Depression. Front. Psychol. 2016, 7, 1848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulis, W. Assessing the continuum between temperament and affective illness: Psychiatric and mathematical perspectives. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170168. [Google Scholar] [CrossRef] [Green Version]
- Trofimova, I.; Sulis, W. There is more to mental illness than negative affect: Comprehensive temperament profiles in depression and generalized anxiety. BMC Psychiatry 2018, 18, 125. [Google Scholar] [CrossRef] [Green Version]
- Trofimova, I. Functional constructivism approach to multilevel nature of biobehavioural diversity. Front. Psychiatry 2021, 12, 641286. [Google Scholar] [CrossRef]
- Trofimova, I. Contingent Tunes of Neurochemical Ensembles in the Norm and Pathology: Can We See the Patterns? Neuropsychobiology 2021, 80, 101–133. [Google Scholar] [CrossRef]
- Wikipedia, List of Neuroscience Databases. 2022. Available online: https://en.wikipedia.org/wiki/List_of_neuroscience_databases (accessed on 20 December 2022).
- Nieuwenhuys, R. Chemoarchitecture of the Brain; Springer: Berlin, Germany, 1985; 246p. [Google Scholar]
- Robbins, T. Arousal systems and attentional processes. Biol. Psychol. 1997, 45, 57–71. [Google Scholar] [CrossRef]
- Robbins, T.W. 5.1 From Behavior to Cognition: Functions of Mesostriatal, Mesolimbic, and Mesocortical Dopamine Systems. In Dopamine Handbook; Oxford Academic Press: Oxford, UK, 2009; pp. 203–214. [Google Scholar] [CrossRef]
- Robbins, T.W.; Dalley, J.W. Dissecting Impulsivity: Brain Mechanisms and Neuropsychiatric Implications. Nebr. Symp. Motiv. 2017, 64, 201–226. [Google Scholar] [CrossRef]
- Trofimova, I. The interlocking between functional aspects of activities and a neurochemical model of adult temperament. In Temperaments: Individual Differences, Social and Environmental Influences and Impact on Quality of Life; Arnold, M.C., Ed.; Nova Science Publishers: New York, NY, USA, 2016; pp. 77–147. [Google Scholar]
- Trofimova, I.; Robbins, T.W. Temperament and arousal systems: A new synthesis of differential psychology and functional neurochemistry. Neurosci. Biobehav. Rev. 2016, 64, 382–402. [Google Scholar] [CrossRef] [PubMed]
- Trofimova, I. Functionality versus dimensionality in psychological taxonomies, and a puzzle of emotional valence. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20170167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trofimova, I. An overlap between mental abilities and temperament traits. In General and Specific Mental Abilities; McFarland, D., Ed.; Cambridge Scholars Publishing: Cambridge, UK, 2019; pp. 77–114. [Google Scholar]
- Trofimova, I.N.; Gaykalova, A.A. Emotionality vs. Other Biobehavioural Traits: A Look at Neurochemical Biomarkers for Their Differentiation. Front. Psychol. 2021, 12, 781631. [Google Scholar] [CrossRef] [PubMed]
- Trofimova, I. Transient nature of stable behavioural patterns, and how we can respect it. Curr. Opin. Behav. Sci. 2022, 44, 101109. [Google Scholar] [CrossRef]
- Coplan, J.D.; Pathangi, V.; Kim, Y. Treating comorbid anxiety and depression: Psychosocial and pharmacological approaches. World J. Pschiatry 2015, 5, 366–378. [Google Scholar] [CrossRef]
- Wittmann, W.; Schunk, E.; Rosskothen, I.; Gaburro, S.; Singewald, N.; Herzog, H.; Schwarzer, C. Prodynorphin-Derived Peptides Are Critical Modulators of Anxiety and Regulate Neurochemistry and Corticosterone. Neuropsychopharmacology 2008, 34, 775–785. [Google Scholar] [CrossRef] [Green Version]
- Lange, K.H.; Juul, A.; Rasmussen, M.H.; Bülow, J.; Kjaer, M. Growth hormone enhances effects of endurance training on oxidative muscle metabolism in elderly women. Am. J. Physiol. Endocrinol. Metab. 2000, 279, E989-96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clarke, H.F.; Walker, S.C.; Dalley, J.; Robbins, T.; Roberts, A. Cognitive Inflexibility after Prefrontal Serotonin Depletion Is Behaviorally and Neurochemically Specific. Cereb. Cortex 2006, 17, 18–27. [Google Scholar] [CrossRef]
- Kehagia, A.; Murray, G.K.; Robbins, T.W. Learning and cognitive flexibility: Frontostriatal function and monoaminergic modulation. Curr. Opin. Neurobiol. 2010, 20, 199–204. [Google Scholar] [CrossRef]
- Bari, A.; Robbins, T.W. Inhibition and impulsivity: Behavioral and neural basis of response control. Prog. Neurobiol. 2013, 108, 44–79. [Google Scholar] [CrossRef]
- Dalley, J.W.; Everitt, B.J.; Robbins, T.W. Impulsivity, Compulsivity, and Top-Down Cognitive Control. Neuron 2011, 69, 680–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyazaki, K.; Miyazaki, K.W.; Doya, K. The role of serotonin in the regulation of patience and impulsivity. Mol. Neurobiol. 2012, 45, 213–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruchas, M.; Land, B.; Chavkin, C. The dynorphin/kappa opioid system as a modulator of stress-induced and pro-addictive behaviors. Brain Res. 2010, 1314, 44–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donaldson, Z.R.; Young, L.J. Oxytocin, Vasopressin, and the Neurogenetics of Sociality. Science 2008, 322, 900–904. [Google Scholar] [CrossRef] [Green Version]
- Barraza, J.A.; Zak, P.J. Empathy toward Strangers Triggers Oxytocin Release and Subsequent Generosity. Ann. N. Y. Acad. Sci. 2009, 1167, 182–189. [Google Scholar] [CrossRef]
- Coyle, J.; Konopask, G. The Neurochemistry of Schizophrenia. Basic Neurochemistry, 8th ed.; Brady, S.T., Siegel, G.J., Wayne Albers, R., Price, D.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1000–1011. [Google Scholar]
- Shabani, S.; Dehghani, M.; Hedayati, M.; Rezaei, O. Relationship of serum serotonin and salivary cortisol with sensation seeking. Int. J. Psychophysiol. 2011, 81, 225–229. [Google Scholar] [CrossRef]
- Zuckerman, M. Sensation Seeking (Psychology Revivals): Beyond the Optimal Level of Arousal; Psychology Press: London, UK, 2014. [Google Scholar] [CrossRef]
- Mesulam, M.; Larry, R. Acetylcholine neurotransmission in CNS. In Encyclopedia of Neuroscience; Elsevier Ltd.: Amsterdam, The Netherlands, 2009; pp. 1–4. [Google Scholar]
- Robbins, T.W.; Roberts, A. Differential Regulation of Fronto-Executive Function by the Monoamines and Acetylcholine. Cereb. Cortex 2007, 17, i151–i160. [Google Scholar] [CrossRef]
- Siegel, G.J.; Albers, R.W.; Brady, S.T.; Price, D.L. Basic Neurochemistry; Siegel, G., Albers, R.W., Brady, S.T., Price, D.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Borroto-Escuela, D.O.; Perez De La Mora, M.; Manger, P.; Narváez, M.; Beggiato, S.; Crespo-Ramírez, M.; Navarro, G.; Wydra, K.; Díaz-Cabiale, Z.; Rivera, A.; et al. Brain Dopamine Transmission in Health and Parkinson’s Disease: Modulation of Synaptic Transmission and Plasticity Through Volume Transmission and Dopamine Heteroreceptors. Front Synaptic Neurosci. 2018, 10, 20. [Google Scholar] [CrossRef]
- Fuxe, K.; Borroto-Escuela, D. Volume transmission and receptor-receptor interactions in heteroreceptor complexes: Understanding the role of new concepts for brain communication. Neural Regen. Res. 2016, 11, 1220–1223. [Google Scholar] [CrossRef]
- Fuxe, K.; Dahlström, A.B.; Jonsson, G.; Marcellino, D.; Guescini, M.; Dam, M.; Manger, P.; Agnati, L. The discovery of central monoamine neurons gave volume transmission to the wired brain. Prog. Neurobiol. 2010, 90, 82–100. [Google Scholar] [CrossRef] [PubMed]
- Agnati, L.F.; Guidolin, D.; Guescini, M.; Genedani, S.; Fuxe, K. Understanding wiring and volume transmission. Brain Res. Rev. 2010, 64, 137–159. [Google Scholar] [CrossRef] [PubMed]
- Descarries, L.; Mechawar, N. Ultrastructural evidence for diffuse transmission by monoamine and acetylcholine neurons of the central nervous system. Prog. Brain Res. 2000, 125, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Sarter, M.; Parikh, V.; Howe, W.M. Phasic acetylcholine release and the volume transmission hypothesis: Time to move on. Nat. Rev. Neurosci. 2009, 10, 383–390. [Google Scholar] [CrossRef] [Green Version]
- Bernstein, N. O Postroenii Dvijeniy [On the Construction of Motions]; Gosizdat, Medic-State Publish House: Moscow, Russia, 1947. [Google Scholar]
- Bernstein, N.A.; Latash, M.L.; Turvey, M. Dexterity and Its Development; Taylor & Francis: New York, NY, USA, 1996. [Google Scholar]
- Hayashibe, M.; Shimoda, S. Synergetic motor control paradigm for optimizing energy efficiency of multijoint reaching via tacit learning. Front. Comput. Neurosci. 2014, 8, 21. [Google Scholar] [CrossRef] [Green Version]
- Bernstein, N. The Co-Ordination and Regulation of Movements; Pergamon Press: New York, NY, USA, 1967. [Google Scholar]
- Vargas, K.J.; Terunuma, M.; Tello, J.A.; Pangalos, M.N.; Moss, S.J.; Couve, A. The availability of surface GABA B receptors is independent of gamma-aminobutyric acid but controlled by glutamate in central neurons. J. Biol. Chem. 2008, 283, 24641–24648. [Google Scholar] [CrossRef] [Green Version]
- Bartlett, F.C. Reprinted from The British Journal of Psychology (1925), 16, 16-27: Feeling, imaging and thinking. Br. J. Psychol. 2009, 100 Pt 1A, 189–198. [Google Scholar] [CrossRef]
- Trofimova, I. Functional Constructivism: In Search of Formal Descriptors. Nonlinear Dyn. Psychol. Life Sci. 2017, 21, 441–474. [Google Scholar]
- Hebb, D.O. Essays on Mind; Psychology Press: New York, NY, USA, 2014. [Google Scholar]
- Quartz, S.R.; Sejnowski, T.J. The neural basis of cognitive development: A constructivist manifesto. Behav. Brain Sci. 1997, 20, 537–556. [Google Scholar] [CrossRef] [Green Version]
- Alexandrov, Y.I.; Sozinov, A.A.; Svarnik, O.E.; Gorkin, A.G.; Kuzina, E.A.; Gavrilov, V.V. Neuronal Bases of Systemic Organization of Behavior. Adv. Neurobiol. 2018, 21, 1–33. [Google Scholar] [CrossRef]
- Waldhoer, M.B.; Whistler, J.E. Opioid receptors. Annu. Rev. Biochem. 2014, 73, 953–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.; Gullapalli, S.; Pan, H.; Ramos-Ortolaza, D.L.; Hayward, M.D.; Low, M.J.; Pintar, J.E.; Devi, L.A.; Gomes, I. Regulation of Opioid Receptors by Their Endogenous Opioid Peptides. Cell. Mol. Neurobiol. 2021, 41, 1103–1118. [Google Scholar] [CrossRef] [PubMed]
- Mains, R.E.; Eipper, B.A. Peptides. In Basic Neurochemistry, 7th ed.; Siegel, G.J., Albers, R.W., Brady, S.T., Price, D.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Freeman, W.J. Mesoscopic neurodynamics: From neuron to brain. J. Physiol. 2000, 94, 303–322. [Google Scholar] [CrossRef] [PubMed]
- Altman, J.M. Evolving Brains. Scientific American Library, 1040-3213; Scientific American Library: New York, NY, USA, 1999. [Google Scholar]
- Liley, D.T.J.; Foster, B.L.; Bojak, I. Co-operative Populations of Neurons: Mean Field Models of Mesoscopic Brain Activity. In Computational Systems Neurobiology; Springer: Dordrecht, The Netherlands, 2012; pp. 317–364. [Google Scholar] [CrossRef]
- Freeman, W.J. Neurodynamics: An Exploration in Mesoscopic Brain Dynamics; Springer: Berlin, Germany, 2000. [Google Scholar]
- Arbib, M.A.; Erdi, P. Precis of Neural organization: Structure, function, and dynamics. Behav. Brain Sci. 2000, 23, 513–533; discussion 533–571. [Google Scholar] [CrossRef] [PubMed]
- Treisman, A. The psychological reality of levels of processing. In Levels of Processing in Human Memory; Psychology Press: New York, NY, USA, 2014; pp. 301–330. [Google Scholar]
- Jog, M.S.; Kubota, Y.; Connolly, C.I.; Hillegaart, V.; Graybiel, A.M. Building Neural Representations of Habits. Science 1999, 286, 1745–1749. [Google Scholar] [CrossRef] [Green Version]
- Blazquez, P.M.; Fujii, N.; Kojima, J.; Graybiel, A.M. A Network Representation of Response Probability in the Striatum. Neuron 2002, 33, 973–982. [Google Scholar] [CrossRef] [Green Version]
- Everitt, B.J.; Robbins, T.W. From the ventral to the dorsal striatum: Devolving views of their roles in drug addiction. Neurosci. Biobehav. Rev. 2013, 37, 1946–1954. [Google Scholar] [CrossRef] [Green Version]
- Ikemoto, S. Dopamine reward circuitry: Two projection systems from the ventral midbrain to the nucleus accumbens–olfactory tubercle complex. Brain Res. Rev. 2007, 56, 27–78. [Google Scholar] [CrossRef] [Green Version]
- Graybiel, A.M. The basal ganglia: Learning new tricks and loving it. Curr. Opin. Neurobiol. 2005, 15, 638–644. [Google Scholar] [CrossRef]
- Smith, K.S.; Graybiel, A.M. Habit formation coincides with shifts in reinforcement representations in the sensorimotor striatum. J. Neurophysiol. 2016, 115, 1487–1498. [Google Scholar] [CrossRef] [Green Version]
- Graybiel, A.M. The Striatum and Decision-Making Based on Value, in Micro-, Meso- and Macro-Dynamics of the Brain; Buzsaki, G., Christen, Y., Eds.; Springer: Cham, Switzerland, 2016; pp. 81–84. [Google Scholar]
- Friston, K.; Buzsáki, G. The Functional Anatomy of Time: What and When in the Brain. Trends Cogn. Sci. 2016, 20, 500–511. [Google Scholar] [CrossRef] [PubMed]
- Cieri, F.; Zhuang, X.; Caldwell, J.Z.K.; Cordes, D. Brain Entropy During Aging Through a Free Energy Principle Approach. Front. Hum. Neurosci. 2021, 15, 647513. [Google Scholar] [CrossRef] [PubMed]
- Sulis, W. Fundamental Concepts of Collective Intelligence. Nonlinear Dyn. Psychol. Life Sci. 1997, 1, 35–53. [Google Scholar] [CrossRef]
- Sulis, W. Stochastic phase decoupling in dynamical networks. Nonlinear Dyn. Psychol. Life Sci. 2008, 12, 327–358. [Google Scholar]
- Sulis, W. Collective Intelligence: Observations and Models; Cambridge University Press: Cambridge, UK, 2008; pp. 41–72. [Google Scholar] [CrossRef]
- Gallant, S. Perceptron-based learning algorithms. IEEE Trans. Neural Netw. 1990, 1, 179–191. [Google Scholar] [CrossRef]
- Freeman, W.J. How Brains Make Up Their Minds; Columbia University Press: New York, NY, USA, 2001; p. 146. [Google Scholar]
- Holt, C.E.; Martin, K.C.; Schuman, E.M. Local translation in neurons: Visualization and function. Nat. Struct. Mol. Biol. 2019, 26, 557–566. [Google Scholar] [CrossRef]
- Freeman, W.J. Chaotic Oscillaions and the Genesis of Meaning in Cerebral Cortex. In Nonlinear Dynamics in Life and Social Sciences; Sulis, W., Trofimova, I., Eds.; IOS Press: Amsterdam, The Netherlands, 2001; pp. 44–62. [Google Scholar]
- Freeman, W.J.; Vitiello, G. Nonlinear brain dynamics as macroscopic manifestation of underlying many-body field dynamics. Phys. Life Rev. 2006, 3, 93–118. [Google Scholar] [CrossRef] [Green Version]
- Freeman, W.J. The place of ‘codes’ in nonlinear neurodynamics. Prog. Brain Res. 2007, 165, 447–462. [Google Scholar]
- Neisser, U. Anticipations, images, and introspection. Cognition 1978, 6, 169–174. [Google Scholar] [CrossRef]
- Ho, P.; Ross, D.A. More Than a Gut Feeling: The Implications of the Gut Microbiota in Psychiatry. Biol. Psychiatry 2017, 81, e35–e37. [Google Scholar] [CrossRef]
- Liu, L.; Zhu, G. Gut-Brain Axis and Mood Disorder. Front Psychiatry 2018, 9, 223. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Brietzke, E.; Rosenblat, J.D.; Musial, N.; Zuckerman, H.; Ragguett, R.M.; Pan, Z.; Rong, C.; Fus, D.; McIntyre, R.S. Probiotics for the treatment of depressive symptoms: An anti-inflammatory mechanism? Brain Behav. Immun. 2018, 73, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Sumich, A.; Heym, N.; Lenzoni, S.; Hunter, K. Gut microbiome-brain axis and inflammation in temperament, personality and psychopathology. Curr. Opin. Behav. Sci. 2022, 44, 101101. [Google Scholar] [CrossRef]
- Shabbir, F.; Patel, A.; Mattison, C.; Bose, S.; Krishnamohan, R.; Sweeney, E.; Sandhu, S.; Nel, W.; Rais, A.; Sandhu, R.; et al. Effect of diet on serotonergic neurotransmission in depression. Neurochem. Int. 2013, 62, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Reuter, M.; Zamoscik, V.; Plieger, T.; Bravo, R.; Ugartemendia, L.; Rodriguez, A.B.; Kirsch, P. Tryptophan-rich diet is negatively associated with depression and positively linked to social cognition. Nutr. Res. 2020, 85, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Pilšáková, L.; Riečanský, I.; Jagla, F. The physiological actions of isoflavone phytoestrogens. Physiol. Res. 2010, 59, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Craig, M.C.; Fletcher, P.C.; Daly, E.M.; Rymer, J.; Brammer, M.; Giampietro, V.; Murphy, D.G. Physiological variation in estradiol and brain function: A functional magnetic resonance imaging study of verbal memory across the follicular phase of the menstrual cycle. Horm. Behav. 2008, 53, 503–508. [Google Scholar] [CrossRef]
- Craig, M.C.; Murphy, D. Estrogen: Effects on normal brain function and neuropsychiatric disorders. Climacteric 2007, 10, 97–104. [Google Scholar] [CrossRef]
- Cui, Y.; Huang, C.; Momma, H.; Niu, K.; Nagatomi, R. Daily dietary isoflavone intake in relation to lowered risk of depressive symptoms among men. J. Affect. Disord. 2019, 261, 121–125. [Google Scholar] [CrossRef]
- Guo, F.; Huang, C.; Cui, Y.; Momma, H.; Niu, K.; Nagatomi, R. Dietary seaweed intake and depressive symptoms in Japanese adults: A prospective cohort study. Nutr. J. 2019, 18, 1–8. [Google Scholar] [CrossRef]
- Dong, J.-Y.; Kimura, T.; Ikehara, S.; Cui, M.; Kawanishi, Y.; Yamagishi, K.; Ueda, K.; Iso, H. The Japan Environment and Children’s Study Group Chocolate consumption and risk of gestational diabetes mellitus: The Japan Environment and Children’s Study. Br. J. Nutr. 2019, 122, 936–941. [Google Scholar] [CrossRef] [PubMed]
- Richards, G.; Smith, A. Caffeine consumption and self-assessed stress, anxiety, and depression in secondary school children. J. Psychopharmacol. 2015, 29, 1236–1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, L.M.; Pipingas, A.; White, D.J.; Gauci, S.; Scholey, A. A Systematic Review and Meta-Analysis of B Vitamin Supplementation on Depressive Symptoms, Anxiety, and Stress: Effects on Healthy and ‘At-Risk’ Individuals. Nutrients 2019, 11, 2232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diggins, K. The Power of Physical Touch. J. Christ. Nurs. 2009, 26, 119. [Google Scholar] [CrossRef] [PubMed]
- Jakubiak, B.K.; Feeney, B.C. Affectionate Touch to Promote Relational, Psychological, and Physical Well-Being in Adulthood: A Theoretical Model and Review of the Research. Pers. Soc. Psychol. Rev. 2016, 21, 228–252. [Google Scholar] [CrossRef] [PubMed]
- Kanitz, E.; Hameister, T.; Tuchscherer, A.; Tuchscherer, M.; Puppe, B. Social Support Modulates Stress-Related Gene Expression in Various Brain Regions of Piglets. Front. Behav. Neurosci. 2016, 10, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuler, J.; Haufler, A.; Ditzen, B. Social Support as a Stress Buffer or Stress Amplifier and the Moderating Role of Implicit Motives: Protocol for a Randomized Study. JMIR Res. Protoc. 2022, 11, e39509. [Google Scholar]
- Bordon, Y. Neuroimmunology: Social support from the immune system. Nat. Rev. Neurosci. 2016, 17, 534–535. [Google Scholar] [CrossRef]
- Samuels, L.T. Body adaptation to change in diet. J. Am. Diet. Assoc. 1946, 22, 843–848. [Google Scholar] [CrossRef]
- Tost, H.; Reichert, M.; Braun, U.; Reinhard, I.; Peters, R.; Lautenbach, S.; Hoell, A.; Schwarz, E.; Ebner-Priemer, U.; Zipf, A.; et al. Neural correlates of individual differences in affective benefit of real-life urban green space exposure. Nat. Neurosci. 2019, 22, 1389–1393. [Google Scholar] [CrossRef]
- Bratman, G.N.; Anderson, C.B.; Berman, M.G.; Cochran, B.; de Vries, S.; Flanders, J.; Folke, C.; Frumkin, H.; Gross, J.J.; Hartig, T.; et al. Nature and mental health: An ecosystem service perspective. Sci. Adv. 2019, 5, eaax0903. [Google Scholar] [CrossRef] [PubMed]
- Wulan, S.N.; Bouwman, F.G.; Westerterp, K.R.; Mariman, E.C.M.; Plasqui, G. Molecular adaptation in adipose tissue in response to overfeeding with a high-fat diet under sedentary conditions in South Asian and Caucasian men. Br. J. Nutr. 2019, 122, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Serino, M.; Luche, E.; Gres, S.; Baylac, A.; Bergé, M.; Cenac, C.; Waget, A.; Klopp, P.; Iacovoni, J.; Klopp, C.; et al. Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut 2011, 61, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Cahill, L. Why sex matters for neuroscience. Nat. Rev. Neurosci. 2006, 7, 477–484. [Google Scholar] [CrossRef]
- Damasio, A.; Dolan, R.J. The feeling of what happens. Nature 1999, 401, 847. [Google Scholar]
- Wilson, E.O. Ecology of ants and termites. Science 1978, 201, 337. [Google Scholar] [CrossRef]
- Lobo, L.; Heras-Escribano, M.; Travieso, D. The History and Philosophy of Ecological Psychology. Front. Psychol. 2018, 9, 2228. [Google Scholar] [CrossRef] [Green Version]
- Trofimova, I. Phenomena of functional differentiation and fractal functionality. Int. J. Des. Nat. Ecodynamics 2016, 11, 508–521. [Google Scholar] [CrossRef]
- Spichak, S.; Bastiaanssen, T.F.; Berding, K.; Vlckova, K.; Clarke, G.; Dinan, T.G.; Cryan, J.F. Mining microbes for mental health: Determining the role of microbial metabolic pathways in human brain health and disease. Neurosci. Biobehav. Rev. 2021, 125, 698–761. [Google Scholar] [CrossRef]
- Emambokus, N.; Granger, A.; Mott, R.; Helenius, T. The Immunometabolism Choreography. Cell Metab. 2017, 26, 1. [Google Scholar] [CrossRef]
- Piccio, L.; Wu, G.F.; Klein, R.S. A new era in neuroimmunology. J. Neuroimmunol. 2021, 351, 577478. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, G.; Subramaniam, S.; Caldwell, L.J.; Fitzgerald, D.; Harrison, N.; Hong, S.; Irani, S.R.; Khandaker, G.M.; Liston, A.; Miron, V.; et al. Research priorities for neuroimmunology: Identifying the key research questions to be addressed by 2030. Wellcome Open Res. 2021, 6, 194. [Google Scholar] [CrossRef]
- Taquet, M.; Geddes, J.R.; Husain, M.; Luciano, S.; Harrison, P.J. 6-month neurological and psychiatric outcomes in 236 379 survivors of COVID-19: A retrospective cohort study using electronic health records. Lancet Psychiatry 2021, 8, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Decety, J.; Smith, K.E.; Norman, G.J.; Halpern, J. A social neuroscience perspective on clinical empathy. World Psychiatry 2014, 13, 233–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunn, A.J.; Ando, T.; Brown, R.F.; Berg, R.D. HPA axis activation and neurochemical responses to bacterial translocation from the gastrointestinal tract. Ann. N. Y. Acad. Sci. 2003, 992, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Deats, S.; Adidharma, W.; Lonstein, J.; Yan, L. Attenuated orexinergic signaling underlies depression-like responses induced by daytime light deficiency. Neuroscience 2014, 272, 252–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthmainah, M.; Gogos, A.; Sumithran, P.; Brown, R.M. Orexins (hypocretins): The intersection between homeostatic and hedonic feeding. J. Neurochem. 2021, 157, 1473–1494. [Google Scholar] [CrossRef] [PubMed]
- Whiting, H.T.A. Human Motor Actions. Bernstein Reassessed. Advances in Psychology; Elsevier Science Publishers: Amsterdam, The Netherlands, 1991; Volume 17. [Google Scholar]
- Agnati, L.F.; Leo, G.; Zanardi, A.; Genedani, S.; Rivera, A.; Fuxe, K.; Guidolin, D. Volume transmission and wiring transmission from cellular to molecular networks: History and perspectives. Acta Physiol. 2006, 187, 329–344. [Google Scholar] [CrossRef]
- Guidolin, D.; Fuxe, K.; Neri, G.; Nussdorfer, G.G.; Agnati, L.F. On the role of receptor–receptor interactions and volume transmission in learning and memory. Brain Res. Rev. 2007, 55, 119–133. [Google Scholar] [CrossRef]
- LeDoux, J. The Emotional Brain: The Mysterious Underpinnings of Emotional Life; Simon and Schuster: New York, NY, USA, 1998. [Google Scholar]
- Zeng, Y.; Tao, F.; Cui, Z.; Wu, L.; Xu, J.; Dong, W.; Liu, C.; Yang, Z.; Qin, S. Dynamic integration and segregation of amygdala subregional functional circuits linking to physiological arousal. NeuroImage 2021, 238, 118224. [Google Scholar] [CrossRef]
- Khrenov, A.I.; Fedorov, A.A.; Sirotkin, V.N.; Zaraiskaya, I.Y.; Leontovich, T.A. Two types of projection neurons in human striatum: Peculiarities of their somatodendritic structure in ventral and dorsal striatum. Bull. Exp. Biol. Med. 2006, 141, 657–661. [Google Scholar] [CrossRef]
- Cairns-Smith, A.G. Genetic Takeover: And the Mineral Origins of Life; Cambridge University Press: Cambridge, UK, 1982. [Google Scholar]
- Trofimova, I. Do Psychological Sex Differences Reflect Evolutionary Bisexual Partitioning? Am. J. Psychol. 2015, 128, 485–514. [Google Scholar] [CrossRef]
- Hampe, C.S.; Mitoma, H.; Manto, M. GABA and Glutamate: Their Transmitter Role in the CNS and Pancreatic Islets; Intech Open: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Veening, J.G.; Gerrits, P.; Barendregt, H.P. Volume transmission of beta-endorphin via the cerebrospinal fluid; a review. Fluids Barriers CNS 2012, 9, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Braitenberg, V. Brain Size and Number of Neurons: An Exercise in Synthetic Neuroanatomy. J. Comput. Neurosci. 2001, 10, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Hassel, B.R.D. Glutamate. In Basic Neurochemistry; Siegel, G., Albers, R.W., Brady, S.T., Price, D.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 267–290. [Google Scholar]
- Granger, A.J.; Wallace, M.L.; Sabatini, B.L. Multi-transmitter neurons in the mammalian central nervous system. Curr. Opin. Neurobiol. 2017, 45, 85–91. [Google Scholar] [CrossRef]
- Bryson, A.; Hatch, R.J.; Zandt, B.-J.; Rossert, C.; Berkovic, S.F.; Reid, C.A.; Grayden, D.B.; Hill, S.L.; Petrou, S. GABA-mediated tonic inhibition differentially modulates gain in functional subtypes of cortical interneurons. Proc. Natl. Acad. Sci. USA 2020, 117, 3192–3202. [Google Scholar] [CrossRef]
- Beane, M.; Marrocco, R. Norepinephrine and acetylcholine mediation of the components of reflexive attention: Implications for attention deficit disorders. Prog. Neurobiol. 2004, 74, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Aston-Jones, G.; Cohen, J.D. An Integrative Theory of Locus Coeruleus-Norepinephrine Function: Adaptive Gain and Optimal Performance. Annu. Rev. Neurosci. 2005, 28, 403–450. [Google Scholar] [CrossRef] [Green Version]
- Robbins, T.; Everitt, B. Arousal Systems and Attention. In The Cognitive Neurosciences; Gazzaniga, M., Ed.; MIT Press: Cambridge, MA, USA, 1996; pp. 703–720. [Google Scholar]
- McClure, S.; Gilzenrat, M.S.; Cohen, J.D. An exploration-exploitation model based on norepinepherine and dopamine activity. Adv. Neural Inf. Process. Syst. 2006, 18, 867. [Google Scholar]
- Dalley, J.W.; McGaughy, J.; O’Connell, M.; Cardinal, R.; Levita, L.; Robbins, T. Distinct Changes in Cortical Acetylcholine and Noradrenaline Efflux during Contingent and Noncontingent Performance of a Visual Attentional Task. J. Neurosci. 2001, 21, 4908–4914. [Google Scholar] [CrossRef] [Green Version]
- Ballinger, E.C.; Ananth, M.; Talmage, D.A.; Role, L.W. Basal Forebrain Cholinergic Circuits and Signaling in Cognition and Cognitive Decline. Neuron 2016, 91, 1199–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ondicova, K.; Mravec, B. Multilevel interactions between the sympathetic and parasympathetic nervous systems: A minireview. Endocr. Regul. 2010, 44, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Tau, G.Z.; Peterson, B.S. Normal Development of Brain Circuits. Neuropsychopharmacology 2009, 35, 147–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavezzi, A.M.; Ottaviani, G.; Terni, L.; Matturri, L. Histological and biological developmental characterization of the human cerebellar cortex. Int. J. Dev. Neurosci. 2006, 24, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Abitz, M.; Nielsen, R.D.; Jones, E.G.; Laursen, H.; Graem, N.; Pakkenberg, B. Excess of Neurons in the Human Newborn Mediodorsal Thalamus Compared with That of the Adult. Cereb. Cortex 2007, 17, 2573–2578. [Google Scholar] [CrossRef] [Green Version]
- Kolk, S.M.; Rakic, P. Development of prefrontal cortex. Neuropsychopharmacology 2021, 47, 41–57. [Google Scholar] [CrossRef]
- Hamel, E.; Beaudet, A. Electron microscopic autoradiographic localization of opioid receptors in rat neostriatum. Nature 1984, 312, 155–157. [Google Scholar] [CrossRef]
- Peckys, D.; Landwehrmeyer, G.B. Expression of mu, kappa, and delta opioid receptor messenger RNA in the human CNS: A 33P in situ hybridization study. Neuroscience 1999, 88, 1093–1135. [Google Scholar] [CrossRef]
- Delay-Goyet, P.; Zajac, J.M.; Javoy-Agid, F.; Agid, Y.; Roques, B.P. Regional distribution of mu, delta and kappa opioid receptors in human brains from controls and parkinsonian subjects. Brain Res. 1987, 414, 8–14. [Google Scholar] [CrossRef]
- Mansour, A.; Khachaturian, H.; Lewis, M.; Akil, H.; Watson, S.J. Autoradiographic differentiation of mu, delta, and kappa opioid receptors in the rat forebrain and midbrain. J. Neurosci. 1987, 7, 2445–2464. [Google Scholar]
- Sharif, N.; Hughes, J. Discrete mapping of brain mu and delta opioid receptors using selective peptides: Quantitative autoradiography, species differences and comparison with kappa receptors. Peptides 1989, 10, 499–522. [Google Scholar] [CrossRef] [PubMed]
- Semendeferi, K.; Teffer, K.; Buxhoeveden, D.P.; Park, M.S.; Bludau, S.; Amunts, K.; Travis, K.; Buckwalter, J. Spatial Organization of Neurons in the Frontal Pole Sets Humans Apart from Great Apes. Cereb. Cortex 2010, 21, 1485–1497. [Google Scholar] [CrossRef] [Green Version]
- Prévot, T.; Sibille, E. Altered GABA-mediated information processing and cognitive dysfunctions in depression and other brain disorders. Mol. Psychiatry 2020, 26, 151–167. [Google Scholar] [CrossRef] [PubMed]
- Granger, A.J.; Wang, W.; Robertson, K.; El-Rifai, M.; Zanello, A.F.; Bistrong, K.; Saunders, A.; Chow, B.W.; Nuñez, V.; García, M.T.; et al. Cortical ChAT+ neurons co-transmit acetylcholine and GABA in a target- and brain-region-specific manner. eLife 2020, 9, 57749. [Google Scholar] [CrossRef] [PubMed]
- Almayev, N.; Murasheva, O.; Petrovich, D. EEG correlates of personality questionnaires: Rusalov’s STQ-150, Trofimova’s STQ-77 and Cloninger’s TCI, Russian version. Psychophysiol. News (Vestn. Psychophisiologii) 2020, 3, 135–138. [Google Scholar]
- Reynolds, S.M.; Berridge, K. Fear and Feeding in the Nucleus Accumbens Shell: Rostrocaudal Segregation of GABA-Elicited Defensive Behavior Versus Eating Behavior. J. Neurosci. 2001, 21, 3261–3270. [Google Scholar] [CrossRef] [Green Version]
- Kanen, J.W.; Robbins, T.W.; Trofimova, I.N. Harnessing temperament to elucidate the complexities of serotonin function. Curr. Opin. Behav. Sci. 2022, 45, 101108. [Google Scholar] [CrossRef]
- Zvereva, N.; Zvereva, M.; Pyatnitskaya, L. Temperament Profiles of Children and Adolescents with Psychotic and Mood Disorders. Neuropsychobiology 2020, 80, 176–184. [Google Scholar] [CrossRef]
- Trofimova, I.; Christiansen, J. Coupling of Temperament with Mental Illness in Four Age Groups. Psychol. Rep. 2016, 118, 387–412. [Google Scholar] [CrossRef]
- Trofimova, I. An investigation into differences between the structure of temperament and the structure of personality. Am. J. Psychol. 2010, 123, 467–480. [Google Scholar] [CrossRef] [Green Version]
- Sallis, H.; Davey Smith, G.; Munafo, M.R. Genetics of biologically based psychological differences. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018, 373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plomin, R.; Asbury, K. Nature and Nurture: Genetic and Environmental Influences on Behavior. Ann. Am. Acad. Political Soc. Sci. 2005, 600, 86–98. [Google Scholar] [CrossRef]
- Trofimova, I.; Netter, P. In Search of Biomarkers for Biobehavioural and Psychiatric Taxonomies. Neuropsychobiology 2021, 80, 79–83. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trofimova, I. Analytic Background in the Neuroscience of the Potential Project “Hippocrates”. Brain Sci. 2023, 13, 39. https://doi.org/10.3390/brainsci13010039
Trofimova I. Analytic Background in the Neuroscience of the Potential Project “Hippocrates”. Brain Sciences. 2023; 13(1):39. https://doi.org/10.3390/brainsci13010039
Chicago/Turabian StyleTrofimova, Irina. 2023. "Analytic Background in the Neuroscience of the Potential Project “Hippocrates”" Brain Sciences 13, no. 1: 39. https://doi.org/10.3390/brainsci13010039
APA StyleTrofimova, I. (2023). Analytic Background in the Neuroscience of the Potential Project “Hippocrates”. Brain Sciences, 13(1), 39. https://doi.org/10.3390/brainsci13010039