Neuronal and Astroglial Localization of Glucocorticoid Receptor GRα in Adult Zebrafish Brain (Danio rerio)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Western Immunoblotting
2.3. Immunohistochemistry and Double Immunofluorescence
2.4. Antibody Characterization
2.5. Brain Microscopy, Photomicrograph Processing
2.6. Westen Blot Quantification and Statistical Analysis
3. Results
3.1. Western Blotting of Glucocorticoid-like Receptors (GRα) in the Adult Zebrafish Brain
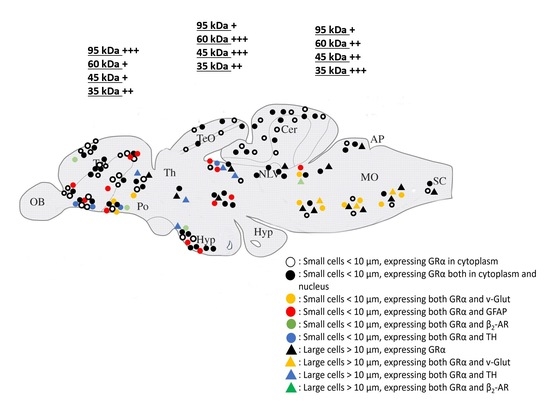
3.2. Cellular Distribution of the Glucocorticoid Receptor GRα in Zebrafish Brain
3.2.1. Telencephalon
3.2.2. Diencephalon
3.2.3. Mesencephalon
3.2.4. Rhombencephalon
3.3. Phenotype of Cells Expressing GRα
4. Discussion
4.1. GRα-Immunoreactive Proteins in Zebrafish Brain
4.2. GRα Immunoreactivity in Stress-Related Brain Areas
4.3. GRα Expression in Social Behavior/Reward Brain Network
4.4. GRα Immunoreactivity in the Cerebellum
4.5. GRα Expression in Astroglial Cells
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sapolsky, R.M.; Romero, L.M.; Munck, A.U. How do glucocorticoids influence stress responses? Integrating permissive.; suppressive.; stimulatory.; and preparative actions. Endocr. Rev. 2000, 21, 55–89. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Anti-inflammatory actions of glucocorticoids: Molecular mechanisms. Clin. Sci. 1998, 94, 557–572. [Google Scholar] [CrossRef] [PubMed]
- Evans, R.M. The steroid and thyroid hormone receptor superfamily. Science 1988, 240, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Galon, J.; Franchimont, D.; Hiroi, N.; Frey, G.; Boettner, A.; Ehrhart-Bornstein, M.; O’shea, J.J.; Chrousos, G.P.; Bornstein, S.R. Gene profiling reveals unknown enhancing and suppressive actions of glucocorticoids on immune cells. FASEB J. 2002, 16, 61–71. [Google Scholar] [CrossRef]
- Lu, N.Z.; Collins, J.B.; Grissom, S.F.; Cidlowski, J.A. Selective regulation of bone cell apoptosis by translational isoforms of the glucocorticoid receptor. Mol. Cell. Biol. 2007, 27, 7143–7160. [Google Scholar] [CrossRef]
- Ren, R.; Oakley, R.H.; Cruz-Topete, D.; Cidlowski, J.A. Dual role for glucocorticoids in cardiomyocyte hypertrophy and apoptosis. Endocrinology 2012, 153, 5346–5360. [Google Scholar] [CrossRef]
- Wendelaar Bonga, S.E. The stress response in fish. Physiol. Rev. 1997, 77, 591–625. [Google Scholar] [CrossRef]
- Bledsoe, R.K.; Montana, V.G.; Stanley, T.B.; Delves, C.J.; Apolito, C.J.; McKee, D.D.; Consler, T.G.; Parks, D.J.; Stewart, E.L.; Willson, T.M.; et al. Crystal structure of the glucocorticoid receptor ligand binding domain reveals a novel mode of receptor dimerization and coactivator recognition. Cell 2002, 110, 93–105. [Google Scholar] [CrossRef]
- Kumar, R.; Thompson, E.B. Gene regulation by the glucocorticoid receptor: Structure: Function relationship. J. Steroid Biochem. Mol. Biol. 2005, 94, 383–394. [Google Scholar] [CrossRef]
- Lu, N.Z.; Cidlowski, J.A. Translational regulatory mechanisms generate N-terminal glucocorticoid receptor isoforms with unique transcriptional target genes. Mol. Cell 2005, 18, 331–342. [Google Scholar] [CrossRef]
- Kino, T.; De Martino, M.U.; Charmandari, E.; Mirani, M.; Chrousos, G.P. Tissue glucocorticoid resistance/hypersensitivity syndromes. J. Steroid Biochem. Mol. Biol. 2003, 85, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Oakley, R.H.; Cidlowski, J.A. Cellular processing of the glucocorticoid receptor gene and protein: New mechanisms for generating tissue-specific actions of glucocorticoids. J. Biol. Chem. 2011, 286, 3177–3184. [Google Scholar] [CrossRef] [PubMed]
- Grad, I.; Picard, D. The glucocorticoid responses are shaped by molecular chaperones. Mol. Cell. Endocrinol. 2007, 275, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Pratt, W.B.; Toft, D.O. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr. Rev. 1997, 18, 306–360. [Google Scholar] [CrossRef]
- Beato, M. Gene regulation by steroid hormones. In Gene Expression; Birkhäuser: Boston, MA, USA, 1993; pp. 43–75. [Google Scholar] [CrossRef]
- Uhlenhaut, N.H.; Barish, G.D.; Ruth, T.Y.; Downes, M.; Karunasiri, M.; Liddle, C.; Schwalie, P.; Hübner, N.; Evans, R.M. Insights into negative regulation by the glucocorticoid receptor from genome-wide profiling of inflammatory cistromes. Mol. Cell 2013, 49, 158–171. [Google Scholar] [CrossRef]
- Yang-Yen, H.F.; Chambard, J.C.; Sun, Y.L.; Smeal, T.; Schmidt, T.J.; Drouin, J.; Karin, M. Transcriptional interference between c-Jun and the glucocorticoid receptor: Mutual inhibition of DNA binding due to direct protein-protein interaction. Cell 1990, 62, 1205–1215. [Google Scholar] [CrossRef]
- Moore, F.L.; Evans, S.J. Steroid hormones use non-genomic mechanisms to control brain functions and behaviors: A review of evidence. Brain. Behav. Evol. 1999, 54, 41–50. [Google Scholar] [CrossRef]
- Gross, K.L.; Cidlowski, J.A. Tissue-specific glucocorticoid action: A family affair. Trends Endocrinol. Metab. 2008, 19, 331–339. [Google Scholar] [CrossRef]
- DeRijk, R.H.; de Kloet, E.R. Corticosteroid receptor polymorphisms: Determinants of vulnerability and resilience. Eur. J. Pharmacol. 2008, 583, 303–311. [Google Scholar] [CrossRef]
- Parker, M.O.; Brock, A.J.; Walton, R.T.; Brennan, C.H. The role of zebrafish (Danio rerio) in dissecting the genetics and neural circuits of executive function. Front. Neural Circuits 2013, 7, 63. [Google Scholar] [CrossRef]
- Alsop, D.; Vijayan, M.M. Development of the corticosteroid stress axis and receptor expression in zebrafish. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2008, 294, R711–R719. [Google Scholar] [CrossRef] [PubMed]
- Bury, N.R.; Sturm, A.; Le Rouzic, P.; Lethimonier, C.; Ducouret, B.; Guiguen, Y.; Robinson-Rechavi, M.; Laudet, V.; Rafestin-Oblin, M.E.; Prunet, P. Evidence for two distinct functional glucocorticoid receptors in teleost fish. J. Mol. Endocrinol. 2003, 31, 141–156. [Google Scholar] [CrossRef] [PubMed]
- Greenwood, A.K.; Butler, P.C.; White, R.B.; DeMarco, U.; Pearce, D.; Fernald, R.D. Multiple corticosteroid receptors in a teleost fish: Distinct sequences.; expression patterns.; and transcriptional activities. Endocrinology 2003, 144, 4226–4236. [Google Scholar] [CrossRef] [PubMed]
- Stolte, E.H.; van Kemenade, B.L.; Savelkoul, H.F.; Flik, G. Evolution of glucocorticoid receptors with different glucocorticoid sensitivity. J. Endocrinol. 2006, 190, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Ziv, L.; Muto, A.; Schoonheim, P.J.; Meijsing, S.H.; Strasser, D.; Ingraham, H.A.; Schaaf, M.J.; Yamamoto, K.R.; Baier, H. An affective disorder in zebrafish with mutation of the glucocorticoid receptor. Mol. Psychiatry 2013, 18, 681–691. [Google Scholar] [CrossRef]
- Patel, P.D.; Lopez, J.F.; Lyons, D.M.; Burke, S.; Wallace, M.; Schatzberg, A.F. Glucocorticoid and mineralocorticoid receptor mRNA expression in squirrel monkey brain. J. Psychiatr. Res. 2000, 34, 383–392. [Google Scholar] [CrossRef]
- Sánchez, M.M.; Young, L.J.; Plotsky, P.M.; Insel, T.R. Distribution of corticosteroid receptors in the rhesus brain: Relative absence of glucocorticoid receptors in the hippocampal formation. J. Neurosci. 2000, 20, 4657–4668. [Google Scholar] [CrossRef]
- Aronsson, M.; Fuxe, K.; Dong, Y.; Agnati, L.F.; Okret, S.; Gustafsson, J.A. Localization of glucocorticoid receptor mRNA in the male rat brain by in situ hybridization. Proc. Natl. Acad. Sci. USA 1988, 85, 9331–9335. [Google Scholar] [CrossRef]
- Morimoto, M.; Morita, N.; Ozawa, H.; Yokoyama, K.; Kawata, M. Distribution of glucocorticoid receptor immunoreactivity and mRNA in the rat brain: An immunohistochemical and in situ hybridization study. Neurosci. Res. 1996, 26, 235–269. [Google Scholar] [CrossRef]
- Carruth, L.L.; Jones, R.E.; Norris, D.O. Cell density and intracellular translocation of glucocorticoid receptor-immunoreactive neurons in the kokanee salmon (Oncorhynchus nerka kennerlyi) brain.; with an emphasis on the olfactory system. Gen. Comp. Endocrinol. 2000, 117, 66–76. [Google Scholar] [CrossRef]
- Teitsma, C.A.; Anglade, I.; Toutirais, G.; Muñoz-cueto, J.A.; Saligaut, D.; Ducouret, B.; Kah, O. Immunohistochemical localization of glucocorticoid receptors in the forebrain of the rainbow trout (Oncorhynchus mykiss). J. Comp. Neurol. 1998, 401, 395–410. [Google Scholar] [CrossRef]
- Sinclair, D.; Webster, M.J.; Wong, J.; Weickert, C.S. Dynamic molecular and anatomical changes in the glucocorticoid receptor in human cortical development. Mol. Psychiatry 2011, 16, 504–515. [Google Scholar] [CrossRef] [PubMed]
- Dickmeis, T.; Lahiri, K.; Nica, G.; Vallone, D.; Santoriello, C.; Neumann, C.J.; Hammerschmidt, M.; Foulkes, N.S. Glucocorticoids play a key role in circadian cell cycle rhythms. PLoS Biol. 2007, 5, e78. [Google Scholar] [CrossRef] [PubMed]
- Cruz, S.A.; Lin, C.H.; Chao, P.L.; Hwang, P.P. Glucocorticoid receptor.; but not mineralocorticoid receptor.; mediates cortisol regulation of epidermal ionocyte development and ion transport in zebrafish (Danio rerio). PloS ONE 2013, 8, e77997. [Google Scholar] [CrossRef]
- Facchinello, N.; Skobo, T.; Meneghetti, G.; Colletti, E.; Dinarello, A.; Tiso, N.; Costa, R.; Gioacchini, G.; Carnevali, O.; Argenton, F.; et al. nr3c1 null mutant zebrafish are viable and reveal DNA-binding-independent activities of the glucocorticoid receptor. Sci. Rep. 2017, 7, 4371. [Google Scholar] [CrossRef]
- Lin, C.H.; Tsai, I.L.; Su, C.H.; Hwang, P.P. Reverse effect of mammalian hypocalcemic cortisol in fish: Cortisol stimulates Ca2+ uptake via glucocorticoid receptor-mediated vitamin D3 metabolism. PLoS ONE 2011, 6, e23689. [Google Scholar] [CrossRef]
- Kumai, Y.; Nesan, D.; Vijayan, M.M.; Perry, S.F. Cortisol regulates Na+ uptake in zebrafish.; Danio rerio.; larvae via the glucocorticoid receptor. Mol. Cell. Endocrinol. 2012, 364, 113–125. [Google Scholar] [CrossRef]
- Tesic, V.; Perovic, M.; Lazic, D.; Kojic, S.; Smiljanic, K.; Ruzdijic, S.; Rakic, L.; Kanazir, S. Long-term intermittent feeding restores impaired GR signaling in the hippocampus of aged rat. J. Steroid Biochem. Mol. Biol. 2015, 149, 43–52. [Google Scholar] [CrossRef]
- Shen, K.; Leung, S.W.; Ji, L.; Huang, Y.; Hou, M.; Xu, A.; Wang, Z.; Vanhoutte, P.M. Notoginsenoside Ft1 activates both glucocorticoid and estrogen receptors to induce endothelium-dependent, nitric oxide-mediated relaxations in rat mesenteric arteries. Biochem. Pharmacol. 2014, 88, 66–74. [Google Scholar] [CrossRef]
- Ampatzis, K.; Dermon, C.R. Regional distribution and cellular localization of β2-adrenoceptors in the adult zebrafish brain (Danio rerio). J. Comp. Neurol. 2010, 518, 1418–1441. [Google Scholar] [CrossRef]
- Yamamoto, K.; Ruuskanen, J.O.; Wullimann, M.F.; Vernier, P. Differential expression of dopaminergic cell markers in the adult zebrafish forebrain. J. Comp. Neurol. 2011, 519, 576–598. [Google Scholar] [CrossRef] [PubMed]
- Ampatzis, K.; Kentouri, M.; Dermon, C.R. Neuronal and glial localization of α2A-adrenoceptors in the adult zebrafish (Danio rerio) brain. J. Comp. Neurol. 2008, 508, 72–93. [Google Scholar] [CrossRef] [PubMed]
- Higashijima, S.I.; Mandel, G.; Fetcho, J.R. Distribution of prospective glutamatergic.; glycinergic.; and GABAergic neurons in embryonic and larval zebrafish. J. Comp. Neurol. 2004, 480, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Filippi, A.; Mueller, T.; Driever, W. vglut2 and gad expression reveal distinct patterns of dual GABAergic versus glutamatergic cotransmitter phenotypes of dopaminergic and noradrenergic neurons in the zebrafish brain. J. Comp. Neurol. 2014, 522, 2019–2037. [Google Scholar] [CrossRef]
- Perdikaris, P.; Dermon, C.R. Behavioral and neurochemical profile of MK-801 adult zebrafish model: Forebrain β2-adrenoceptors contribute to social withdrawal and anxiety-like behavior. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2022, 115, 110494. [Google Scholar] [CrossRef] [PubMed]
- Wullimann, M.F.; Rupp, B.; Reichert, H.; Wullimann, M.F.; Rupp, B.; Reichert, H. The brain of the zebrafish Danio rerio: A neuroanatomical atlas. In Neuroanatomy of the Zebrafish Brain: A Topological Atlas; Springer: Cham, Switzerland, 1996; pp. 19–87. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Wullimann, M.F.; Mueller, T. Teleostean and mammalian forebrains contrasted: Evidence from genes to behavior. J. Comp. Neurol. 2004, 475, 143–162. [Google Scholar] [CrossRef]
- Meek, H.J. Tectal morphology: Connections, neurones and synapses. In The Visual System of Fish; Springer: Cham, Switzerland, 1990; pp. 239–277. [Google Scholar] [CrossRef]
- Guthrie, S.D. The physiology of the teleostean optic tectum. In The Visual System of Fish; Springer: Cham, Switzerland, 1990; pp. 279–343. [Google Scholar] [CrossRef]
- Ma, P.M. Catecholaminergic systems in the zebrafish. III. Organization and projection pattern of medullary dopaminergic and noradrenergic neurons. J. Comp. Neurol. 1997, 381, 411–427. [Google Scholar] [CrossRef]
- Rink, E.; Wullimann, M.F. The teleostean (zebrafish) dopaminergic system ascending to the subpallium (striatum) is located in the basal diencephalon (posterior tuberculum). Brain Res. 2001, 889, 316–330. [Google Scholar] [CrossRef]
- Kaslin, J.A.; Panula, P. Comparative anatomy of the histaminergic and other aminergic systems in zebrafish (Danio rerio). J. Comp. Neurol. 2001, 440, 342–377. [Google Scholar] [CrossRef]
- Mueckler, M.; Caruso, C.; Baldwin, S.A.; Panico, M.; Blench, I.; Morris, H.R.; Allard, W.J.; Lienhard, G.E.; Lodish, H.F. Sequence and structure of a human glucose transporter. Science 1985, 229, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Jacque, C.M.; Vinner, C.; Kujas, M.; Raoul, M.; Racadot, J.; Baumann, N.A. Determination of glial fibrillary acidic, page 185 protein (GFAP) in human brain tumors. J. Neurol. Sci. 1978, 35, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, A.L.; Jørgensen, A.L. Structural and functional characterization of the zebrafish gene for glial fibrillary acidic protein, GFAP. Gene 2003, 310, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Schaaf, M.J.; Chatzopoulou, A.; Spaink, H.P. The zebrafish as a model system for glucocorticoid receptor research. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2009, 153, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Chatzopoulou, A.; Roy, U.; Meijer, A.H.; Alia, A.; Spaink, H.P.; Schaaf, M.J. Transcriptional and metabolic effects of glucocorticoid receptor α and β signaling in zebrafish. Endocrinology 2015, 156, 1757–1769. [Google Scholar] [CrossRef]
- Chin, J.S.; Phan, T.A.; Albert, L.T.; Keene, A.C.; Duboué, E.R. Long lasting anxiety following early life stress is dependent on glucocorticoid signaling in zebrafish. Sci. Rep. 2022, 12, 12826. [Google Scholar] [CrossRef]
- Nesan, D.; Vijayan, M.M. The transcriptomics of glucocorticoid receptor signaling in developing zebrafish. PLoS ONE 2013, 8, e80726. [Google Scholar] [CrossRef]
- Dinarello, A.; Licciardello, G.; Fontana, C.M.; Tiso, N.; Argenton, F.; Dalla Valle, L. Glucocorticoid receptor activities in the zebrafish model: A review. J. Endocrinol. 2020, 247, R63–R82. [Google Scholar] [CrossRef]
- Ahima, R.S.; Harlan, R.E. Charting of type II glucocorticoid receptor-like immunoreactivity in the rat central nervous system. Neuroscience 1990, 39, 579–604. [Google Scholar] [CrossRef]
- YAU, J.; Seckl, J.R. Local amplification of glucocorticoids in the aging brain and impaired spatial memory. Spatial memory–a unique window into healthy and pathological ageing. Front. Aging Neurosci. 2015, 4, 24. [Google Scholar] [CrossRef]
- Wang, Q.; Van Heerikhuize, J.; Aronica, E.; Kawata, M.; Seress, L.; Joels, M.; Swaab, D.F.; Lucassen, P.J. Glucocorticoid receptor protein expression in human hippocampus; stability with age. Neurobiol. Aging 2013, 34, 1662–1673. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; Hu, F.; Denver, R.J. Distribution and corticosteroid regulation of glucocorticoid receptor in the brain of Xenopus laevis. J. Comp. Neurol. 2008, 508, 967–982. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, K.J.; Westphal, H.M.; Peczely, P. Distribution of glucocorticoid receptor-like immunoreactivity in the brain.; and its relation to CRF and ACTH immunoreactivity in the hypothalamus of the japanese quail.; Coturnix coturnix japonica. Brain Res. 1989, 505, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Godowski, P.J.; Rusconi, S.; Miesfeld, R.; Yamamoto, K.R. Glucocorticoid receptor mutants that are constitutive activators of transcriptional enhancement. Nature 1987, 325, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Saif, Z.; Hodyl, N.A.; Stark, M.J.; Fuller, P.J.; Cole, T.; Lu, N.; Clifton, V.L. Expression of eight glucocorticoid receptor isoforms in the human preterm placenta vary with fetal sex and birthweight. Placenta 2015, 36, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Saif, Z.; Dyson, R.M.; Palliser, H.K.; Wright, I.M.; Lu, N.; Clifton, V.L. Identification of eight different isoforms of the glucocorticoid receptor in guinea pig placenta: Relationship to preterm delivery.; sex and betamethasone exposure. PloS ONE 2016, 11, e0148226. [Google Scholar] [CrossRef]
- Cuffe, J.S.; Saif, Z.; Perkins, A.V.; Moritz, K.M.; Clifton, V.L. Dexamethasone and sex regulate placental glucocorticoid receptor isoforms in mice. J. Endocrinol. 2017, 234, 89–100. [Google Scholar] [CrossRef]
- Dickmeis, T. Glucocorticoids and the circadian clock. J. Endocrinol. 2009, 200, 3. [Google Scholar] [CrossRef]
- Gray, T.S.; Bingaman, E.W. The amygdala: Corticotropin-releasing factor.; steroids.; and stress. Critical Reviews™ in Neurobiology. Crit. Rev. Neurobiol. 1996, 10, 155–168. [Google Scholar] [CrossRef]
- Makino, S.; Gold, P.W.; Schulkin, J. Effects of corticosterone on CRH mRNA and content in the bed nucleus of the stria terminalis; comparison with the effects in the central nucleus of the amygdala and the paraventricular nucleus of the hypothalamus. Brain Res. 1994, 657, 141–149. [Google Scholar] [CrossRef]
- Shepard, J.D.; Barron, K.W.; Myers, D.A. Corticosterone delivery to the amygdala increases corticotropin-releasing factor mRNA in the central amygdaloid nucleus and anxiety-like behavior. Brain Res. 2000, 861, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, L.; Sapolsky, R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr. Rev. 1991, 12, 118–134. [Google Scholar] [CrossRef] [PubMed]
- Diorio, D.; Viau, V.; Meaney, M.J. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J. Neurosci. 1993, 13, 3839–3847. [Google Scholar] [CrossRef]
- Portavella, M.; Vargas, J.P.; Torres, B.; Salas, C. The effects of telencephalic pallial lesions on spatial, temporal.; and emotional learning in goldfish. Brain Res. Bull. 2002, 57, 397–399. [Google Scholar] [CrossRef]
- Mueller, T.; Dong, Z.; Berberoglu, M.A.; Guo, S. The dorsal pallium in zebrafish.; Danio rerio (Cyprinidae.; Teleostei). Brain Res. 2011, 1381, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Von Trotha, J.W.; Vernier, P.; Bally-Cuif, L. Emotions and motivated behavior converge on an amygdala-like structure in the zebrafish. Eur. J. Neurosci. 2014, 40, 3302–3315. [Google Scholar] [CrossRef]
- Broglio, C.; Gómez, A.; Durán, E.; Ocana, F.M.; Jiménez-Moya, F.; Rodríguez, F.; Salas, C. Hallmarks of a common forebrain vertebrate plan: Specialized pallial areas for spatial, temporal and emotional memory in actinopterygian fish. Brain Res. Bull. 2005, 66, 277–281. [Google Scholar] [CrossRef]
- Ampatzis, K.; Makantasi, P.; Dermon, C.R. Cell proliferation pattern in adult zebrafish forebrain is sexually dimorphic. Neuroscience 2012, 226, 367–381. [Google Scholar] [CrossRef]
- Wang, Q.; Verweij, E.W.; Krugers, H.J.; Joels, M.; Swaab, D.F.; Lucassen, P.J. Distribution of the glucocorticoid receptor in the human amygdala; changes in mood disorder patients. Brain Struct. Funct. 2014, 219, 1615–1626. [Google Scholar] [CrossRef]
- Ohnishi, K. Effects of telencephalic ablation on short-term memory and attention in goldfish. Behav. Brain Res. 1997, 86, 191–199. [Google Scholar] [CrossRef]
- Vargas, J.P.; Rodrıguez, F.; Lopez, J.C.; Arias, J.L.; Salas, C. Spatial learning-induced increase in the argyrophilic nucleolar organizer region of dorsolateral telencephalic neurons in goldfish. Brain Res. 2000, 865, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Joëls, M. Corticosteroid effects in the brain: U-shape it. Trends Pharmacol. Sci. 2006, 27, 244–250. [Google Scholar] [CrossRef]
- Gould, E.; McEwen, B.S.; Tanapat, P.; Galea, L.A.; Fuchs, E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J. Neurosci. 1997, 17, 2492–2498. [Google Scholar] [CrossRef] [PubMed]
- Sørensen, C.; Bohlin, L.C.; Øverli, Ø.; Nilsson, G.E. Cortisol reduces cell proliferation in the telencephalon of rainbow trout (Oncorhynchus mykiss). Physiol. Behav. 2011, 102, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Fokos, S.; Pavlidis, M.; Yiotis, T.; Tsalafouta, A.; Papandroulakis, N.; Dermon, C.R. Early life low intensity stress experience modifies acute stress effects on juvenile brain cell proliferation of European sea bass (D. Labrax). Behav. Brain Res. 2017, 317, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Vindas, M.A.; Fokos, S.; Pavlidis, M.; Höglund, E.; Dionysopoulou, S.; Ebbesson, L.O.; Papandroulakis, N.; Dermon, C.R. Early life stress induces long-term changes in limbic areas of a teleost fish: The role of catecholamine systems in stress coping. Sci. Rep. 2018, 8, 5638. [Google Scholar] [CrossRef]
- O’Connell, L.A.; Hofmann, H.A. Evolution of a vertebrate social decision-making network. Science 2012, 336, 1154–1157. [Google Scholar] [CrossRef]
- Stednitz, S.J.; McDermott, E.M.; Ncube, D.; Tallafuss, A.; Eisen, J.S.; Washbourne, P. Forebrain control of behaviorally driven social orienting in zebrafish. Curr. Biol. 2018, 28, 2445–2451. [Google Scholar] [CrossRef]
- Ampatzis, K.; Dermon, C.R. Sexual dimorphisms in swimming behavior.; cerebral metabolic activity and adrenoceptors in adult zebrafish (Danio rerio). Behav. Brain Res. 2016, 312, 385–393. [Google Scholar] [CrossRef]
- Zikopoulos, B.; Dermon, C.R. Comparative anatomy of α2 and β adrenoceptors in the adult and developing brain of the marine teleost the red porgy (Pagrus pagrus.; Sparidae): [3H] clonidine and [3H] dihydroalprenolol quantitative autoradiography and receptor subtypes immunohistochemistry. J. Comp. Neurol. 2005, 489, 217–240. [Google Scholar] [CrossRef]
- Rodriguez, F.; Durán, E.; Gómez, A.; Ocana, F.M.; Alvarez, E.; Jiménez-Moya, F.; Broglio, C.; Salas, C. Cognitive and emotional functions of the teleost fish cerebellum. Brain Res. Bull. 2005, 66, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.C. Central distribution of octavolateral afferents and efferents in a teleost (Mormyridae). J. Comp. Neurol. 1981, 195, 391–414. [Google Scholar] [CrossRef] [PubMed]
- Dohaku, R.; Yamaguchi, M.; Yamamoto, N.; Shimizu, T.; Osakada, F.; Hibi, M. Tracing of afferent connections in the zebrafish cerebellum using recombinant rabies virus. Front. Neural Circuits 2019, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Senft, R.A.; Meddle, S.L.; Baugh, A.T. Distribution and abundance of glucocorticoid and mineralocorticoid receptors throughout the brain of the great tit (Parus major). PLoS ONE 2016, 11, e0148516. [Google Scholar] [CrossRef]
- Lu, C.L.; Ren, J.; Mo, J.W.; Fan, J.; Guo, F.; Chen, L.Y.; Wen, Y.L.; Li, S.J.; Fang, Y.Y.; Wu, Z.F.; et al. Glucocorticoid Receptor–Dependent Astrocytes Mediate Stress Vulnerability. Biol. Psychiatry 2022, 92, 204–215. [Google Scholar] [CrossRef]
- Grupp, L.; Wolburg, H.; Mack, A.F. Astroglial structures in the zebrafish brain. J. Comp. Neurol. 2010, 518, 4277–4287. [Google Scholar] [CrossRef]
- März, M.; Chapouton, P.; Diotel, N.; Vaillant, C.; Hesl, B.; Takamiya, M.; Lam, C.S.; Kah., O.; Bally-Cuif, L.; Strähle, U. Heterogeneity in progenitor cell subtypes in the ventricular zone of the zebrafish adult telencephalon. Glia 2010, 58, 870–888. [Google Scholar] [CrossRef]
- Middeldorp, J.; Hol, E.M. GFAP in health and disease. Prog. Neurobiol. 2011, 93, 421–443. [Google Scholar] [CrossRef]
- Ito, Y.; Tanaka, H.; Okamoto, H.; Ohshima, T. Characterization of neural stem cells and their progeny in the adult zebrafish optic tectum. Dev. Biol. 2010, 342, 26–38. [Google Scholar] [CrossRef]
- Jurisch-Yaksi, N.; Yaksi, E.; Kizil, C. Radial glia in the zebrafish brain: Functional.; structural.; and physiological comparison with the mammalian glia. Glia 2020, 68, 2451–2470. [Google Scholar] [CrossRef]
- Yang, L.; Wang, J.; Wang, D.; Hu, G.; Liu, Z.; Yan, D.; Serikuly, N.; Alpyshov, E.T.; Demin, K.A.; Strekalova, T.; et al. Delayed behavioral and genomic responses to acute combined stress in zebrafish.; potentially relevant to PTSD and other stress-related disorders: Focus on neuroglia.; neuroinflammation.; apoptosis and epigenetic modulation. Behav. Brain Res. 2020, 389, 112644. [Google Scholar] [CrossRef] [PubMed]
- Tertil, M.; Skupio, U.; Barut, J.; Dubovyk, V.; Wawrzczak-Bargiela, A.; Soltys, Z.; Golda, S.; Kudla, L.; Wiktorowska, L.; Szklarczyk, K.; et al. Glucocorticoid receptor signaling in astrocytes is required for aversive memory formation. Transl. Psychiatry 2018, 28, 255. [Google Scholar] [CrossRef] [PubMed]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natsaridis, E.; Perdikaris, P.; Fokos, S.; Dermon, C.R. Neuronal and Astroglial Localization of Glucocorticoid Receptor GRα in Adult Zebrafish Brain (Danio rerio). Brain Sci. 2023, 13, 861. https://doi.org/10.3390/brainsci13060861
Natsaridis E, Perdikaris P, Fokos S, Dermon CR. Neuronal and Astroglial Localization of Glucocorticoid Receptor GRα in Adult Zebrafish Brain (Danio rerio). Brain Sciences. 2023; 13(6):861. https://doi.org/10.3390/brainsci13060861
Chicago/Turabian StyleNatsaridis, Evangelos, Panagiotis Perdikaris, Stefanos Fokos, and Catherine R. Dermon. 2023. "Neuronal and Astroglial Localization of Glucocorticoid Receptor GRα in Adult Zebrafish Brain (Danio rerio)" Brain Sciences 13, no. 6: 861. https://doi.org/10.3390/brainsci13060861
APA StyleNatsaridis, E., Perdikaris, P., Fokos, S., & Dermon, C. R. (2023). Neuronal and Astroglial Localization of Glucocorticoid Receptor GRα in Adult Zebrafish Brain (Danio rerio). Brain Sciences, 13(6), 861. https://doi.org/10.3390/brainsci13060861