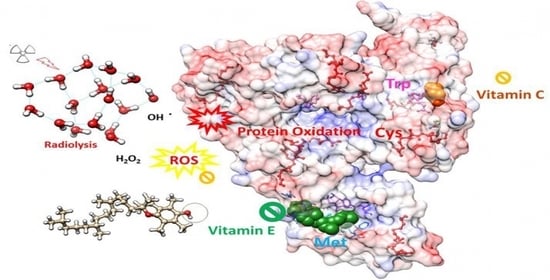
Radioprotective Role of Vitamins C and E against the Gamma Ray-Induced Damage to the Chemical Structure of Bovine Serum Albumin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Apparatus
2.2. Sample Preparation and Gamma Irradiation
2.3. Circular Dichroism (CD) and Fluorescence Spectroscopy
2.4. Molecular Docking
3. Results
3.1. Effect of Gamma Radiation at Therapeutic Doses on the Secondary and Tertiary Structure of BSA
3.2. Investigation of the Effect of Gamma Rays at the 3 Gy Dose on the Structure BSA in the Presence of Natural Protectors
3.2.1. Radioprotection of the Secondary Structure of IR-BSA with Vitamin C
3.2.2. Radioprotection of the Tertiary Structure of IR-BSA with Vitamin C
3.2.3. Radioprotection of the Secondary Structure of IR-BSA with Vitamin E
3.2.4. Radioprotection of the Tertiary Structure of IR-BSA with Vitamin E
3.3. Fluorescence Quenching Mechanism and the Binding of Vitamins C and E to BSA
3.4. Molecular Docking Analysis
- The chemical effect, in which case vitamins scavenge radicals and thus prevent them from interacting with the protein.
- The steric effect, in which case the interaction of vitamins with BSA, causes steric alterations at or around the binding site, thereby inhibiting radicals from interacting with the protein. This scenario is important when the binding pocket is near the sensitive amino acids.
- The colloidal effect, in which case the presence of vitamins stabilizes the protein dispersion behavior and prevents aggregation.
4. Discussion
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cash, H.; Dean, D. The effects of low-dose radiation on articular cartilage: A review. J. Biol. Eng. 2019, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Mohammadkarim, A.; Mokhtari-Dizaji, M.; Kazemian, A.; Saberi, H.; Khani, M.M.; Bakhshandeh, M. Dose-dependent 60Co γ-radiation Effects on Human Endothelial Cell Mechanical Properties. Cell Biochem. Biophys. 2019, 77, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Olubukola Sinbad, O.; Folorunsho, A.A.; Olabisi, O.L.; Abimbola Ayoola, O.; Johnson Temitope, E. Vitamins as Antioxidants. J. Food Sci. Nutr. Res. 2019, 2, 214–235. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [Green Version]
- Gaber, M.H. Effect of γ-irradiation on the molecular properties of bovine serum albumin. J. Biosci. Bioeng. 2005, 100, 203–206. [Google Scholar] [CrossRef]
- Lee, Y.; Song, K. Bin Effect of gamma-irradiation on the molecular properties of myoglobin. J. Biochem. Mol. Biol. 2002, 35, 590–594. [Google Scholar] [CrossRef] [Green Version]
- Tutar, Y. Role of Protein Aggregation in Neurodegenerative Diseases. Özgür, A., Ed.; IntechOpen: Rijeka, Croatia, 2013. [Google Scholar]
- Singh, V.K.; Seed, T.M. The efficacy and safety of amifostine for the acute radiation syndrome. Expert Opin. Drug Saf. 2019, 18, 1077–1090. [Google Scholar] [CrossRef] [Green Version]
- Rades, D.; Fehlauer, F.; Bajrovic, A.; Mahlmann, B.; Richter, E.; Alberti, W. Serious adverse effects of amifostine during radiotherapy in head and neck cancer patients. Radiother. Oncol. J. Eur. Soc. Ther. Radiol. Oncol. 2004, 70, 261–264. [Google Scholar] [CrossRef]
- Chaghouri, P.; Maalouf, N.; Peters, S.L.; Nowak, P.J.; Peczek, K.; Zasowska-Nowak, A.; Nowicki, M. Two Faces of Vitamin C in Hemodialysis Patients: Relation to Oxidative Stress and Inflammation. Nutrients 2021, 13, 791. [Google Scholar] [CrossRef]
- Bendich, A.; Machlin, L.J.; Scandurra, O.; Burton, G.W.; Wayner, D.D.M. The antioxidant role of vitamin C. Adv. Free Radic. Biol. Med. 1986, 2, 419–444. [Google Scholar] [CrossRef]
- Kamal-Eldin, A.; Appelqvist, L.A. The chemistry and antioxidant properties of tocopherols and tocotrienols. Lipids 1996, 31, 671–701. [Google Scholar] [CrossRef]
- Geng, C.; Zhang, X.; Tang, X.; Liu, Y.; Chen, W.; He, J.; Gong, C. Research on a wide-range biodosimeter based on the irradiation damage effect of proteins for γ radiation. Radiat. Phys. Chem. 2020, 166, 108477. [Google Scholar] [CrossRef]
- Bahreinipour, M.; Zarei, H.; Dashtestani, F.; Rashidiani, J.; Eskandari, K.; Zarandi, S.A.M.; Ardestani, S.K.; Watabe, H. Radioprotective effect of nanoceria and magnetic flower-like iron oxide microparticles on gamma radiation-induced damage in BSA protein: Running Title: Radioprotective Effect of Nanostructures on BSA. AIMS Biophys. 2021, 8, 124–142. [Google Scholar] [CrossRef]
- Zarei, H.; Bahreinipour, M.; Eskandari, K.; MousaviZarandi, S.A.; Ardestani, S.K. Spectroscopic study of gamma irradiation effect on the molecular structure of bovine serum albumin. Vacuum 2017, 136, 91–96. [Google Scholar] [CrossRef]
- Greenfield, N.J. Using circular dichroism spectra to estimate protein secondary structure. Nat. Protoc. 2006, 1, 2876–2890. [Google Scholar] [CrossRef]
- Yang, H.; Xiao, X.; Zhao, X.; Wu, Y. Intrinsic Fluorescence Spectra of Tryptophan, Tyrosine and Phenyloalanine. Proc. SPIE 2015, 224–233. [Google Scholar] [CrossRef] [Green Version]
- Kar, T.; Basak, P.; Ghosh, R.K.; Bhattacharyya, M. Protective effects of curcumin against gamma ray induced conformational change of human serum albumin. Int. J. Biol. Macromol. 2017, 99, 600–607. [Google Scholar] [CrossRef]
- Huang, H.-Y.; Appel, L.J.; Croft, K.D.; Miller, E.R., 3rd; Mori, T.A.; Puddey, I.B. Effects of vitamin C and vitamin E on in vivo lipid peroxidation: Results of a randomized controlled trial. Am. J. Clin. Nutr. 2002, 76, 549–555. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Suh, J.; Carr, A.C.; Morrow, J.D.; Zeind, J.; Frei, B. Vitamin C suppresses oxidative lipid damage in vivo, even in the presence of iron overload. Am. J. Physiol. Metab. 2000, 279, E1406–E1412. [Google Scholar] [CrossRef]
- Chin, K.-Y.; Ima-Nirwana, S. The Role of Vitamin E in Preventing and Treating Osteoarthritis—A Review of the Current Evidence. Front. Pharmacol. 2018, 9, 946. [Google Scholar] [CrossRef] [Green Version]
- Bujacz, A. Structures of bovine, equine and leporine serum albumin. Acta Crystallogr. D. Biol. Crystallogr. 2012, 68, 1278–1289. [Google Scholar] [CrossRef]
- Jurrus, E.; Engel, D.; Star, K.; Monson, K.; Brandi, J.; Felberg, L.E.; Brookes, D.H.; Wilson, L.; Chen, J.; Liles, K.; et al. Improvements to the APBS biomolecular solvation software suite. Protein Sci. 2018, 27, 112–128. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Grimm, M.; Dai, W.; Hou, M.; Xiao, Z.-X.; Cao, Y. CB-Dock: A web server for cavity detection-guided protein–ligand blind docking. Acta Pharmacol. Sin. 2020, 41, 138–144. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]
- Laskowski, R.A.; Swindells, M.B. LigPlot+: Multiple ligand-protein interaction diagrams for drug discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef]
- Krissinel, E.; Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 2007, 372, 774–797. [Google Scholar] [CrossRef]
- Willard, L.; Ranjan, A.; Zhang, H.; Monzavi, H.; Boyko, R.F.; Sykes, B.D.; Wishart, D.S. VADAR: A web server for quantitative evaluation of protein structure quality. Nucleic Acids Res. 2003, 31, 3316–3319. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Ray, D.; Paul, B.K.; Guchhait, N. Effect of biological confinement on the photophysics and dynamics of a proton-transfer phototautomer: An exploration of excitation and emission wavelength-dependent photophysics of the protein-bound drug. Phys. Chem. Chem. Phys. 2012, 14, 12182–12192. [Google Scholar] [CrossRef]
- Shen, L.; Tang, C.-H. Microfluidization as a potential technique to modify surface properties of soy protein isolate. Food Res. Int. 2012, 48, 108–118. [Google Scholar] [CrossRef]
- Lu, R.; Li, W.-W.; Katzir, A.; Raichlin, Y.; Yu, H.-Q.; Mizaikoff, B. Probing the secondary structure of bovine serum albumin during heat-induced denaturation using mid-infrared fiberoptic sensors. Analyst 2015, 140, 765–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, R.G.; Feldhoff, R.C.; Clute, O.L.; Peters, T. Fragments of bovine serum albumin produced by limited proteolysis. Conformation and ligand binding. Biochemistry 1975, 14, 4578–4583. [Google Scholar] [CrossRef] [PubMed]
- Zolghadri, S.; Saboury, A.A.; Amin, E.; Moosavi-Movahedi, A.A. A spectroscopic study on the interaction between ferric oxide nanoparticles and human hemoglobin. J. Iran. Chem. Soc. 2010, 7, S145–S153. [Google Scholar] [CrossRef]
- Li, X.; Wang, G.; Chen, D.; Lu, Y. Binding of ascorbic acid and α-tocopherol to bovine serum albumin: A comparative study. Mol. Biosyst. 2014, 10, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Suryawanshi, V.D.; Walekar, L.S.; Gore, A.H.; Anbhule, P.V.; Kolekar, G.B. Spectroscopic analysis on the binding interaction of biologically active pyrimidine derivative with bovine serum albumin. J. Pharm. Anal. 2016, 6, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Liu, E.-H.; Qi, L.-W.; Li, P. Structural relationship and binding mechanisms of five flavonoids with bovine serum albumin. Molecules 2010, 15, 9092–9103. [Google Scholar] [CrossRef] [Green Version]
- Farasat, M.; Arjmand, S.; Ranaei Siadat, S.O.; Sefidbakht, Y.; Ghomi, H. The effect of non-thermal atmospheric plasma on the production and activity of recombinant phytase enzyme. Sci. Rep. 2018, 8, 16647. [Google Scholar] [CrossRef]
- Suto, D.; Ikeda, Y.; Fujii, J.; Ohba, Y. Structural Analysis of Amino Acids, Oxidized by Reactive Oxygen Species and an Antibody against N-Formylkynurenine. J. Clin. Biochem. Nutr. 2006, 38, 107–111. [Google Scholar] [CrossRef]
- Sefidbakht, Y.; Moosavi-Movahedi, A.A.; Hosseinkhani, S.; Khodagholi, F.; Torkzadeh-Mahani, M.; Foolad, F.; Faraji-Dana, R. Effects of 940 MHz EMF on bioluminescence and oxidative response of stable luciferase producing HEK cells. Photochem. Photobiol. Sci. 2014, 13, 1082–1092. [Google Scholar] [CrossRef]
- Girod, M.; Enjalbert, Q.; Brunet, C.; Antoine, R.; Lemoine, J.; Lukac, I.; Radman, M.; Krisko, A.; Dugourd, P. Structural Basis of Protein Oxidation Resistance: A Lysozyme Study. PLoS ONE 2014, 9, e101642. [Google Scholar] [CrossRef]
- Deshmukh, A.R.; Kim, B.S. Chitosan-Vitamin C Nanoparticles. KSBB J. 2019, 34, 221–232. [Google Scholar] [CrossRef]
- Abu Teir, M.; Abu Awwad, I.; Abu-Hadid, M.; Darwish, S. Study the Interaction of Hydrophobic Vitamins (Vitamin E and Vitamin D) with HSA Using Spectroscopic Techniques. 2018. Available online: http://dspace.alquds.edu/handle/20.500.12213/885 (accessed on 29 October 2021).
- Fukuzawa, K.; Gebicki, J.M. Oxidation of alpha-tocopherol in micelles and liposomes by the hydroxyl, perhydroxyl, and superoxide free radicals. Arch. Biochem. Biophys. 1983, 226, 242–251. [Google Scholar] [CrossRef]
- Nimse, S.B.; Pal, D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015, 5, 27986–28006. [Google Scholar] [CrossRef] [Green Version]
- Rahman, F.; Fontés, M. Ascorbic acid: Binding proteins and pathophysiology. In Vitamin-Binding Proteins: Functional Consequences; CRC Press: Boca Raton, FL, USA, 2013; pp. 257–278. [Google Scholar] [CrossRef]
- Bouillon, R.; Schuit, F.; Antonio, L.; Rastinejad, F. Vitamin D Binding Protein: A Historic Overview. Front. Endocrinol. 2020, 10, 910. [Google Scholar] [CrossRef]
- Yamauchi, R. Vitamin E: Mechanism of Its Antioxidant Activity. Food Sci. Technol. Int. Tokyo 1997, 3, 301–309. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Hayek, M.G.; Meydani, S.N. Symposium: Molecular mechanisms of protective effects of vitamin E in atherosclerosis: Vitamin E and macrophage cyclooxygenase regulation in the aged. J. Nutr. 2001, 131, 369–373. [Google Scholar] [CrossRef] [Green Version]
- Brand, M.; Sommer, M.; Ellmann, S.; Wuest, W.; May, M.S.; Eller, A.; Vogt, S.; Lell, M.M.; Kuefner, M.A.; Uder, M. Influence of Different Antioxidants on X-Ray Induced DNA Double-Strand Breaks (DSBs) Using γ-H2AX Immunofluorescence Microscopy in a Preliminary Study. PLoS ONE 2015, 10, e0127142. [Google Scholar]
- Alcaraz, M.; Armero, D.; Martínez-Beneyto, Y.; Castillo, J.; Benavente-García, O.; Fernandez, H.; Alcaraz-Saura, M.; Canteras, M. Chemical genoprotection: Reducing biological damage to as low as reasonably achievable levels. Dentomaxillofac. Radiol. 2011, 40, 310–314. [Google Scholar] [CrossRef]
- Akbay, E.; Erdem, B.; Ünlü, A.; Durukan, A.B.; Onur, M.A. Effects of N-acetyl cysteine, vitamin E and vitamin C on liver glutathione levels following amiodarone treatment in rats. Kardiochirurgia Torakochirurgia Pol.=Polish J. Cardio-Thoracic Surg. 2019, 16, 88–92. [Google Scholar] [CrossRef]
- Irato, P.; Santovito, G. Enzymatic and Non-Enzymatic Molecules with Antioxidant Function. Antioxidants 2021, 10, 579. [Google Scholar] [CrossRef]















| Dose (Gy) | α-Helix (%) | β-Sheet (%) | β-Turn (%) | Random Coil (%) |
|---|---|---|---|---|
| 0 | 60.10 | 7.50 | 12.98 | 17.40 |
| 0.1 | 54.73 | 8.90 | 13.70 | 19.83 |
| 0.5 | 54.78 | 8.88 | 13.68 | 19.88 |
| 1 | 54.23 | 9.03 | 13.78 | 20.15 |
| 2 | 54.10 | 9.08 | 13.80 | 20.15 |
| 3 | 53.32 | 9.25 | 13.85 | 20.65 |
| Complex | KSV (M−1) | Kq (M−1s−1) | Ka (M−1) | K (M−1) | n |
|---|---|---|---|---|---|
| BSA–Vit C | 25,270 | 2.527 × 1012 | 27,315 | 20,792 | 0.99 |
| IR-BSA–Vit C | 21,134 | 2.1134 × 1012 | 15,593 | 29,580 | 1.04 |
| BSA–Vit E | 15,241 | 1.5241 × 1012 | 12,371 | 12,103 | 0.97 |
| IR-BSA–Vit E | 1592 | 1.592 × 1011 | -- | 1.5 | 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zarei, H.; Bahreinipour, M.; Sefidbakht, Y.; Rezaei, S.; Gheisari, R.; Ardestani, S.K.; Uskoković, V.; Watabe, H. Radioprotective Role of Vitamins C and E against the Gamma Ray-Induced Damage to the Chemical Structure of Bovine Serum Albumin. Antioxidants 2021, 10, 1875. https://doi.org/10.3390/antiox10121875
Zarei H, Bahreinipour M, Sefidbakht Y, Rezaei S, Gheisari R, Ardestani SK, Uskoković V, Watabe H. Radioprotective Role of Vitamins C and E against the Gamma Ray-Induced Damage to the Chemical Structure of Bovine Serum Albumin. Antioxidants. 2021; 10(12):1875. https://doi.org/10.3390/antiox10121875
Chicago/Turabian StyleZarei, Hajar, Mostean Bahreinipour, Yahya Sefidbakht, Shokouh Rezaei, Rouhollah Gheisari, Susan Kabudanian Ardestani, Vuk Uskoković, and Hiroshi Watabe. 2021. "Radioprotective Role of Vitamins C and E against the Gamma Ray-Induced Damage to the Chemical Structure of Bovine Serum Albumin" Antioxidants 10, no. 12: 1875. https://doi.org/10.3390/antiox10121875
APA StyleZarei, H., Bahreinipour, M., Sefidbakht, Y., Rezaei, S., Gheisari, R., Ardestani, S. K., Uskoković, V., & Watabe, H. (2021). Radioprotective Role of Vitamins C and E against the Gamma Ray-Induced Damage to the Chemical Structure of Bovine Serum Albumin. Antioxidants, 10(12), 1875. https://doi.org/10.3390/antiox10121875