Antioxidant and Anti-Inflammatory Activity of Citrus Flavanones Mix and Its Stability after In Vitro Simulated Digestion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. In Vitro Cell-Free Antioxidant and Anti-Inflammatory Screening
2.2.1. Antioxidant Assays
Ferric Reducing Antioxidant Power (FRAP) Assay
Oxygen Radical Absorbance Capacity (ORAC) Assay
Trolox Equivalent Antioxidant Capacity (TEAC) Assay
DPPH (2,2-Difenil-1-Picrylhydrazyl) Assay
2.2.2. Anti-Inflammatory Assays
Albumin Denaturation Assay (ADA)
Anti-Protease Activity (APA)
2.3. Flavanones Mix Preparation
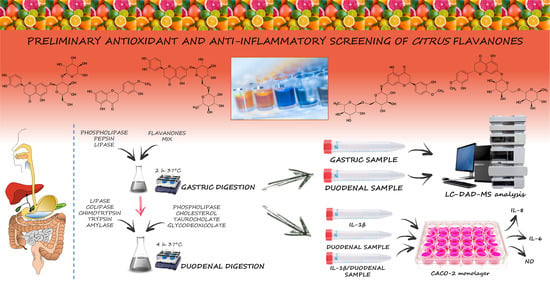
2.4. In Vitro Simulated Human Digestion
2.5. Pre- and Post-Digestion Analyses
2.6. Anti-Inflammatory Activity on In Vitro Cell-Based Model
2.6.1. Cell Model
2.6.2. Anti-Inflammatory Assay
2.6.3. Determination of Inflammatory Markers
2.6.4. Cell Viability
2.7. Statistical Analysis
3. Results
3.1. Antioxidant and Anti-Inflammatory Screening of Flavanones and Flavanones Mix
3.2. Pre- and Post-Digestion Analysis of Flavanones Mix
3.3. Anti-Inflammatory Activity of Flavanones Mix on Caco-2 Cells
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cheynier, V. Phenolic compounds: From plants to foods. Phytochem. Rev. 2012, 11, 153–177. [Google Scholar] [CrossRef]
- Khan, M.K.; Huma, Z.E.; Dangles, O. A comprehensive review on flavanones, the major Citrus polyphenols. J. Food Compos. Anal. 2014, 33, 85–104. [Google Scholar] [CrossRef]
- Barreca, D.; Gattuso, G.; Bellocco, E.; Calderaro, A.; Trombetta, D.; Smeriglio, A.; Laganà, G.; Daglia, M.; Meneghini, S.; Nabavi, S.M. Flavanones: Citrus phytochemical with health-promoting properties. BioFactors 2017, 43, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Mandalari, G.; Calderaro, A.; Smeriglio, A.; Trombetta, D.; Felice, M.R.; Gattuso, G. Citrus flavones: An update on sources, biological functions, and health promoting properties. Plants 2020, 9, 288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.; Ikram, M.; Hahm, J.R.; Kim, M.O. Antioxidant and anti-inflammatory effects of citrus flavonoid hesperetin: Special focus on neurological disorders. Antioxidants 2020, 9, 609. [Google Scholar] [CrossRef] [PubMed]
- Bagetta, D.; Maruca, A.; Lupia, A.; Mesiti, F.; Catalano, R.; Romeo, I.; Moraca, F.; Ambrosio, F.A.; Costa, G.; Artese, A.; et al. Mediterranean products as promising source of multi-target agents in the treatment of metabolic syndrome. Eur. J. Med. Chem. 2020, 186, 111903. [Google Scholar] [CrossRef] [PubMed]
- Rees, A.; Dodd, G.F.; Spencer, J.P.E. The effects of flavonoids on cardiovascular health: A review of human intervention trials and implications for cerebrovascular function. Nutrients 2018, 10, 1852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoud, A.M.; Hernández Bautista, R.J.; Sandhu, M.A.; Hussein, O.E. Beneficial effects of Citrus flavonoids on cardiovascular and metabolic health. Oxid. Med. Cell. Longev. 2019, 2019, 5484138. [Google Scholar] [CrossRef] [Green Version]
- Musumeci, L.; Maugeri, A.; Cirmi, S.; Lombardo, G.E.; Russo, C.; Gangemi, S.; Calapai, G.; Navarra, M. Citrus fruits and their flavonoids in inflammatory bowel disease: An overview. Nat. Prod. Res. 2020, 34, 122–136. [Google Scholar] [CrossRef]
- Stevens, Y.; Rymenant, E.V.; Grootaert, C.; Camp, J.V.; Possemiers, S.; Masclee, A.; Jonkers, D. The intestinal fate of Citrus flavanones and their effects on gastrointestinal health. Nutrients 2019, 11, 1464. [Google Scholar] [CrossRef] [Green Version]
- Tanveer, A.; Akram, K.; Farooq, U.; Hayat, Z.; Shafi, A. Management of diabetic complications through fruit flavonoids as a natural remedy. Crit. Rev. Food Sci. Nutr. 2017, 57, 1411–1422. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, G.R.; Vasconcelos, A.B.S.; Wu, D.T.; Li, H.B.; Antony, P.J.; Li, H.; Geng, F.; Gurgel, R.Q.; Narain, N.; Gan, R.Y. Citrus flavonoids as promising phytochemicals targeting diabetes and related complications: A systematic review of in vitro and in vivo studies. Nutrients 2020, 12, 2907. [Google Scholar] [CrossRef] [PubMed]
- Koolaji, N.; Shammugasamy, B.; Schindeler, A.; Dong, Q.; Dehghani, F.; Valtchev, P. Citrus peel flavonoids as potential cancer prevention agents. Curr. Dev. Nutr. 2020, 4, nzaa025. [Google Scholar] [CrossRef] [PubMed]
- Salaritabar, A.; Darvishi, B.; Hadjiakhoondi, F.; Manayi, A.; Sureda, A.; Nabavi, S.F.; Fitzpatrick, L.R.; Nabavi, S.M.; Bishayee, A. Therapeutic potential of flavonoids in inflammatory bowel disease: A comprehensive review. World J. Gastroenterol. 2017, 23, 5097–5114. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Garand, M.; Al Khodor, S. Integrating omics for a better understanding of inflammatory bowel disease: A step towards personalized medicine. J. Transl. Med. 2019, 17, 419. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.; Lei, L.; Zhou, Y.; Ye, F.; Zhao, G. Dietary flavonoids and the risk of colorectal cancer: An updated meta-analysis of epidemiological studies. Nutrients 2018, 10, 950. [Google Scholar] [CrossRef] [Green Version]
- Fusco, R.; Cirmi, S.; Gugliandolo, E.; Di Paola, R.; Cuzzocrea, S.; Navarra, M. A flavonoid-rich extract of orange juice reduced oxidative stress in an experimental model of inflammatory bowel disease. J. Func. Foods 2017, 30, 168–178. [Google Scholar] [CrossRef]
- Gholap, P.A.; Nirmal, S.A.; Pattan, S.R.; Pal, S.C.; Mandal, S.C. Potential of Moringa oleifera root and Citrus sinensis fruit rind extracts in the treatment of ulcerative colitis in mice. Pharm. Biol. 2012, 50, 1297–1302. [Google Scholar] [CrossRef]
- Khan, R.A.; Mallick, N.; Feroz, Z. Anti-inflammatory effects of Citrus sinensis L., Citrus paradisi L. and their combinations. Pak. J. Pharm. Sci. 2016, 29, 843–852. [Google Scholar]
- He, W.; Li, Y.; Liu, M.; Yu, H.; Chen, Q.; Chen, Y.; Ruan, J.; Ding, Z.; Zhang, Y.; Wang, T. Citrus aurantium L. and its flavonoids regulate TNBS-induced inflammatory bowel disease through antiinflammation and suppressing isolated jejunum contraction. Int. J. Mol. Sci. 2018, 19, 3057. [Google Scholar] [CrossRef]
- Abe, H.; Ishioka, M.; Fujita, Y.; Umeno, A.; Yasunaga, M.; Sato, A.; Ohnishi, S.; Suzuki, S.; Ishida, N.; Shichiri, M. Yuzu (Citrus junos Tanaka) peel attenuates dextran sulfate sodium-induced murine experimental colitis. J. Oleo Sci. 2018, 67, 335–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamiloglu, S.; Capanoglu, E.; Bilen, F.D.; Gonzales, G.B.; Grootaert, C.; Van de Wiele, T.; Van Camp, J. Bioaccessibility of polyphenols from plant-processing byproducts of black carrot (Daucus carota L.). J. Agric. Food Chem. 2016, 64, 2450–2458. [Google Scholar] [CrossRef]
- Smeriglio, A.; Bonasera, S.; Germanò, M.P.; D’angelo, V.; Barreca, D.; Denaro, M.; Monforte, M.T.; Galati, E.M.; Trombetta, D. Opuntia ficus-indica (L.) Mill. fruit as source of betalains with antioxidant, cytoprotective, and anti-angiogenic properties. Phytoter. Res. 2019, 33, 1526–1537. [Google Scholar] [CrossRef]
- Muscarà, C.; Smeriglio, A.; Trombetta, D.; Mandalari, G.; La Camera, E.; Occhiuto, C.; Grassi, G.; Circosta, C. Antioxidant and antimicrobial activity of two standardized extracts from a new Chinese accession of non-psychotropic Cannabis sativa L. Phytother. Res. 2020. [Google Scholar] [CrossRef]
- Bazzicalupo, M.; Burlando, B.; Denaro, M.; Barreca, D.; Trombetta, D.; Smeriglio, A.; Cornara, L. Polyphenol characterization and skin-preserving properties of hydroalcoholic flower extract from Himantoglossum robertianum (Orchidaceae). Plants 2019, 8, 502. [Google Scholar] [CrossRef] [Green Version]
- Smeriglio, A.; Cornara, L.; Denaro, M.; Barreca, D.; Burlando, B.; Xiao, J.; Trombetta, D. Antioxidant and cytoprotective activities of an ancient Mediterranean citrus (Citrus lumia Risso) albedo extract: Microscopic observations and polyphenol characterization. Food Chem. 2019, 279, 347–355. [Google Scholar] [CrossRef]
- Smeriglio, A.; Denaro, M.; D’Angelo, V.; Germano, M.P.; Trombetta, D. Antioxidant, anti-inflammatory and anti-angiogenic properties of Citrus lumia juice. Front. Pharmacol. 2020, 11, 593506. [Google Scholar] [CrossRef]
- Trombetta, D.; Smeriglio, A.; Denaro, M.; Zagami, R.; Tomassetti, M.; Pilolli, R.; De Angelis, E.; Monaci, L.; Mandalari, G. Understanding the fate of almond (Prunus dulcis (Mill.) D.A. Webb) oleosomes during simulated digestion. Nutrients 2020, 12, 3397. [Google Scholar] [CrossRef]
- ICH Q2(R1). Validation of Analytical Procedures, Definitions and Terminology; International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use: Geneva, Switzerland, 1996. [Google Scholar]
- Denaro, M.; Smeriglio, A.; De Francesco, C.; Xiao, J.; Cornara, L.; Trombetta, D. In vitro intestinal transport and anti-inflammatory properties of ideain across Caco-2 transwell model. Fitoterapia 2020, 146, 104723. [Google Scholar] [CrossRef]
- Tesoriere, L.; Attanzio, A.; Allegra, M.; Gentile, C.; Livrea, M.A. Indicaxanthin inhibits NADPH oxidase (NOX)-1 activation and NF-κB-dependent release of inflammatory mediators and prevents the increase of epithelial permeability in IL-1β-exposed Caco-2 cells. Br. J. Nutr. 2014, 111, 415–423. [Google Scholar] [CrossRef] [Green Version]
- Kenzaoui, B.H.; Vilà, M.R.; Miquel, J.P.; Cengelli, F.; Juillerat-Jeanneret, L. Evaluation of uptake and transport of cationic and anionic ultrasmall iron oxide nanoparticles by human colon cells. Int. J. Nanomed. 2012, 1275–1286. [Google Scholar]
- Sun, H.; Chow, E.C.; Liu, S.; Du, Y.; Pang, K.S. The Caco-2 cell monolayer: Usefulness and limitations. Expert Opin. Drug Metab. Toxicol. 2008, 4, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Kamiloglu, S.; Capanoglu, E.; Grootaert, C.; Van Camp, J. Anthocyanin absorption and metabolism by human intestinal Caco-2 cells–a review. Int. J. Mol. Sci. 2015, 16, 21555–21574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tejada, S.; Pinya, S.; Martorell, M.; Capó, X.; Tur, J.A.; Pons, A.; Sureda, A. Potential anti-inflammatory effects of hesperidin from the genus Citrus. Curr. Med. Chem. 2018, 25, 4929–4945. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Fokou, P.V.T.; Sharifi-Rad, M.; Zucca, P.; Pezzani, R.; Martins, N.; Sharifi-Rad, J. The therapeutic potential of naringenin: A review of clinical trials. Pharmaceuticals 2019, 12, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira de Oliveira, J.M.P.; Santos, C.; Fernandes, E. Therapeutic potential of hesperidin and its aglycone hesperetin: Cell cycle regulation and apoptosis induction in cancer models. Phytomedicine 2020, 73, 152887. [Google Scholar] [CrossRef]
- Arafah, A.; Rehman, U.; Mir, T.M.; Wali, A.F.; Ali, R.; Qamar, W.; Khan, R.; Ahmad, A.; Aga, S.S.; Alqahtani, S.; et al. Multi-Therapeutic Potential of Naringenin (4’,5,7-Trihydroxyflavonone): Experimental Evidence and Mechanisms. Plants 2020, 9, 1784. [Google Scholar] [CrossRef]
- Smeriglio, A.; Giofrè, S.V.; Galati, E.M.; Monforte, M.T.; Cicero, N.; D’Angelo, V.; Grassi, G.; Circosta, C. Inhibition of aldose reductase activity by Cannabis sativa chemotypes extracts with high content of cannabidiol or cannabigerol. Fitoterapia 2018, 127, 101–108. [Google Scholar] [CrossRef]
- Coelho, J.; Barros, L.; Dias, M.I.; Finimundy, T.C.; Amaral, J.S.; Alves, M.J.; Calhelha, R.C.; Santos, P.F.; Ferreira, I.C.F.R. Echinacea purpurea (L.) Moench: Chemical Characterization and Bioactivity of Its Extracts and Fractions. Pharmaceuticals 2020, 13, 125. [Google Scholar] [CrossRef]
- Giménez-Bastida, J.A.; Martínez-Florensa, M.; Espín, J.C.; Tomás-Barberán, F.A.; García-Conesa, M.T. A Citrus extract containing flavanones represses plasminogen activator inhibitor-1 (PAI-1) expression and regulates multiple inflammatory, tissue repair, and fibrosis genes in human colon fibroblasts. J. Agric. Food Chem. 2009, 57, 9305–9315. [Google Scholar] [CrossRef]
- Kobayashi, S.; Tanabe, S.; Sugiyama, M.; Konishi, Y. Transepithelial transport of hesperetin and hesperidin in intestinal Caco-2 cell monolayers. BBA 2008, 1778, 33–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walle, T.; Browning, A.M.; Steed, L.L.; Reed, S.G.; Walle, U.K. Flavonoid glucosides are hydrolyzed and thus activated in the oral cavity in humans. J. Nutr. 2005, 135, 48–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viswanatha Swamy, A.H.M. Effect of some clinically used proteolytic enzymes on inflammation in rats. Indian J. Pharm. Sci. 2008, 70, 114–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manthey, J.A.; Grohmann, K. Concentrations of hesperidin and other orange peel flavonoids in Citrus processing byproducts. J. Agric. Food Chem. 1996, 44, 811–814. [Google Scholar] [CrossRef]
- Parhiz, H.; Roohbakhsh, A.; Soltani, F.; Rezaee, R.; Iranshahi, M. Antioxidant and anti-inflammatory properties of the citrus flavonoids hesperidin and hesperetin: An updated review of their molecular mechanisms and experimental models. Phytother. Res. 2015, 29, 323–331. [Google Scholar] [CrossRef]
- Bodduluru, L.N.; Kasala, E.R.; Madhana, R.M.; Barua, C.C.; Hussain, M.I.; Haloi, P.; Borah, P. Naringenin ameliorates inflammation and cell proliferation in benzo(a)pyrene induced pulmonary carcinogenesis by modulating CYP1A1, NFjB and PCNA expression. Int. Immunopharmacol. 2016, 30, 102–110. [Google Scholar] [CrossRef]
- Guazelli, C.F.S.; Fattori, V.; Ferraz, C.R.; Borghi, S.M.; Casagrande, R.; Baracat, M.M.; Verri, W.A., Jr. Antioxidant and anti-inflammatory effects of hesperidin methyl chalcone in experimental ulcerative colitis. Chem. Biol. Interact. 2021, 333, 109315. [Google Scholar] [CrossRef]
- Wedlake, L.; Slack, N.; Andreyev, H.J.; Whelan, K. Fiber in the treatment and maintenance of inflammatory bowel disease: A systematic review of randomized controlled trials. Inflamm. Bowel. Dis. 2014, 20, 576–586. [Google Scholar] [CrossRef]
- Li, C.; Zhang, J.; Lv, F.; Ge, X.; Li, G. Naringin protects against bone loss in steroid-treated inflammatory bowel disease in a rat model. Arch. Biochem. Biophys. 2018, 650, 22–29. [Google Scholar] [CrossRef]





| Flavanone | Calibration Range (µM) | Equation | Linearity (R2) | R.S.D. 1 (%), n = 6 Within-Day | R.S.D. (%), n = 6 Between-Day | LOD 2 (µM) | LOQ 3 (µM) | Recovery (%) |
|---|---|---|---|---|---|---|---|---|
| Eriocitrin | 0.625–20.0 | y = 19.891x | 0.9993 | 0.177 | 0.177 | 0.014 | 0.042 | 95.28 |
| Neoeriocitrin | 0.625–20.0 | y = 9.627x | 0.9998 | 0.240 | 0.240 | 0.027 | 0.081 | 94.79 |
| Hesperidin | 0.625–20.0 | y = 22.944x | 0.9999 | 0.230 | 0.230 | 0.016 | 0.050 | 99.48 |
| Neohesperidin | 0.625–20.0 | y = 17.954x | 0.9994 | 0.275 | 0.275 | 0.015 | 0.045 | 97.83 |
| Hesperetin | 0.625–20.0 | y = 26.473x | 0.9998 | 0.243 | 0.243 | 0.049 | 0.148 | 90.36 |
| Flavanone | RT 1 | λmax 2 | MW 3 | [M−H]− (m/z) | MS/MS (m/z) | FM 4 (µM) | GFM 5 (µM) | DFM 6 (µM) |
|---|---|---|---|---|---|---|---|---|
| Eriocitrin | 32.460 | 284; 336 | 596.5 | 595 | 459; 287 | 10 | 9.05 ± 0.12 | 8.80 ± 0.03 |
| Neoeriocitrin | 33.256 | 284; 334 | 596.5 | 595 | 459; 287 | 10 | 8.92 ± 0.08 | 8.77 ± 0.16 |
| Hesperidin | 36.183 | 284; 332 | 610.5 | 609 | 301; 286 | 10 | 9.95 ± 0.04 | 9.86 ± 0.13 |
| Neohesperidin | 37.520 | 284; 334 | 610.5 | 609 | 301; 286 | 10 | 9.19 ± 0.22 | 9.04 ± 0.18 |
| Hesperetin | 48.077 | 288; 336 | 302.2 | 301 | 286 | 10 | 9.38 ± 0.18 | 8.76 ± 0.22 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denaro, M.; Smeriglio, A.; Trombetta, D. Antioxidant and Anti-Inflammatory Activity of Citrus Flavanones Mix and Its Stability after In Vitro Simulated Digestion. Antioxidants 2021, 10, 140. https://doi.org/10.3390/antiox10020140
Denaro M, Smeriglio A, Trombetta D. Antioxidant and Anti-Inflammatory Activity of Citrus Flavanones Mix and Its Stability after In Vitro Simulated Digestion. Antioxidants. 2021; 10(2):140. https://doi.org/10.3390/antiox10020140
Chicago/Turabian StyleDenaro, Marcella, Antonella Smeriglio, and Domenico Trombetta. 2021. "Antioxidant and Anti-Inflammatory Activity of Citrus Flavanones Mix and Its Stability after In Vitro Simulated Digestion" Antioxidants 10, no. 2: 140. https://doi.org/10.3390/antiox10020140
APA StyleDenaro, M., Smeriglio, A., & Trombetta, D. (2021). Antioxidant and Anti-Inflammatory Activity of Citrus Flavanones Mix and Its Stability after In Vitro Simulated Digestion. Antioxidants, 10(2), 140. https://doi.org/10.3390/antiox10020140