Effect on the Antioxidant, Lipoperoxyl Radical Scavenger Capacity, Nutritional, Sensory and Microbiological Traits of an Ovine Stretched Cheese Produced with Grape Pomace Powder Addition
Abstract
:1. Introduction
2. Materials and Methods
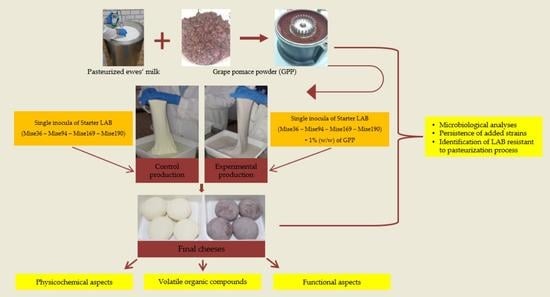
2.1. Grape Pomace Powder Production and Natural Milk Starter Culture Preparation
2.2. Cheese Production and Sample Collection
2.3. Microbiological Analyses
2.4. Persistence of Added Strains and Identification of the Survival Indigenous Milk LAB
2.5. Physicochemical Analyses of Cheeses
2.6. Volatile Organic Compounds
2.7. Sensory Evaluation
2.8. In Vitro Gastrointestinal Digestion
2.9. ABTS + Radical Cation Decolorization Assay
2.10. Lipid Peroxidation Assay
2.11. Statistical Analyses
3. Results and Discussion
3.1. Acidification Kinetics of Curds
3.2. Microbiological Analyses
3.3. Persistence of LAB Inoculums
3.4. Physicochemical Analyses of Cheeses
3.5. Volatile Organic Compounds
3.6. Sensory Test
3.7. Functional Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mikovà, K. The regulation of antioxidants in foods. In Handbook of Food Preservation; Rahman, M., Ed.; CRC Press: Boca Raton, FL, USA, 2007; Volume 2, pp. 83–267. [Google Scholar]
- Torri, L.; Piochi, M.; Marchiani, R.; Zeppa, G.; Dinnella, C.; Monteleone, E. A sensory-and consumer-based approach to optimize cheese enrichment with grape skin powders. J. Dairy Sci. 2016, 99, 194–204. [Google Scholar] [CrossRef] [Green Version]
- Bachmann, P. Cheese analogues: A review. Int. Dairy J. 2001, 11, 505–515. [Google Scholar] [CrossRef]
- Tomas, M.; Zhang, L.; Zengin, G.; Rocchetti, G.; Capanoglu, E.; Lucini, L. Metabolomic insight into the profile, in vitro bioaccessibility and bioactive properties of polyphenols and glucosinolates from four Brassicaceae microgreens. Food Res. Int. 2021, 140, 110039. [Google Scholar] [CrossRef]
- Elejalde, E.; Villarán, M.C.; Alonso, R.M. Grape polyphenols supplementation for exercise-induced oxidative stress. J. Int. Soc. Sports Nutr. 2021, 18, 1–12. [Google Scholar] [CrossRef]
- Lavelli, V.; Gallotti, F.; Pedrali, D. Application of compounds from grape processing by-products: Formulation of dietary fiber and encapsulated bioactive compounds. In Food Waste Recovery; Galanakis, C.M., Ed.; Academic Press: Gaithersburg, MD, USA, 2021; pp. 355–366. [Google Scholar]
- Marchiani, R.; Bertolino, M.; Ghirardello, D.; McSweeney, P.L.; Zeppa, G. Physicochemical and nutritional qualities of grape pomace powder-fortified semi-hard cheeses. J. Food Sci. Technol. 2016, 53, 1585–1596. [Google Scholar] [CrossRef] [Green Version]
- Theagarajan, R.; Narayanaswamy, M.L.; Dutta, S.; Moses, J.A.; Chinnaswamy, A. Valorisation of grape pomace (cv. Muscat) for development of functional cookies. Int. J. Food Sci. Technol. 2019, 54, 1299–1305. [Google Scholar] [CrossRef]
- Beres, C.; Costa, G.N.; Cabezudo, I.; da Silva-James, N.K.; Teles, A.S.; Cruz, A.P.; Mellinger-Silva, C.; Tonon, R.V.; Cabral, L.M.C.; Freitas, S.P. Towards integral utilization of grape pomace from winemaking process: A review. Waste Manag. 2017, 68, 581–594. [Google Scholar] [CrossRef] [PubMed]
- Fontana, A.R.; Antoniolli, A.; Bottini, R. Grape pomace as a sustainable source of bioactive compounds: Extraction, characterization, and biotechnological applications of phenolics. J. Agric. Food Chem. 2013, 61, 8987–9003. [Google Scholar] [CrossRef]
- Aghamirzaei, M.; Peighambardoust, S.H.; Azadmard-Damirchi, S.; Majzoobi, M. Effects of grape seed powder as a functional ingredient on flour physicochemical characteristics and dough rheological properties. J. Agric. Sci. Technol. 2015, 17, 365–373. [Google Scholar]
- García-Lomillo, J.; Gonzalez-SanJose, M.L.; Del Pino-García, R.; Ortega-Heras, M.; Muñiz-Rodríguez, P. Antioxidant effect of seasonings derived from wine pomace on lipid oxidation in refrigerated and frozen beef patties. LWT Food Sci. Technol. 2017, 77, 85–91. [Google Scholar] [CrossRef]
- Sánchez-Alonso, I.; Jiménez-Escrig, A.; Saura-Calixto, F.; Borderías, A.J. Effect of grape antioxidant dietary fibre on the prevention of lipid oxidation in minced fish: Evaluation by different methodologies. Food Chem. 2007, 101, 372–378. [Google Scholar] [CrossRef]
- Tseng, A.; Zhao, Y. Wine grape pomace as antioxidant dietary fibre for enhancing nutritional value and improving storability of yogurt and salad dressing. Food Chem. 2013, 138, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Barbaccia, P.; Francesca, N.; Di Gerlando, R.; Busetta, G.; Moschetti, G.; Gaglio, R.; Settanni, L. Biodiversity and dairy traits of indigenous milk lactic acid bacteria grown in presence of the main grape polyphenols. FEMS Microbiol. Lett. 2020, 367, fnaa066. [Google Scholar] [CrossRef] [PubMed]
- Cruciata, M.; Gaglio, R.; Todaro, M.; Settanni, L. Ecology of Vastedda della valle del Belìce cheeses: A review and recent findings to stabilize the traditional production. Food Rev. Int. 2019, 35, 90–103. [Google Scholar] [CrossRef]
- ISO (International Organization for Standardization). Enumeration of Colony-Forming Units of Micro-Organism-Colony-Count Technique at 30 Degrees C. Milk and milk Products; International Standardization Organization (ISO): Geneva, Switzerland, 1992; Volume ISO 6610. [Google Scholar]
- Settanni, L.; Di Grigoli, A.; Tornambè, G.; Bellina, V.; Francesca, N.; Moschetti, G.; Bonanno, A. Persistence of wild Streptococcus thermophilus strains on wooden vat and during the manufacture of a Caciocavallo type cheese. Int. J. Food Microbiol. 2012, 155, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Alfonzo, A.; Urso, V.; Corona, O.; Francesca, N.; Amato, G.; Settanni, L.; Di Miceli, G. Development of a method for the direct fermentation of semolina by selected sourdough lactic acid bacteria. Int. J. Food Microbiol. 2016, 239, 65–78. [Google Scholar] [CrossRef] [Green Version]
- Cruciata, M.; Gaglio, R.; Scatassa, M.L.; Sala, G.; Cardamone, C.; Palmeri, M.; Moschetti, G.; La Mantia, T.; Settanni, L. Formation and characterization of early bacterial biofilms on different wood typologies applied in dairy production. Appl. Environ. Microbiol. 2018, 84, e02107–e02117. [Google Scholar] [CrossRef] [Green Version]
- Weisburg, W.; Barns, S.M.; Pelletier, D.A.; Lane, D.J. 16S ribosomal DNA amplification for phylogenetic study. J. Bacteriol. 1991, 173, 697–703. [Google Scholar] [CrossRef] [Green Version]
- IDF (International Dairy Federation). Cheese and Processed Cheese Product. Determination of the Total Solids Content. Standard FIL-IDF 4A; International Dairy Federation: Brussels, Belgium, 1982. [Google Scholar]
- IDF (International Dairy Federation). Cheese and Processed Cheese Product. Determination of Fat Content-Gravimetric Method. Standard FIL-IDF 5B; International Dairy Federation: Brussels, Belgium, 1986. [Google Scholar]
- IDF (International Dairy Federation). Determination of the Protein Content of Processed Cheese Products. Standard FIL-IDF 25; International Dairy Federation: Brussels, Belgium, 1964. [Google Scholar]
- IDF (International Dairy Federation). Determination of the Ash Content of Processed Cheese Products. Standard FIL-IDF 27; International Dairy Federation: Brussels, Belgium, 1964. [Google Scholar]
- Bonanno, A.; Di Grigoli, A.; Vitale, F.; Di Miceli, G.; Todaro, M.; Alabiso, M.; Gargano, M.L.; Venturella, G.; Anike, F.N.; Isikhuemhen, O.S. Effects of feeding diets supplemented with medicinal mushrooms myceliated grains on some production, health and oxidation traits of dairy ewes. Int. J. Med. Mushrooms 2019, 21, 89–103. [Google Scholar] [CrossRef]
- ISO (International Organization for Standardization). Sensory Analysis e General Guidance for the Design of Test Rooms; International Standardization Organization (ISO): Geneva, Switzerland, 2007; Volume 8589. [Google Scholar]
- Costa, C.; Lucera, A.; Marinelli, V.; Del Nobile, M.A.; Conte, A. Influence of different by-products addition on sensory and physicochemical aspects of Primosale cheese. J. Food Sci. Technol. 2018, 55, 4174–4183. [Google Scholar] [CrossRef]
- Faccia, M.; Angiolillo, L.; Mastromatteo, M.; Conte, A.; Del Nobile, M.A. The effect of incorporating calcium lactate in the saline solution on improving the shelf life of fiordilatte cheese. Int. J. Dairy Technol. 2013, 66, 373–381. [Google Scholar]
- Attanzio, A.; Diana, P.; Barraja, P.; Carbone, A.; Spanò, V.; Parrino, B.; Cascioferro, S.M.; Allegra, M.; Cirrincione, G.; Tesoriere, L.; et al. Quality, functional and sensory evaluation of pasta fortified with extracts from Opuntia ficus-indica cladodes. J. Sci. Food Agric. 2019, 99, 4242–4247. [Google Scholar] [CrossRef]
- Attanzio, A.; Tesoriere, L.; Allegra, M.; Livrea, M.A. Monofloral honeys by Sicilian black honeybee (Apis mellifera ssp. sicula) have high reducing power and antioxidant capacity. Heliyon 2016, 2, e00193. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Gaglio, R.; Scatassa, M.L.; Cruciata, M.; Miraglia, V.; Corona, O.; Di Gerlando, R.; Portolano, B.; Moschetti, G.; Settanni, L. In vivo application and dynamics of lactic acid bacteria for the four-season production of Vastedda-like cheese. Int. J. Food Microbiol. 2014, 177, 37–48. [Google Scholar] [CrossRef] [Green Version]
- Mainente, F.; Menin, A.; Alberton, A.; Zoccatelli, G.; Rizzi, C. Evaluation of the sensory and physical properties of meat and fish derivatives containing grape pomace powders. Int. J. Food Sci. Technol. 2019, 54, 952–958. [Google Scholar] [CrossRef]
- Franciosi, E.; Settanni, L.; Cologna, N.; Cavazza, A.; Poznanski, E. Microbial analysis of raw cows’ milk used for cheese-making: Influence of storage treatments on microbial composition and other technological traits. World J. Microbiol. Biotechnol. 2011, 27, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Rynne, N.M.; Beresford, T.P.; Kelly, A.L.; Guinee, T.P. Effect of milk pasteurisation temperature on age-related changes in lactose metabolism, pH and the growth of non-starter lactic acid bacteria in half-fat Cheddar cheese. Food Chem. 2007, 100, 375–382. [Google Scholar] [CrossRef]
- Gaglio, R.; Gentile, C.; Bonanno, A.; Vintaloro, L.; Perrone, A.; Mazza, F.; Barbaccia, P.; Settanni, L.; Di Grigoli, A. Effect of saffron addition on the microbiological, physicochemical, antioxidant and sensory characteristics of yoghurt. Int. J. Dairy Technol. 2019, 72, 208–217. [Google Scholar] [CrossRef]
- Dumalisile, P.; Witthuhn, R.C.; Britz, T.J. Impact of different pasteurization temperatures on the survival of microbial contaminants isolated from pasteurized milk. Int. J. Dairy Technol. 2005, 58, 74–82. [Google Scholar] [CrossRef]
- Settanni, L.; Gaglio, R.; Guarcello, R.; Francesca, N.; Carpino, S.; Sannino, C.; Todaro, M. Selected lactic acid bacteria as a hurdle to the microbial spoilage of cheese: Application on a traditional raw ewes’ milk cheese. Int. Dairy J. 2013, 32, 126–132. [Google Scholar] [CrossRef] [Green Version]
- Gaglio, R.; Cruciata, M.; Di Gerlando, R.; Scatassa, M.L.; Cardamone, C.; Mancuso, I.; Sardina, M.T.; Moschetti, G.; Portolano, B.; Settanni, L. Microbial activation of wooden vats used for traditional cheese production and evolution of the neo-formed biofilms. Appl. Environ. Microbiol. 2016, 82, 585–595. [Google Scholar] [CrossRef] [Green Version]
- Gaglio, R.; Cruciata, M.; Scatassa, M.L.; Tolone, M.; Mancuso, I.; Cardamone, C.; Corona, O.; Todaro, M.; Settanni, L. Influence of the early bacterial biofilms developed on vats made with seven wood types on PDO Vastedda della valle del Belìce cheese characteristics. Int. J. Food Microbiol. 2019, 291, 91–103. [Google Scholar] [CrossRef]
- Settanni, L.; Moschetti, G. Non-starter lactic acid bacteria used to improve cheese quality and provide health benefits. Food Microbiol. 2010, 27, 691–697. [Google Scholar] [CrossRef]
- Todaro, M.; Palmeri, M.; Settanni, L.; Scatassa, M.L.; Mazza, F.; Bonanno, A.; Di Grigoli, A. Effect of refrigerated storage on microbiological, chemical and sensory characteristics of a ewes’ raw milk stretched cheese. Food Packag. Shelf Life 2017, 11, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, L.F.; Ribani, R.H.; Francisco, T.M.G.; Soares, A.A.; Pontarolo, R.; Haminiuk, C.W.I. Profile of bioactive compounds from grape pomace (Vitis vinifera and Vitis labrusca) by spectrophotometric, chromatographic and spectral analyses. J. Chromatogr. B 2015, 1007, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Guarrasi, V.; Sannino, C.; Moschetti, M.; Bonanno, A.; Di Grigoli, A.; Settanni, L. The individual contribution of starter and non starter lactic acid bacteria to the volatile organic compound composition of Caciocavallo Palermitano cheese. Int. J. Food Microbiol. 2017, 259, 35–42. [Google Scholar] [CrossRef]
- Kırmacı, H.A.; Hayaloğlu, A.A.; Özer, H.B.; Atasoy, A.F.; Levent, O. Effects of wild-type starter culture (artisanal strains) on volatile profile of urfa cheese made from ewe milk. Int. J. Food Prop. 2015, 18, 1915–1929. [Google Scholar] [CrossRef]
- Smit, G.; Smit, B.A.; Engels, W.J. Flavour formation by lactic acid bacteria and biochemical flavour profiling of cheese products. FEMS Microbiol. Rev. 2005, 29, 591–610. [Google Scholar] [CrossRef]
- Thierry, A.; Collins, Y.F.; Mukdsi, M.C.A.; McSweeney, P.L.H.; Wilkinson, M.G.; Spinnler, H.E. Lipolysis and metabolism of fatty acids in cheese. In Cheese: Chemistry, Physics, and Microbiology; McSweeney, P.L.H., Fox, P.F., Cotter, P.D., Everett, D.W., Eds.; Academic Press: Gaithersburg, MD, USA, 2017; Volume 1, pp. 423–444. [Google Scholar]
- Todaro, M.; Palmeri, M.; Cardamone, C.; Settanni, L.; Mancuso, I.; Mazza, F.; Corona, O. Impact of packaging on the microbiological, physicochemical and sensory characteristics of a “pasta filata” cheese. Food Packag. Shelf Life 2018, 17, 85–90. [Google Scholar] [CrossRef]
- Muñoz, N.; Ortigosa, M.; Torre, P.; Izco, J.M. Free amino acids and volatile compounds in an ewe’s milk cheese as affected by seasonal and cheese-making plant variations. Food Chem. 2003, 83, 329–338. [Google Scholar] [CrossRef]
- Abilleira, E.; de Renobales, M.; Nájera, A.I.; Virto, M.; de Gordoa, J.C.R.; Pérez-Elortondo, F.J.; Albisu, M.; Barron, L.J.R. An accurate quantitative method for the analysis of terpenes in milk fat by headspace solid-phase microextraction coupled to gas chromatography–mass spectrometry. Food Chem. 2010, 120, 1162–1169. [Google Scholar] [CrossRef]
- Abd Elhamid, A.M. Physicochemical, rheological and sensory properties of Egyptian Kariesh cheese containing wheat bran. Int. J. Dairy Technol. 2016, 69, 425–432. [Google Scholar] [CrossRef]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [Green Version]
- Helal, A.; Tagliazucchi, D.; Verzelloni, E.; Conte, A. Gastro-pancreatic release of phenolic compounds incorporated in a polyphenols-enriched cheese-curd. Food Sci. Technol. 2015, 60, 957–963. [Google Scholar] [CrossRef] [Green Version]
- Petrat-Melin, B.; Andersen, P.; Rasmussen, J.T.; Poulsen, N.A.; Larsen, L.B.; Young, J.F. In vitro digestion of purified b-casein variants A1, A2, B, and I: Effects on antioxidant and angiotensin-converting enzyme inhibitory capacity. J. Dairy Sci. 2014, 98, 15–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, A.; Mann, B.; Kumar, R.; Sangwan, R.B. Antioxidant activity of Cheddar cheeses at different stages of ripening. Int. J. Dairy Technol. 2009, 62, 339–347. [Google Scholar] [CrossRef]
- Lucas, A.; Agabriel, C.; Martin, B.; Ferlay, A.; Verdier-Metz, I.; Coulon, J.B.; Rock, E. Relationships between the conditions of cow’s milk production and the contents of components of nutritional interest in raw milk farmhouse cheese. Le Lait 2006, 86, 177–202. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Helal, A.; Verzelloni, E.; Conte, A. The type and concentration of milk increased the in vitro bioaccessibility of coffee chlorogenic acids. J. Agric. Food Chem. 2012, 60, 11056–11064. [Google Scholar] [CrossRef]
- Ursini, F.; Sevanian, A. Postprandial oxidative stress. Biol. Chem. 2002, 383, 599–605. [Google Scholar] [CrossRef]





| Analyses | Sampling Points | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BM | PBM | IM | Curd t0 | Acidified Curds | Cheese at t0 | Cheese at 15 d | |||||
| Ctr | Exp | Ctr | Exp | Ctr | Exp | Ctr | Exp | ||||
| pH | ■ | ■ | ■ | ■ | ■ | ■ | |||||
| plate counts | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ |
| molecular typing | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | ■ | |
| physical aspects | ■ | ■ | |||||||||
| chemical composition | ■ | ■ | |||||||||
| VOCs | ■ | ■ | |||||||||
| sensory tests | ■ | ■ | |||||||||
| functional properties | ■ | ■ | |||||||||
| Treatment (TR) | Natural Milk Starter Culture (NMSC) | Significance p< | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MISE36 | MISE94 | MISE169 | MISE190 | SEM | TR | NMSC | TR*NMSC | ||||
| Inoculated milk | TMM | 7.23 ab | 7.33 a | 6.94 ab | 7.08 b | 0.088 | 0.0442 | ||||
| MCLAB | 7.30 | 6.95 | 7.04 | 7.18 | 0.14 | 0.3546 | |||||
| Curd t0 | TMM | Ctr | 7.93 | 8.12 | 7.98 | 7.83 | 7.77 | 0.10 | 0.0227 | 0.0096 | 0.7364 |
| Exp | 7.75 | 7.99 | 7.71 | 7.61 | 7.71 | ||||||
| Tot | 8.06 a | 7.84 ab | 7.72 b | 7.74 b | |||||||
| MCLAB | Ctr | 7.77 | 7.89 | 7.81 | 7.74 | 7.63 | 0.13 | 0.7777 | 0.3440 | 0.7577 | |
| Exp | 7.74 | 7.78 | 7.89 | 7.60 | 7.70 | ||||||
| Tot | 7.83 | 7.85 | 7.67 | 7.66 | |||||||
| Acidified curd | TMM | Ctr | 9.13 | 9.01 | 9.23 | 9.15 | 9.14 | 0.12 | 0.7408 | 0.2539 | 0.8490 |
| Exp | 9.16 | 9.07 | 9.33 | 9.20 | 9.04 | ||||||
| Tot | 9.04 | 9.28 | 9.18 | 9.09 | |||||||
| MCLAB | Ctr | 9.44 | 9.47 | 9.35 | 9.44 | 9.49 | 0.10 | 0.5935 | 0.3304 | 0.1127 | |
| Exp | 9.48 | 9.38 | 9.53 | 9.69 | 9.30 | ||||||
| Tot | 9.42 | 9.44 | 9.57 | 9.39 | |||||||
| Cheese at t0 | TMM | Ctr | 8.66 | 8.66 | 8.63 | 8.69 | 8.66 | 0.13 | 0.3307 | 0.9658 | 0.9431 |
| Exp | 8.57 | 8.57 | 8.55 | 8.50 | 8.64 | ||||||
| Tot | 8.62 | 8.59 | 8.59 | 8.65 | |||||||
| MCLAB | Ctr | 8.62 | 8.70 | 8.52 | 8.77 | 8.49 | 0.13 | 0.8256 | 0.4783 | 0.8644 | |
| Exp | 8.60 | 8.61 | 8.56 | 8.65 | 8.57 | ||||||
| Tot | 8.65 | 8.54 | 8.71 | 8.53 | |||||||
| Cheese at t15 | TMM | Ctr | 8.73 | 8.62 | 8.98 | 8.60 | 8.71 | 0.075 | 0.8795 | 0.0074 | 0.2972 |
| Exp | 8.74 | 8.72 | 8.81 | 8.63 | 8.79 | ||||||
| Tot | 8.67 b | 8.89 a | 8.62 b | 8.75 ab | |||||||
| MCLAB | Ctr | 8.76 | 8.70 | 8.89 | 8.69 | 8.76 | 0.071 | 0.8147 | 0.5271 | 0.3911 | |
| Exp | 8.75 | 8.77 | 8.74 | 8.75 | 8.74 | ||||||
| Tot | 8.73 | 8.82 | 8.72 | 8.75 | |||||||
| Treatment (TR) | Natural Milk Starter Culture (NMSC) | Significance p< | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MISE 36 | MISE 94 | MISE 169 | MISE 190 | SEM | TR | NMSC | TR*NMSC | ||||
| External colour | lightness (L*) | Ctr | 82.47 | 82.27 | 83.43 | 80.33 | 83.86 | 2.96 | <0.0001 | 0.4523 | 0.5840 |
| Exp | 51.60 | 53.91 | 47.51 | 49.99 | 54.98 | ||||||
| Tot | 68.09 | 65.47 | 65.16 | 69.42 | |||||||
| redness (a*) | Ctr | −5.35 | −5.21 | −5.27 | −5.64 | −5.29 | 0.30 | <0.0001 | 0.6749 | 0.3080 | |
| Exp | 4.90 | 4.52 | 5.25 | 5.18 | 4.65 | ||||||
| Tot | −0.35 | −0.01 | −0.23 | −0.32 | |||||||
| yellowness (*) | Ctr | 18.10 | 17.77 | 18.31 | 18.80 | 17.54 | 0.63 | <0.0001 | 0.3406 | 0.8874 | |
| Exp | 4.31 | 4.59 | 4.32 | 4.78 | 3.57 | ||||||
| Tot | 11.18 | 11.31 | 11.79 | 10.55 | |||||||
| Internal colour | lightness (L*) | Ctr | 85.02 | 84.19 | 86.66 | 83.59 | 85.66 | 1.71 | <0.0001 | 0.7484 | 0.1583 |
| Exp | 56.49 | 59.09 | 53.24 | 56.99 | 56.65 | ||||||
| Tot | 71.64 | 69.95 | 70.29 | 71.15 | |||||||
| redness (a*) | Ctr | −2.87 | −2.92 | −2.81 | −2.85 | −2.91 | 0.35 | <0.0001 | 0.6809 | 0.7180 | |
| Exp | 5.19 | 4.88 | 5.44 | 4.91 | 5.54 | ||||||
| Tot | 0.98 | 1.31 | 1.03 | 1.32 | |||||||
| yellowness (*) | Ctr | 10.68 | 10.77 | 10.63 | 10.42 | 10.92 | 0.17 | <0.0001 | 0.5230 | 0.0896 | |
| Exp | 3.61 | 3.76 | 3.38 | 3.90 | 3.42 | ||||||
| Tot | 7.26 | 7.00 | 7.16 | 7.17 | |||||||
| Hardness, N/mm2 | Ctr | 0.44 | 0.50 a | 0.40 Bab | 0.52 Ba | 0.36 Bb | 0.021 | <0.0001 | 0.0059 | 0.0044 | |
| Exp | 0.64 | 0.58 b | 0.67 Aab | 0.69 Aa | 0.64 Aab | ||||||
| Tot | 0.54 b | 0.53 b | 0.61 a | 0.50 b | |||||||
| Chemical composition | Dry matter (DM), % | Ctr | 55.34 | 55.77 a | 55.13 b | 54.24 Bc | 56.21 Aa | 0.13 | <0.0001 | 0.0007 | <0.0001 |
| Exp | 55.85 | 55.94 b | 55.42 bc | 56.82 Aa | 55.24 Bc | ||||||
| Tot | 55.85 a | 55.27 b | 55.53 ab | 55.72 a | |||||||
| Ash, % DM | Ctr | 5.16 | 5.40 | 4.81 | 5.47 | 4.96 | 0.12 | 0.0015 | 0.0007 | 0.2336 | |
| Exp | 5.47 | 5.74 | 5.37 | 5.53 | 5.25 | ||||||
| Tot | 5.57 a | 5.09 b | 5.50 a | 5.11 b | |||||||
| Protein, % DM | Ctr | 46.01 | 45.18 b | 44.42 Bb | 50.17 a | 44.28 Bb | 0.74 | 0.0020 | 0.0007 | 0.0013 | |
| Exp | 47.94 | 45.81 | 48.99 A | 48.41 | 48.54 A | ||||||
| Tot | 45.49 b | 46.70 b | 49.29 a | 46.41 b | |||||||
| Fat, % DM | Ctr | 46.41 | 47.40 Ab | 48.54 Aa | 41.90 c | 47.78 Ab | 0.12 | <0.0001 | <0.0001 | <0.0001 | |
| Exp | 42.42 | 44.08 Ba | 40.83 Bd | 41.96 c | 42.82 Bb | ||||||
| Tot | 45.74 a | 44.69 c | 41.93 d | 45.30 b | |||||||
| TBARS, μg MDA/kg DM | Ctr | 18.74 | 17.57 | 20.92 | 19.54 | 16.94 | 3.65 | <0.0461 | 0.9722 | 0.7790 | |
| Exp | 24.82 | 24.31 | 22.83 | 25.75 | 26.41 | ||||||
| Tot | 20.94 | 21.88 | 22.65 | 21.67 | |||||||
| Chemical Compounds a | Samples | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| GPP | CCh1536 | ECh1536 | CCh1594 | ECh1594 | CCh15169 | ECh15169 | CCh15190 | ECh15190 | |
| Acids | |||||||||
| Acetic acid | n.d. | 9.5 | 16.2 | 14.1 | 14.9 | 9.2 | 11.8 | 18.7 | 10.4 |
| Butanoic acid | n.d. | 9.2 | 7.5 | 10.9 | 7.4 | 5.7 | 5.7 | 9.0 | 6.0 |
| 4-Hydroxybutanoic acid | 4.1 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Hexanoic acid | 1.6 | 5.9 | 5.0 | 8.2 | 4.3 | 4.3 | 4.0 | 7.1 | 4.3 |
| Pentanoinc acid-2-hydroxy-4-methyl | n.d. | 16.0 | 2.5 | 12.2 | 7.2 | 8.0 | 5.2 | 13.0 | 4.7 |
| 3-Methylbutanoic acid | n.d. | n.d. | 0.5 | n.d. | 0.3 | n.d. | 0.2 | n.d. | 0.4 |
| Nonanoic acid | 3.4 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Ketons | |||||||||
| 2-Pentanone | n.d. | 2.8 | 0.6 | 2.4 | 0.7 | 1.8 | 0.6 | 2.3 | 0.5 |
| 2-Heptanone | n.d. | 2.0 | 0.5 | 1.9 | 0.7 | 2.2 | 0.6 | 2.4 | 0.5 |
| p-Phenylacetophenone | 4.2 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Alcohol | |||||||||
| Isoamyl alcohol | 4.9 | 23.7 | 42.3 | 27.8 | 50.0 | 44.3 | 51.6 | 21.5 | 59.9 |
| 2-Pentanol | n.d. | n.d. | 0.4 | n.d. | 0.2 | n.d. | 0.2 | n.d. | 0.8 |
| 2-Butanol | n.d. | 1.3 | 0.5 | 1.4 | 0.5 | 1.7 | 0.6 | 2.3 | 0.2 |
| 2-Phenylethanol | 11.3 | n.d. | 2.6 | n.d. | 2.3 | n.d. | 3.9 | n.d. | 2.7 |
| Hydrocarbons | |||||||||
| Hexane 2-methyl | n.d. | n.d. | 0.3 | n.d. | 0.5 | n.d. | 0.3 | n.d. | 0.3 |
| Heptane 2,4-dimethyl | 3.2 | 12.2 | 3.4 | 5.6 | 3.2 | 9.5 | 4.6 | 8.5 | 5.1 |
| Octane 4-methyl | n.d. | 4.4 | 0.5 | 1.8 | 0.5 | 3.6 | 0.6 | 2.5 | 0.3 |
| Nonane | 2.2 | n.d. | 0.4 | n.d. | 0.5 | n.d. | 0.7 | n.d. | 0.4 |
| Nonane 2,5-methyl | 2.3 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Decane | 1.8 | n.d. | 0.4 | n.d. | 0.3 | n.d. | 0.5 | n.d. | 0.2 |
| Dodecane | 2.3 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Hexadecane | 1.7 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Aldeyde | |||||||||
| Hexanal | 3.2 | 2.6 | 0.2 | 1.7 | 0.4 | 2.0 | 0.2 | 2.8 | 0.2 |
| Heptanal | n.d. | 1.9 | 0.5 | 1.9 | 0.4 | 1.8 | 0.1 | 2.5 | 0.2 |
| Nonanal | 1.7 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Monoterpene | |||||||||
| Phellandrene | n.d. | 1.8 | n.d. | 2.5 | n.d. | 1.9 | n.d. | 2.4 | n.d. |
| D-Limonene | 6.3 | n.d. | 12.7 | n.d. | 2.1 | n.d. | 5.0 | n.d. | 0.5 |
| α-Pinene | 2.1 | 6.7 | 2.6 | 7.6 | 3.1 | 4.1 | 2.6 | 5.0 | 1.9 |
| Carene | 1.5 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Esters | |||||||||
| Octanoinc acid, ethyl ester | 9.6 | n.d. | 0.4 | n.d. | 0.7 | n.d. | 0.7 | n.d. | 0.6 |
| Butanedioic acid, diethyl ester | 2.2 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Decanoic acid, ethyl ester | 9.7 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Diol | |||||||||
| 2,3-Butanediol | 20.6 | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. |
| Digestion Step | CCh1594 | ECh1594 |
|---|---|---|
| µmol TE/g | ||
| Post-Oral | 0.192 ± 0.011 A | 0.215 ± 0.012 A |
| Post-Gastric | 0.202 ± 0.012 A | 0.304 ± 0.014 Ba |
| Post-Intestinal | 0.317 ± 0.015 B | 0.557 ± 0.022 Cb |
| Bioaccesible fraction | 0.320 ± 0.012 B | 0.556 ± 0.023 Cb |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaglio, R.; Restivo, I.; Barbera, M.; Barbaccia, P.; Ponte, M.; Tesoriere, L.; Bonanno, A.; Attanzio, A.; Di Grigoli, A.; Francesca, N.; et al. Effect on the Antioxidant, Lipoperoxyl Radical Scavenger Capacity, Nutritional, Sensory and Microbiological Traits of an Ovine Stretched Cheese Produced with Grape Pomace Powder Addition. Antioxidants 2021, 10, 306. https://doi.org/10.3390/antiox10020306
Gaglio R, Restivo I, Barbera M, Barbaccia P, Ponte M, Tesoriere L, Bonanno A, Attanzio A, Di Grigoli A, Francesca N, et al. Effect on the Antioxidant, Lipoperoxyl Radical Scavenger Capacity, Nutritional, Sensory and Microbiological Traits of an Ovine Stretched Cheese Produced with Grape Pomace Powder Addition. Antioxidants. 2021; 10(2):306. https://doi.org/10.3390/antiox10020306
Chicago/Turabian StyleGaglio, Raimondo, Ignazio Restivo, Marcella Barbera, Pietro Barbaccia, Marialetizia Ponte, Luisa Tesoriere, Adriana Bonanno, Alessandro Attanzio, Antonino Di Grigoli, Nicola Francesca, and et al. 2021. "Effect on the Antioxidant, Lipoperoxyl Radical Scavenger Capacity, Nutritional, Sensory and Microbiological Traits of an Ovine Stretched Cheese Produced with Grape Pomace Powder Addition" Antioxidants 10, no. 2: 306. https://doi.org/10.3390/antiox10020306
APA StyleGaglio, R., Restivo, I., Barbera, M., Barbaccia, P., Ponte, M., Tesoriere, L., Bonanno, A., Attanzio, A., Di Grigoli, A., Francesca, N., Moschetti, G., & Settanni, L. (2021). Effect on the Antioxidant, Lipoperoxyl Radical Scavenger Capacity, Nutritional, Sensory and Microbiological Traits of an Ovine Stretched Cheese Produced with Grape Pomace Powder Addition. Antioxidants, 10(2), 306. https://doi.org/10.3390/antiox10020306