Functions of ROS in Macrophages and Antimicrobial Immunity
Abstract
:1. Reactive Oxygen Species
2. Cellular Redox Balance
3. Cellular ROS Sources
3.1. NADPH Oxidases
3.2. Mitochondria
3.3. Other ROS Sources
3.3.1. Xanthine Oxidase
3.3.2. Peroxisomes
3.3.3. Cyclooxygenases and Lipoxygenases
3.3.4. Cytochrome P450 Enzymes
4. Macrophages and ROS
4.1. Direct Antimicrobial Functions of ROS in Macrophages
4.1.1. ROS vs. Bacteria
4.1.2. ROS vs. Parasites
4.1.3. ROS vs. Viruses
4.2. Immune-Regulatory Functions of ROS
4.2.1. Immune-Regulatory Functions of mtROS
4.2.2. Regulatory Functions of Nox-Derived ROS
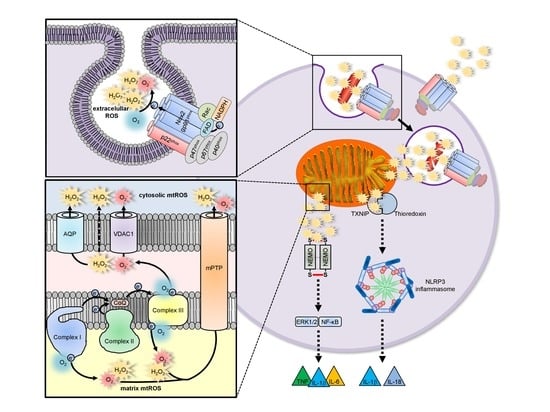
4.3. ROS and Inflammasomes
4.3.1. Nox-Derived ROS in Inflammasome Activation
4.3.2. mtROS in Inflammasome Activation
5. ROS Probes
5.1. Diffusable ROS Probes (Total Cellular ROS Detection)
5.2. Nondiffusable ROS Probes (Compartment-Specific ROS Detection)
6. ROS Scavengers
7. ROS Source Inhibitors
8. Concluding Remarks
- usage of only one type of ROS probe without explanation of the rationale behind the choice, such as compartment or ROS subspecies specificity or no specificity at all (“total cellular ROS”);
- usage of unspecific inhibitors of ROS production or usage of only globally working ROS scavengers;
- no genetic evidence for the ROS source (especially in case of Nox enzymes);
- inconsistent use of different stimuli and macrophage cell types for experiments in one study.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Fenton, H.J.H. LXXIII.—Oxidation of tartaric acid in presence of iron. J. Chem. Soc. Trans. 1894, 65, 899–910. [Google Scholar] [CrossRef] [Green Version]
- Haber, F.; Weiss, J.; Pope, W.J. The catalytic decomposition of hydrogen peroxide by iron salts. Proc. R. Soc. Lond. Ser. A Math. Phys. Sci. 1997, 147, 332–351. [Google Scholar]
- Prousek, J. Fenton chemistry in biology and medicine. Pure Appl. Chem. 2007, 79, 2325–2338. [Google Scholar] [CrossRef]
- Das, K.; Roychoudhury, A. Reactive oxygen species (ros) and response of antioxidants as ros-scavengers during environmental stress in plants. Front. Environ. Sci. 2014, 2, 53. [Google Scholar] [CrossRef] [Green Version]
- Hatz, S.; Lambert, J.D.; Ogilby, P.R. Measuring the lifetime of singlet oxygen in a single cell: Addressing the issue of cell viability. Photochem. Photobiol. Sci. 2007, 6, 1106–1116. [Google Scholar] [CrossRef]
- Aratani, Y. Myeloperoxidase: Its role for host defense, inflammation, and neutrophil function. Arch. Biochem. Biophys. 2018, 640, 47–52. [Google Scholar] [CrossRef]
- Odobasic, D.; Kitching, A.R.; Holdsworth, S.R. Neutrophil-mediated regulation of innate and adaptive immunity: The role of myeloperoxidase. J. Immunol. Res. 2016, 2016, 2349817. [Google Scholar] [CrossRef] [Green Version]
- Chavez, V.; Mohri-Shiomi, A.; Garsin, D.A. Ce-duox1/bli-3 generates reactive oxygen species as a protective innate immune mechanism in caenorhabditis elegans. Infect. Immun. 2009, 77, 4983–4989. [Google Scholar] [CrossRef] [Green Version]
- Niethammer, P.; Grabher, C.; Look, A.T.; Mitchison, T.J. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature 2009, 459, 996–999. [Google Scholar] [CrossRef]
- Pendyala, S.; Gorshkova, I.A.; Usatyuk, P.V.; He, D.; Pennathur, A.; Lambeth, J.D.; Thannickal, V.J.; Natarajan, V. Role of nox4 and nox2 in hyperoxia-induced reactive oxygen species generation and migration of human lung endothelial cells. Antioxid. Redox Signal. 2009, 11, 747–764. [Google Scholar] [CrossRef] [Green Version]
- Ha, E.M.; Oh, C.T.; Ryu, J.H.; Bae, Y.S.; Kang, S.W.; Jang, I.H.; Brey, P.T.; Lee, W.J. An antioxidant system required for host protection against gut infection in drosophila. Dev. Cell 2005, 8, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Levine, A.; Tenhaken, R.; Dixon, R.; Lamb, C. H2o2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 1994, 79, 583–593. [Google Scholar] [CrossRef]
- Lambeth, J.D.; Neish, A.S. Nox enzymes and new thinking on reactive oxygen: A double-edged sword revisited. Annu. Rev. Pathol. 2014, 9, 119–145. [Google Scholar] [CrossRef]
- Cheeseman, K.H.; Slater, T.F. An introduction to free radical biochemistry. Br. Med. Bull. 1993, 49, 481–493. [Google Scholar] [CrossRef]
- Shoshan-Barmatz, V.; De Pinto, V.; Zweckstetter, M.; Raviv, Z.; Keinan, N.; Arbel, N. Vdac, a multi-functional mitochondrial protein regulating cell life and death. Mol. Asp. Med. 2010, 31, 227–285. [Google Scholar] [CrossRef] [PubMed]
- Gutteridge, J.M.C.; Halliwell, B. Mini-review: Oxidative stress, redox stress or redox success? Biochem. Biophys. Res. Commun. 2018, 502, 183–186. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Davies, K.J. Adaptive homeostasis. Mol. Asp. Med. 2016, 49, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Berlett, B.S.; Stadtman, E.R. Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 1997, 272, 20313–20316. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, H.R.; Dias, I.H.; Willetts, R.S.; Devitt, A. Redox regulation of protein damage in plasma. Redox Biol. 2014, 2, 430–435. [Google Scholar] [CrossRef] [Green Version]
- Kelly, F.J.; Mudway, I.S. Protein oxidation at the air-lung interface. Amino Acids 2003, 25, 375–396. [Google Scholar] [CrossRef] [PubMed]
- Cadet, J.; Wagner, J.R. DNA base damage by reactive oxygen species, oxidizing agents, and uv radiation. Cold Spring Harb. Perspect. Biol. 2013, 5, a012559. [Google Scholar] [CrossRef]
- Girotti, A.W. Mechanisms of lipid peroxidation. J. Free Radic. Biol. Med. 1985, 1, 87–95. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Lipid peroxidation in brain homogenates: The role of iron and hydroxyl radicals. J. Neurochem. 1997, 69, 1330–1331. [Google Scholar] [CrossRef]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive oxygen species-induced lipid peroxidation in apoptosis, autophagy, and ferroptosis. Oxid. Med. Cell Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Azad, M.B.; Gibson, S.B. Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ. 2009, 16, 1040–1052. [Google Scholar] [CrossRef] [Green Version]
- Sies, H.; Jones, D.P. Reactive oxygen species (ros) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Herb, M.; Gluschko, A.; Wiegmann, K.; Farid, A.; Wolf, A.; Utermohlen, O.; Krut, O.; Kronke, M.; Schramm, M. Mitochondrial reactive oxygen species enable proinflammatory signaling through disulfide linkage of nemo. Sci. Signal. 2019, 12. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. Ros and ros-mediated cellular signaling. Oxid Med. Cell Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [Green Version]
- Marinho, H.S.; Cyrne, L.; Cadenas, E.; Antunes, F. The cellular steady-state of h2o2: Latency concepts and gradients. Methods Enzym. 2013, 527, 3–19. [Google Scholar]
- Veal, E.A.; Day, A.M.; Morgan, B.A. Hydrogen peroxide sensing and signaling. Mol. Cell 2007, 26, 1–14. [Google Scholar] [CrossRef]
- Bienert, G.P.; Schjoerring, J.K.; Jahn, T.P. Membrane transport of hydrogen peroxide. Biochim. Biophys. Acta 2006, 1758, 994–1003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antunes, F.; Cadenas, E. Estimation of h2o2 gradients across biomembranes. FEBS Lett. 2000, 475, 121–126. [Google Scholar] [CrossRef] [Green Version]
- Sies, H. Oxidative eustress: On constant alert for redox homeostasis. Redox Biol. 2021, 101867. [Google Scholar] [CrossRef]
- Beretta, M.; Santos, C.X.; Molenaar, C.; Hafstad, A.D.; Miller, C.C.; Revazian, A.; Betteridge, K.; Schroder, K.; Streckfuss-Bomeke, K.; Doroshow, J.H.; et al. Nox4 regulates insp3 receptor-dependent ca(2+) release into mitochondria to promote cell survival. EMBO J. 2020, 39, e103530. [Google Scholar] [CrossRef]
- Wang, H.; Schoebel, S.; Schmitz, F.; Dong, H.; Hedfalk, K. Characterization of aquaporin-driven hydrogen peroxide transport. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183065. [Google Scholar] [CrossRef]
- Bienert, G.P.; Chaumont, F. Aquaporin-facilitated transmembrane diffusion of hydrogen peroxide. Biochim. Biophys. Acta 2014, 1840, 1596–1604. [Google Scholar] [CrossRef]
- Herscovitch, M.; Comb, W.; Ennis, T.; Coleman, K.; Yong, S.; Armstead, B.; Kalaitzidis, D.; Chandani, S.; Gilmore, T.D. Intermolecular disulfide bond formation in the nemo dimer requires cys54 and cys347. Biochem. Biophys. Res. Commun. 2008, 367, 103–108. [Google Scholar] [CrossRef] [Green Version]
- Jones, A.I.; Meshulam, T.; Oliveira, M.F.; Burritt, N.; Corkey, B.E. Extracellular redox regulation of intracellular reactive oxygen generation, mitochondrial function and lipid turnover in cultured human adipocytes. PLoS ONE 2016, 11, e0164011. [Google Scholar] [CrossRef] [PubMed]
- Short, J.D.; Downs, K.; Tavakoli, S.; Asmis, R. Protein thiol redox signaling in monocytes and macrophages. Antioxid. Redox Signal. 2016, 25, 816–835. [Google Scholar] [CrossRef] [Green Version]
- Rhee, S.G. Cell signaling. H2o2, a necessary evil for cell signaling. Science 2006, 312, 1882–1883. [Google Scholar] [CrossRef] [PubMed]
- Chiarugi, P.; Fiaschi, T.; Taddei, M.L.; Talini, D.; Giannoni, E.; Raugei, G.; Ramponi, G. Two vicinal cysteines confer a peculiar redox regulation to low molecular weight protein tyrosine phosphatase in response to platelet-derived growth factor receptor stimulation. J. Biol. Chem. 2001, 276, 33478–33487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero, L.C.; Aroca, M.A.; Laureano-Marin, A.M.; Moreno, I.; Garcia, I.; Gotor, C. Cysteine and cysteine-related signaling pathways in arabidopsis thaliana. Mol. Plant 2014, 7, 264–276. [Google Scholar] [CrossRef] [Green Version]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Poole, L.B. The basics of thiols and cysteines in redox biology and chemistry. Free Radic. Biol. Med. 2015, 80, 148–157. [Google Scholar] [CrossRef] [Green Version]
- Kamata, H.; Honda, S.; Maeda, S.; Chang, L.; Hirata, H.; Karin, M. Reactive oxygen species promote tnfalpha-induced death and sustained jnk activation by inhibiting map kinase phosphatases. Cell 2005, 120, 649–661. [Google Scholar] [CrossRef] [Green Version]
- Tonks, N.K. Redox redux: Revisiting ptps and the control of cell signaling. Cell 2005, 121, 667–670. [Google Scholar] [CrossRef] [Green Version]
- Meng, T.C.; Fukada, T.; Tonks, N.K. Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol. Cell 2002, 9, 387–399. [Google Scholar] [CrossRef]
- Lee, S.R.; Kwon, K.S.; Kim, S.R.; Rhee, S.G. Reversible inactivation of protein-tyrosine phosphatase 1b in a431 cells stimulated with epidermal growth factor. J. Biol. Chem. 1998, 273, 15366–15372. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Yeo, A.T.; Ballarano, C.; Weber, U.; Allen, K.N.; Gilmore, T.D.; Whitty, A. Disulfide-mediated stabilization of the ikappab kinase binding domain of nf-kappab essential modulator (nemo). Biochemistry 2014, 53, 7929–7944. [Google Scholar] [CrossRef]
- Holmstrom, K.M.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef]
- Barford, D. The role of cysteine residues as redox-sensitive regulatory switches. Curr. Opin. Struct. Biol. 2004, 14, 679–686. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Hampton, M.B. Thiol chemistry and specificity in redox signaling. Free Radic. Biol. Med. 2008, 45, 549–561. [Google Scholar] [CrossRef]
- Kelley, E.E.; Khoo, N.K.; Hundley, N.J.; Malik, U.Z.; Freeman, B.A.; Tarpey, M.M. Hydrogen peroxide is the major oxidant product of xanthine oxidase. Free Radic. Biol. Med. 2010, 48, 493–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nohl, H.; Kozlov, A.V.; Gille, L.; Staniek, K. Cell respiration and formation of reactive oxygen species: Facts and artefacts. Biochem. Soc. Trans. 2003, 31, 1308–1311. [Google Scholar] [CrossRef] [PubMed]
- Gluschko, A.; Herb, M.; Wiegmann, K.; Krut, O.; Neiss, W.F.; Utermohlen, O.; Kronke, M.; Schramm, M. The beta2 integrin mac-1 induces protective lc3-associated phagocytosis of listeria monocytogenes. Cell Host Microbe 2018, 23, 324–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schramm, M.; Wiegmann, K.; Schramm, S.; Gluschko, A.; Herb, M.; Utermohlen, O.; Kronke, M. Riboflavin (vitamin b2) deficiency impairs nadph oxidase 2 (nox2) priming and defense against listeria monocytogenes. Eur. J. Immunol. 2014, 44, 728–741. [Google Scholar] [CrossRef]
- Hoeven, R.; McCallum, K.C.; Cruz, M.R.; Garsin, D.A. Ce-duox1/bli-3 generated reactive oxygen species trigger protective skn-1 activity via p38 mapk signaling during infection in c. Elegans. PLoS Pathog. 2011, 7, e1002453. [Google Scholar]
- West, A.P.; Brodsky, I.E.; Rahner, C.; Woo, D.K.; Erdjument-Bromage, H.; Tempst, P.; Walsh, M.C.; Choi, Y.; Shadel, G.S.; Ghosh, S. Tlr signalling augments macrophage bactericidal activity through mitochondrial ros. Nature 2011, 472, 476–480. [Google Scholar] [CrossRef] [Green Version]
- Babior, B.M. The activity of leukocyte nadph oxidase: Regulation by p47phox cysteine and serine residues. Antioxid. Redox Signal. 2002, 4, 35–38. [Google Scholar] [CrossRef]
- Bulua, A.C.; Simon, A.; Maddipati, R.; Pelletier, M.; Park, H.; Kim, K.Y.; Sack, M.N.; Kastner, D.L.; Siegel, R.M. Mitochondrial reactive oxygen species promote production of proinflammatory cytokines and are elevated in tnfr1-associated periodic syndrome (traps). J. Exp. Med. 2011, 208, 519–533. [Google Scholar] [CrossRef]
- Kim, J.J.; Lee, S.B.; Park, J.K.; Yoo, Y.D. Tnf-alpha-induced ros production triggering apoptosis is directly linked to romo1 and bcl-x(l). Cell Death Differ. 2010, 17, 1420–1434. [Google Scholar] [CrossRef] [Green Version]
- Kirkman, H.N.; Gaetani, G.F. Mammalian catalase: A venerable enzyme with new mysteries. Trends Biochem. Sci. 2007, 32, 44–50. [Google Scholar] [CrossRef]
- Poljsak, B.; Suput, D.; Milisav, I. Achieving the balance between ros and antioxidants: When to use the synthetic antioxidants. Oxid. Med. Cell Longev. 2013, 2013, 956792. [Google Scholar] [CrossRef]
- Fridovich, I. Superoxide anion radical (o2-.), superoxide dismutases, and related matters. J. Biol. Chem. 1997, 272, 18515–18517. [Google Scholar] [CrossRef] [Green Version]
- Melov, S.; Coskun, P.; Patel, M.; Tuinstra, R.; Cottrell, B.; Jun, A.S.; Zastawny, T.H.; Dizdaroglu, M.; Goodman, S.I.; Huang, T.T.; et al. Mitochondrial disease in superoxide dismutase 2 mutant mice. Proc. Natl. Acad. Sci. USA 1999, 96, 846–851. [Google Scholar] [CrossRef] [Green Version]
- Brigelius-Flohe, R.; Maiorino, M. Glutathione peroxidases. Biochim. Biophys. Acta 2013, 1830, 3289–3303. [Google Scholar] [CrossRef]
- Nakamura, H. Thioredoxin and its related molecules: Update 2005. Antioxid. Redox Signal. 2005, 7, 823–828. [Google Scholar] [CrossRef]
- Holmgren, A. Antioxidant function of thioredoxin and glutaredoxin systems. Antioxid. Redox Signal. 2000, 2, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; McLellan, L.I. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. Free Radic. Res. 1999, 31, 273–300. [Google Scholar] [CrossRef]
- Couto, N.; Wood, J.; Barber, J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic. Biol. Med. 2016, 95, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Alleva, R.; Tomasetti, M.; Battino, M.; Curatola, G.; Littarru, G.P.; Folkers, K. The roles of coenzyme q10 and vitamin e on the peroxidation of human low density lipoprotein subfractions. Proc. Natl. Acad. Sci. USA 1995, 92, 9388–9391. [Google Scholar] [CrossRef] [Green Version]
- Niki, E. Oxidative stress and antioxidants: Distress or eustress? Arch. Biochem. Biophys. 2016, 595, 19–24. [Google Scholar] [CrossRef]
- Thimmulappa, R.K.; Lee, H.; Rangasamy, T.; Reddy, S.P.; Yamamoto, M.; Kensler, T.W.; Biswal, S. Nrf2 is a critical regulator of the innate immune response and survival during experimental sepsis. J. Clin. Investig. 2006, 116, 984–995. [Google Scholar] [CrossRef] [Green Version]
- Rangasamy, T.; Guo, J.; Mitzner, W.A.; Roman, J.; Singh, A.; Fryer, A.D.; Yamamoto, M.; Kensler, T.W.; Tuder, R.M.; Georas, S.N.; et al. Disruption of nrf2 enhances susceptibility to severe airway inflammation and asthma in mice. J. Exp. Med. 2005, 202, 47–59. [Google Scholar] [CrossRef]
- Cross, C.E.; Halliwell, B.; Borish, E.T.; Pryor, W.A.; Ames, B.N.; Saul, R.L.; McCord, J.M.; Harman, D. Oxygen radicals and human disease. Ann. Intern. Med. 1987, 107, 526–545. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Kettle, A.J. Redox reactions and microbial killing in the neutrophil phagosome. Antioxid. Redox Signal. 2013, 18, 642–660. [Google Scholar] [CrossRef]
- Nathan, C.; Cunningham-Bussel, A. Beyond oxidative stress: An immunologist’s guide to reactive oxygen species. Nat. Rev. Immunol. 2013, 13, 349–361. [Google Scholar] [CrossRef] [Green Version]
- Tai, T.C.; Wong-Faull, D.C.; Claycomb, R.; Wong, D.L. Hypoxic stress-induced changes in adrenergic function: Role of hif1 alpha. J. Neurochem. 2009, 109, 513–524. [Google Scholar] [CrossRef]
- Reczek, C.R.; Chandel, N.S. Ros-dependent signal transduction. Curr. Opin. Cell Biol. 2014, 33, 8–13. [Google Scholar] [CrossRef] [Green Version]
- Bedard, K.; Krause, K.H. The nox family of ros-generating nadph oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- West, A.P.; Shadel, G.S.; Ghosh, S. Mitochondria in innate immune responses. Nat. Rev. Immunol. 2011, 11, 389–402. [Google Scholar] [CrossRef] [Green Version]
- Brand, M.D. The sites and topology of mitochondrial superoxide production. Exp. Gerontol. 2010, 45, 466–472. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Cheng, G.; Cao, Z.; Xu, X.; van Meir, E.G.; Lambeth, J.D. Homologs of gp91phox: Cloning and tissue expression of nox3, nox4, and nox5. Gene 2001, 269, 131–140. [Google Scholar] [CrossRef]
- Nakano, Y.; Longo-Guess, C.M.; Bergstrom, D.E.; Nauseef, W.M.; Jones, S.M.; Banfi, B. Mutation of the cyba gene encoding p22phox causes vestibular and immune defects in mice. J. Clin. Investig. 2008, 118, 1176–1185. [Google Scholar] [CrossRef]
- Kawahara, T.; Ritsick, D.; Cheng, G.; Lambeth, J.D. Point mutations in the proline-rich region of p22phox are dominant inhibitors of nox1- and nox2-dependent reactive oxygen generation. J. Biol. Chem. 2005, 280, 31859–31869. [Google Scholar] [CrossRef] [Green Version]
- Lambeth, J.D.; Kawahara, T.; Diebold, B. Regulation of nox and duox enzymatic activity and expression. Free Radic. Biol. Med. 2007, 43, 319–331. [Google Scholar] [CrossRef] [Green Version]
- Banfi, B.; Molnar, G.; Maturana, A.; Steger, K.; Hegedus, B.; Demaurex, N.; Krause, K.H. A ca(2+)-activated nadph oxidase in testis, spleen, and lymph nodes. J. Biol. Chem. 2001, 276, 37594–37601. [Google Scholar] [CrossRef] [Green Version]
- Aguirre, J.; Lambeth, J.D. Nox enzymes from fungus to fly to fish and what they tell us about nox function in mammals. Free Radic. Biol. Med. 2010, 49, 1342–1353. [Google Scholar] [CrossRef] [Green Version]
- Pollock, J.D.; Williams, D.A.; Gifford, M.A.; Li, L.L.; Du, X.; Fisherman, J.; Orkin, S.H.; Doerschuk, C.M.; Dinauer, M.C. Mouse model of x-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat. Genet. 1995, 9, 202–209. [Google Scholar] [CrossRef]
- Carnesecchi, S.; Deffert, C.; Donati, Y.; Basset, O.; Hinz, B.; Preynat-Seauve, O.; Guichard, C.; Arbiser, J.L.; Banfi, B.; Pache, J.C.; et al. A key role for nox4 in epithelial cell death during development of lung fibrosis. Antioxid. Redox Signal. 2011, 15, 607–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dikalova, A.E.; Gongora, M.C.; Harrison, D.G.; Lambeth, J.D.; Dikalov, S.; Griendling, K.K. Upregulation of nox1 in vascular smooth muscle leads to impaired endothelium-dependent relaxation via enos uncoupling. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H673–H679. [Google Scholar] [CrossRef] [Green Version]
- Donko, A.; Ruisanchez, E.; Orient, A.; Enyedi, B.; Kapui, R.; Peterfi, Z.; de Deken, X.; Benyo, Z.; Geiszt, M. Urothelial cells produce hydrogen peroxide through the activation of duox1. Free Radic. Biol. Med. 2010, 49, 2040–2048. [Google Scholar] [CrossRef] [PubMed]
- Gavazzi, G.; Banfi, B.; Deffert, C.; Fiette, L.; Schappi, M.; Herrmann, F.; Krause, K.H. Decreased blood pressure in nox1-deficient mice. FEBS Lett. 2006, 580, 497–504. [Google Scholar] [CrossRef]
- Banfi, B.; Malgrange, B.; Knisz, J.; Steger, K.; Dubois-Dauphin, M.; Krause, K.H. Nox3, a superoxide-generating nadph oxidase of the inner ear. J. Biol. Chem. 2004, 279, 46065–46072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, A.; Herb, M.; Schramm, M.; Langmann, T. The tspo-nox1 axis controls phagocyte-triggered pathological angiogenesis in the eye. Nat. Commun. 2020, 11, 2709. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ros generation and uncoupling (review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bezawork-Geleta, A.; Rohlena, J.; Dong, L.; Pacak, K.; Neuzil, J. Mitochondrial complex ii: At the crossroads. Trends Biochem. Sci. 2017, 42, 312–325. [Google Scholar] [CrossRef]
- Brand, M.D.; Goncalves, R.L.; Orr, A.L.; Vargas, L.; Gerencser, A.A.; Borch Jensen, M.; Wang, Y.T.; Melov, S.; Turk, C.N.; Matzen, J.T.; et al. Suppressors of superoxide-h2o2 production at site iq of mitochondrial complex i protect against stem cell hyperplasia and ischemia-reperfusion injury. Cell Metab. 2016, 24, 582–592. [Google Scholar] [CrossRef] [Green Version]
- Hadrava Vanova, K.; Kraus, M.; Neuzil, J.; Rohlena, J. Mitochondrial complex ii and reactive oxygen species in disease and therapy. Redox Rep. Commun. Free Radic. Res. 2020, 25, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Ohnishi, T. Structure of the succinate-ubiquinone oxidoreductase (complex ii). In Current Topics in Bioenergetics; Lee, C.P., Ed.; Elsevier: Amsterdam, The Netherlands, 1987; Volume 15, pp. 37–65. [Google Scholar]
- Muller, F.L.; Liu, Y.; Abdul-Ghani, M.A.; Lustgarten, M.S.; Bhattacharya, A.; Jang, Y.C.; Van Remmen, H. High rates of superoxide production in skeletal-muscle mitochondria respiring on both complex i- and complex ii-linked substrates. Biochem. J. 2008, 409, 491–499. [Google Scholar] [CrossRef]
- Liu, Y.; Fiskum, G.; Schubert, D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J. Neurochem. 2002, 80, 780–787. [Google Scholar] [CrossRef]
- Votyakova, T.V.; Reynolds, I.J. Deltapsi(m)-dependent and -independent production of reactive oxygen species by rat brain mitochondria. J. Neurochem. 2001, 79, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Sanchez, R.; Hernandez-Esquivel, L.; Rivero-Segura, N.A.; Marin-Hernandez, A.; Neuzil, J.; Ralph, S.J.; Rodriguez-Enriquez, S. Reactive oxygen species are generated by the respiratory complex ii--evidence for lack of contribution of the reverse electron flow in complex i. FEBS J. 2013, 280, 927–938. [Google Scholar] [CrossRef]
- Quinlan, C.L.; Orr, A.L.; Perevoshchikova, I.V.; Treberg, J.R.; Ackrell, B.A.; Brand, M.D. Mitochondrial complex ii can generate reactive oxygen species at high rates in both the forward and reverse reactions. J. Biol. Chem. 2012, 287, 27255–27264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siebels, I.; Drose, S. Q-site inhibitor induced ros production of mitochondrial complex ii is attenuated by tca cycle dicarboxylates. Biochim. Biophys. Acta 2013, 1827, 1156–1164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonke, E.; Zwicker, K.; Drose, S. Manganese ions induce h2o2 generation at the ubiquinone binding site of mitochondrial complex ii. Arch. Biochem. Biophys. 2015, 580, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Kowaltowski, A.J.; de Souza-Pinto, N.C.; Castilho, R.F.; Vercesi, A.E. Mitochondria and reactive oxygen species. Free Radic. Biol. Med. 2009, 47, 333–343. [Google Scholar] [CrossRef]
- Drose, S. Differential effects of complex ii on mitochondrial ros production and their relation to cardioprotective pre- and postconditioning. Biochim. Biophys. Acta 2013, 1827, 578–587. [Google Scholar] [CrossRef] [Green Version]
- Yankovskaya, V.; Horsefield, R.; Tornroth, S.; Luna-Chavez, C.; Miyoshi, H.; Leger, C.; Byrne, B.; Cecchini, G.; Iwata, S. Architecture of succinate dehydrogenase and reactive oxygen species generation. Science 2003, 299, 700–704. [Google Scholar] [CrossRef] [Green Version]
- Bardella, C.; Pollard, P.J.; Tomlinson, I. Sdh mutations in cancer. Biochim. Biophys. Acta 2011, 1807, 1432–1443. [Google Scholar] [CrossRef] [Green Version]
- Iverson, T.M.; Maklashina, E.; Cecchini, G. Structural basis for malfunction in complex ii. J. Biol. Chem. 2012, 287, 35430–35438. [Google Scholar] [CrossRef] [Green Version]
- Ralph, S.J.; Moreno-Sanchez, R.; Neuzil, J.; Rodriguez-Enriquez, S. Inhibitors of succinate: Quinone reductase/complex ii regulate production of mitochondrial reactive oxygen species and protect normal cells from ischemic damage but induce specific cancer cell death. Pharm. Res. 2011, 28, 2695–2730. [Google Scholar] [CrossRef] [PubMed]
- Hoekstra, A.S.; Bayley, J.P. The role of complex ii in disease. Biochim. Biophys. Acta 2013, 1827, 543–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cecchini, G. Respiratory complex ii: Role in cellular physiology and disease. Biochim. Biophys. Acta 2013, 1827, 541–542. [Google Scholar] [CrossRef] [Green Version]
- Muller, F.L.; Liu, Y.; Van Remmen, H. Complex iii releases superoxide to both sides of the inner mitochondrial membrane. J. Biol. Chem. 2004, 279, 49064–49073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, D.; Antunes, F.; Canali, R.; Rettori, D.; Cadenas, E. Voltage-dependent anion channels control the release of the superoxide anion from mitochondria to cytosol. J. Biol. Chem. 2003, 278, 5557–5563. [Google Scholar] [CrossRef] [Green Version]
- Hernansanz-Agustin, P.; Choya-Foces, C.; Carregal-Romero, S.; Ramos, E.; Oliva, T.; Villa-Pina, T.; Moreno, L.; Izquierdo-Alvarez, A.; Cabrera-Garcia, J.D.; Cortes, A.; et al. Na(+) controls hypoxic signalling by the mitochondrial respiratory chain. Nature 2020, 586, 287–291. [Google Scholar] [CrossRef]
- Herb, M.; Farid, A.; Gluschko, A.; Kronke, M.; Schramm, M. Highly efficient transfection of primary macrophages with in vitro transcribed mrna. J. Vis. Exp. 2019, 153, e60143. [Google Scholar] [CrossRef]
- Lanciano, P.; Khalfaoui-Hassani, B.; Selamoglu, N.; Ghelli, A.; Rugolo, M.; Daldal, F. Molecular mechanisms of superoxide production by complex iii: A bacterial versus human mitochondrial comparative case study. Biochim. Biophys. Acta 2013, 1827, 1332–1339. [Google Scholar] [CrossRef]
- Al-Mehdi, A.B.; Pastukh, V.M.; Swiger, B.M.; Reed, D.J.; Patel, M.R.; Bardwell, G.C.; Pastukh, V.V.; Alexeyev, M.F.; Gillespie, M.N. Perinuclear mitochondrial clustering creates an oxidant-rich nuclear domain required for hypoxia-induced transcription. Sci. Signal. 2012, 5, ra47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tormos, K.V.; Anso, E.; Hamanaka, R.B.; Eisenbart, J.; Joseph, J.; Kalyanaraman, B.; Chandel, N.S. Mitochondrial complex iii ros regulate adipocyte differentiation. Cell Metab. 2011, 14, 537–544. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Liu, J.; Deng, H.; Ma, R.; Liao, J.Y.; Liang, H.; Hu, J.; Li, J.; Guo, Z.; Cai, J.; et al. Targeting mitochondria-located circrna scar alleviates nash via reducing mros output. Cell 2020, 183, 76–93.e22. [Google Scholar] [CrossRef] [PubMed]
- Briston, T.; Roberts, M.; Lewis, S.; Powney, B.; Staddon, J.M.; Szabadkai, G.; Duchen, M.R. Mitochondrial permeability transition pore: Sensitivity to opening and mechanistic dependence on substrate availability. Sci. Rep. 2017, 7, 10492. [Google Scholar] [CrossRef] [PubMed]
- Roca, F.J.; Ramakrishnan, L. Tnf dually mediates resistance and susceptibility to mycobacteria via mitochondrial reactive oxygen species. Cell 2013, 153, 521–534. [Google Scholar] [CrossRef] [Green Version]
- Koterski, J.F.; Nahvi, M.; Venkatesan, M.M.; Haimovich, B. Virulent shigella flexneri causes damage to mitochondria and triggers necrosis in infected human monocyte-derived macrophages. Infect. Immun. 2005, 73, 504–513. [Google Scholar] [CrossRef] [Green Version]
- Hos, N.J.; Ganesan, R.; Gutierrez, S.; Hos, D.; Klimek, J.; Abdullah, Z.; Kronke, M.; Robinson, N. Type i interferon enhances necroptosis of salmonella typhimurium-infected macrophages by impairing antioxidative stress responses. J. Cell Biol. 2017, 216, 4107–4121. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Karakhanova, S.; Hartwig, W.; D’Haese, J.G.; Philippov, P.P.; Werner, J.; Bazhin, A.V. Mitochondria and mitochondrial ros in cancer: Novel targets for anticancer therapy. J. Cell Physiol. 2016, 231, 2570–2581. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fang, P.; Mai, J.; Choi, E.T.; Wang, H.; Yang, X.F. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J. Hematol. Oncol. 2013, 6, 19. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Zhou, Y.; Wang, W.; Li, J.; Xie, G.; Zhao, Y.; Xu, D.; Shen, L. Activation of tlr4 signaling promotes gastric cancer progression by inducing mitochondrial ros production. Cell Death Dis. 2013, 4, e794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dillin, A.; Hsu, A.L.; Arantes-Oliveira, N.; Lehrer-Graiwer, J.; Hsin, H.; Fraser, A.G.; Kamath, R.S.; Ahringer, J.; Kenyon, C. Rates of behavior and aging specified by mitochondrial function during development. Science 2002, 298, 2398–2401. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ros) and ros-induced ros release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [Green Version]
- Al-Shehri, S.S.; Duley, J.A.; Bansal, N. Xanthine oxidase-lactoperoxidase system and innate immunity: Biochemical actions and physiological roles. Redox Biol. 2020, 34, 101524. [Google Scholar] [CrossRef]
- Harris, C.M.; Massey, V. The reaction of reduced xanthine dehydrogenase with molecular oxygen. Reaction kinetics and measurement of superoxide radical. J. Biol. Chem. 1997, 272, 8370–8379. [Google Scholar] [CrossRef] [Green Version]
- Huber, R.; Hof, P.; Duarte, R.O.; Moura, J.J.; Moura, I.; Liu, M.Y.; LeGall, J.; Hille, R.; Archer, M.; Romao, M.J. A structure-based catalytic mechanism for the xanthine oxidase family of molybdenum enzymes. Proc. Natl. Acad. Sci. USA 1996, 93, 8846–8851. [Google Scholar] [CrossRef] [Green Version]
- Nomura, J.; Busso, N.; Ives, A.; Matsui, C.; Tsujimoto, S.; Shirakura, T.; Tamura, M.; Kobayashi, T.; So, A.; Yamanaka, Y. Xanthine oxidase inhibition by febuxostat attenuates experimental atherosclerosis in mice. Sci. Rep. 2014, 4, 4554. [Google Scholar] [CrossRef] [Green Version]
- George, J.; Struthers, A. The role of urate and xanthine oxidase in vascular oxidative stress: Future directions. Clin. Risk Manag. 2009, 5, 799–803. [Google Scholar] [CrossRef] [Green Version]
- Feig, D.I.; Soletsky, B.; Johnson, R.J. Effect of allopurinol on blood pressure of adolescents with newly diagnosed essential hypertension: A randomized trial. Jama 2008, 300, 924–932. [Google Scholar] [CrossRef] [Green Version]
- Kao, M.P.; Ang, D.S.; Gandy, S.J.; Nadir, M.A.; Houston, J.G.; Lang, C.C.; Struthers, A.D. Allopurinol benefits left ventricular mass and endothelial dysfunction in chronic kidney disease. J. Am. Soc. Nephrol. JASN 2011, 22, 1382–1389. [Google Scholar] [CrossRef] [Green Version]
- Ives, A.; Nomura, J.; Martinon, F.; Roger, T.; LeRoy, D.; Miner, J.N.; Simon, G.; Busso, N.; So, A. Xanthine oxidoreductase regulates macrophage il1beta secretion upon nlrp3 inflammasome activation. Nat. Commun. 2015, 6, 6555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, N.H.; Choi, S.; Han, E.J.; Hong, B.K.; Choi, S.Y.; Kwon, H.M.; Hwang, S.Y.; Cho, C.S.; Kim, W.U. The xanthine oxidase-nfat5 pathway regulates macrophage activation and tlr-induced inflammatory arthritis. Eur. J. Immunol. 2014, 44, 2721–2736. [Google Scholar] [CrossRef]
- Ty, M.C.; Zuniga, M.; Gotz, A.; Kayal, S.; Sahu, P.K.; Mohanty, A.; Mohanty, S.; Wassmer, S.C.; Rodriguez, A. Malaria inflammation by xanthine oxidase-produced reactive oxygen species. EMBO Mol. Med. 2019, 11, e9903. [Google Scholar] [CrossRef]
- Shai, N.; Yifrach, E.; van Roermund, C.W.T.; Cohen, N.; Bibi, C.; IJlst, L.; Cavellini, L.; Meurisse, J.; Schuster, R.; Zada, L.; et al. Systematic mapping of contact sites reveals tethers and a function for the peroxisome-mitochondria contact. Nat. Commun. 2018, 9, 1761. [Google Scholar] [CrossRef]
- Fransen, M.; Lismont, C.; Walton, P. The peroxisome-mitochondria connection: How and why? Int. J. Mol. Sci. 2017, 18, 1126. [Google Scholar] [CrossRef]
- Schrader, M.; Fahimi, H.D. Peroxisomes and oxidative stress. Biochim. Biophys. Acta 2006, 1763, 1755–1766. [Google Scholar] [CrossRef] [Green Version]
- Schumann, U.; Subramani, S. Special delivery from mitochondria to peroxisomes. Trends Cell Biol. 2008, 18, 253–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lismont, C.; Nordgren, M.; Van Veldhoven, P.P.; Fransen, M. Redox interplay between mitochondria and peroxisomes. Front. Cell Dev. Biol. 2015, 3, 35. [Google Scholar] [CrossRef] [Green Version]
- Fransen, M.; Nordgren, M.; Wang, B.; Apanasets, O. Role of peroxisomes in ros/rns-metabolism: Implications for human disease. Biochim. Biophys. Acta 2012, 1822, 1363–1373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, R.; Liu, J.; Wang, S.; Hu, J. Peroxisomes: Versatile organelles with diverse roles in plants. New Phytol. 2020, 225, 1410–1427. [Google Scholar] [CrossRef] [Green Version]
- Jedlitschky, G.; Mayatepek, E.; Keppler, D. Peroxisomal leukotriene degradation: Biochemical and clinical implications. Adv. Enzym. Regul. 1993, 33, 181–194. [Google Scholar] [CrossRef]
- Ferdinandusse, S.; Rusch, H.; van Lint, A.E.; Dacremont, G.; Wanders, R.J.; Vreken, P. Stereochemistry of the peroxisomal branched-chain fatty acid alpha- and beta-oxidation systems in patients suffering from different peroxisomal disorders. J. Lipid Res. 2002, 43, 438–444. [Google Scholar] [CrossRef]
- Diczfalusy, U.; Vesterqvist, O.; Kase, B.F.; Lund, E.; Alexson, S.E. Peroxisomal chain-shortening of thromboxane b2: Evidence for impaired degradation of thromboxane b2 in zellweger syndrome. J. Lipid Res. 1993, 34, 1107–1113. [Google Scholar] [CrossRef]
- Peters-Golden, M.; Canetti, C.; Mancuso, P.; Coffey, M.J. Leukotrienes: Underappreciated mediators of innate immune responses. J. Immunol. 2005, 174, 589–594. [Google Scholar] [CrossRef] [Green Version]
- Harris, S.G.; Padilla, J.; Koumas, L.; Ray, D.; Phipps, R.P. Prostaglandins as modulators of immunity. Trends Immunol. 2002, 23, 144–150. [Google Scholar] [CrossRef]
- Serhan, C.N.; Dalli, J.; Colas, R.A.; Winkler, J.W.; Chiang, N. Protectins and maresins: New pro-resolving families of mediators in acute inflammation and resolution bioactive metabolome. Biochim. Biophys. Acta 2015, 1851, 397–413. [Google Scholar] [CrossRef] [Green Version]
- Bannenberg, G.L. Resolvins: Current understanding and future potential in the control of inflammation. Curr. Opin. Drug Discov. Dev. 2009, 12, 644–658. [Google Scholar]
- Serhan, C.N.; Levy, B.D. Resolvins in inflammation: Emergence of the pro-resolving superfamily of mediators. J. Clin. Investig. 2018, 128, 2657–2669. [Google Scholar] [CrossRef]
- Di Cara, F.; Rachubinski, R.A.; Simmonds, A.J. Distinct roles for peroxisomal targeting signal receptors pex5 and pex7 in drosophila. Genetics 2019, 211, 141–149. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, A.R.; Marques, M.; Ribeiro, D. Peroxisomes and innate immunity: Antiviral response and beyond. Int. J. Mol. Sci. 2019, 20, 3795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixit, E.; Boulant, S.; Zhang, Y.; Lee, A.S.; Odendall, C.; Shum, B.; Hacohen, N.; Chen, Z.J.; Whelan, S.P.; Fransen, M.; et al. Peroxisomes are signaling platforms for antiviral innate immunity. Cell 2010, 141, 668–681. [Google Scholar] [CrossRef] [Green Version]
- Di Cara, F.; Sheshachalam, A.; Braverman, N.E.; Rachubinski, R.A.; Simmonds, A.J. Peroxisome-mediated metabolism is required for immune response to microbial infection. Immunity 2017, 47, 93–106. [Google Scholar] [CrossRef] [Green Version]
- Smith, W.L.; DeWitt, D.L.; Garavito, R.M. Cyclooxygenases: Structural, cellular, and molecular biology. Annu. Rev. Biochem. 2000, 69, 145–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricciotti, E.; FitzGerald, G.A. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 986–1000. [Google Scholar] [CrossRef]
- Wang, D.; Buchanan, F.G.; Wang, H.; Dey, S.K.; DuBois, R.N. Prostaglandin e2 enhances intestinal adenoma growth via activation of the ras-mitogen-activated protein kinase cascade. Cancer Res. 2005, 65, 1822–1829. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Ubreva, J.; Catala-Moll, F.; Obermajer, N.; Alvarez-Errico, D.; Ramirez, R.N.; Company, C.; Vento-Tormo, R.; Moreno-Bueno, G.; Edwards, R.P.; Mortazavi, A.; et al. Prostaglandin e2 leads to the acquisition of dnmt3a-dependent tolerogenic functions in human myeloid-derived suppressor cells. Cell Rep. 2017, 21, 154–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, C.S.; Mann, M.; DuBois, R.N. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 1999, 18, 7908–7916. [Google Scholar] [CrossRef] [Green Version]
- Langenbach, R.; Morham, S.G.; Tiano, H.F.; Loftin, C.D.; Ghanayem, B.I.; Chulada, P.C.; Mahler, J.F.; Davis, B.J.; Lee, C.A. Disruption of the mouse cyclooxygenase 1 gene. Characteristics of the mutant and areas of future study. Adv. Exp. Med. Biol. 1997, 407, 87–92. [Google Scholar] [PubMed]
- Zarghi, A.; Arfaei, S. Selective cox-2 inhibitors: A review of their structure-activity relationships. Iran. J. Pharm. Res. 2011, 10, 655–683. [Google Scholar]
- Fu, J.Y.; Masferrer, J.L.; Seibert, K.; Raz, A.; Needleman, P. The induction and suppression of prostaglandin h2 synthase (cyclooxygenase) in human monocytes. J. Biol. Chem. 1990, 265, 16737–16740. [Google Scholar] [CrossRef]
- Hsueh, W.; Gonzalez-Crussi, F.; Hanneman, E. Prostaglandin synthesis in different phases of phagocytosis in lung macrophages. Nature 1980, 283, 80–82. [Google Scholar] [CrossRef] [PubMed]
- Bray, M.A.; Gordon, D. Prostaglandin production by macrophages and the effect of anti-inflammatory drugs. Br. J. Pharm. 1978, 63, 635–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, T.; Scambler, T.E.; Smallie, T.; Cunliffe, H.E.; Ross, E.A.; Rosner, D.R.; O’Neil, J.D.; Clark, A.R. Macrophage responses to lipopolysaccharide are modulated by a feedback loop involving prostaglandin e2, dual specificity phosphatase 1 and tristetraprolin. Sci. Rep. 2017, 7, 4350. [Google Scholar] [CrossRef]
- Russell, S.W.; Pace, J.L. Gamma interferon interferes with the negative regulation of macrophage activation by prostaglandin e2. Mol. Immunol. 1984, 21, 249–254. [Google Scholar] [CrossRef]
- Hull, M.A.; Booth, J.K.; Tisbury, A.; Scott, N.; Bonifer, C.; Markham, A.F.; Coletta, P.L. Cyclooxygenase 2 is up-regulated and localized to macrophages in the intestine of min mice. Br. J. Cancer 1999, 79, 1399–1405. [Google Scholar] [CrossRef] [Green Version]
- Tetsuka, T.; Daphna-Iken, D.; Srivastava, S.K.; Baier, L.D.; DuMaine, J.; Morrison, A.R. Cross-talk between cyclooxygenase and nitric oxide pathways: Prostaglandin e2 negatively modulates induction of nitric oxide synthase by interleukin 1. Proc. Natl. Acad. Sci. USA 1994, 91, 12168–12172. [Google Scholar] [CrossRef] [Green Version]
- Eliopoulos, A.G.; Dumitru, C.D.; Wang, C.C.; Cho, J.; Tsichlis, P.N. Induction of cox-2 by lps in macrophages is regulated by tpl2-dependent creb activation signals. EMBO J. 2002, 21, 4831–4840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrios-Rodiles, M.; Tiraloche, G.; Chadee, K. Lipopolysaccharide modulates cyclooxygenase-2 transcriptionally and posttranscriptionally in human macrophages independently from endogenous il-1 beta and tnf-alpha. J. Immunol. 1999, 163, 963–969. [Google Scholar] [PubMed]
- Giroux, M.; Descoteaux, A. Cyclooxygenase-2 expression in macrophages: Modulation by protein kinase c-alpha. J. Immunol. 2000, 165, 3985–3991. [Google Scholar] [CrossRef] [Green Version]
- Reiner, N.E.; Malemud, C.J. Arachidonic acid metabolism by murine peritoneal macrophages infected with leishmania donovani: In vitro evidence for parasite-induced alterations in cyclooxygenase and lipoxygenase pathways. J. Immunol. 1985, 134, 556–563. [Google Scholar] [PubMed]
- Matte, C.; Maion, G.; Mourad, W.; Olivier, M. Leishmania donovani-induced macrophages cyclooxygenase-2 and prostaglandin e2 synthesis. Parasite Immunol. 2001, 23, 177–184. [Google Scholar] [CrossRef]
- Barbieri, S.S.; Eligini, S.; Brambilla, M.; Tremoli, E.; Colli, S. Reactive oxygen species mediate cyclooxygenase-2 induction during monocyte to macrophage differentiation: Critical role of nadph oxidase. Cardiovasc. Res. 2003, 60, 187–197. [Google Scholar] [CrossRef] [Green Version]
- Kiritoshi, S.; Nishikawa, T.; Sonoda, K.; Kukidome, D.; Senokuchi, T.; Matsuo, T.; Matsumura, T.; Tokunaga, H.; Brownlee, M.; Araki, E. Reactive oxygen species from mitochondria induce cyclooxygenase-2 gene expression in human mesangial cells: Potential role in diabetic nephropathy. Diabetes 2003, 52, 2570–2577. [Google Scholar] [CrossRef]
- Porta, H.; Rocha-Sosa, M. Plant lipoxygenases. Physiological and molecular features. Plant Physiol. 2002, 130, 15–21. [Google Scholar] [CrossRef] [Green Version]
- Radmark, O.; Werz, O.; Steinhilber, D.; Samuelsson, B. 5-lipoxygenase: Regulation of expression and enzyme activity. Trends Biochem. Sci. 2007, 32, 332–341. [Google Scholar] [CrossRef]
- Lewis, R.A.; Austen, K.F.; Soberman, R.J. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. New Engl. J. Med. 1990, 323, 645–655. [Google Scholar] [PubMed]
- Harrison, K.A.; Murphy, R.C. Isoleukotrienes are biologically active free radical products of lipid peroxidation. J. Biol. Chem. 1995, 270, 17273–17278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.Y.; Kim, T.B.; Moon, K.A.; Kim, T.J.; Shin, D.; Cho, Y.S.; Moon, H.B.; Lee, K.Y. Regulation of pro-inflammatory responses by lipoxygenases via intracellular reactive oxygen species in vitro and in vivo. Exp. Mol. Med. 2008, 40, 461–476. [Google Scholar] [CrossRef] [Green Version]
- Los, M.; Schenk, H.; Hexel, K.; Baeuerle, P.A.; Droge, W.; Schulze-Osthoff, K. Il-2 gene expression and nf-kappa b activation through cd28 requires reactive oxygen production by 5-lipoxygenase. EMBO J. 1995, 14, 3731–3740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Felts, K.A.; Parry, G.C.; Armacost, L.M.; Cobb, R.R. Inhibition of 5-lipoxygenase blocks il-1 beta-induced vascular adhesion molecule-1 gene expression in human endothelial cells. J. Immunol. 1997, 158, 3401–3407. [Google Scholar] [PubMed]
- Omura, T.; Sato, R. The carbon monoxide-binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J. Biol. Chem. 1964, 239, 2370–2378. [Google Scholar] [CrossRef]
- Katagiri, M.; Ganguli, B.N.; Gunsalus, I.C. A soluble cytochrome p-450 functional in methylene hydroxylation. J. Biol. Chem. 1968, 243, 3543–3546. [Google Scholar] [CrossRef]
- Omura, T.; Sato, R. A new cytochrome in liver microsomes. J. Biol. Chem. 1962, 237, 1375–1376. [Google Scholar] [CrossRef]
- Klingenberg, M. Pigments of rat liver microsomes. Arch. Biochem. Biophys. 1958, 75, 376–386. [Google Scholar] [CrossRef]
- Hofer, R.; Briesen, I.; Beck, M.; Pinot, F.; Schreiber, L.; Franke, R. The arabidopsis cytochrome p450 cyp86a1 encodes a fatty acid omega-hydroxylase involved in suberin monomer biosynthesis. J. Exp. Bot. 2008, 59, 2347–2360. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.D.; Peng, Y.Q.; Zhao, J.; Li, Q.; Yu, X.J.; Acevedo-Rocha, C.G.; Li, A.T. Bacterial cytochrome p450-catalyzed regio- and stereoselective steroid hydroxylation enabled by directed evolution and rational design. Bioresour. Bioprocess. 2020, 7, 2. [Google Scholar] [CrossRef]
- Nelson, D.; Werck-Reichhart, D. A p450-centric view of plant evolution. Plant J. Cell Mol. Biol. 2011, 66, 194–211. [Google Scholar] [CrossRef] [PubMed]
- Maves, S.A.; Sligar, S.G. Understanding thermostability in cytochrome p450 by combinatorial mutagenesis. Protein Sci. Publ. Protein Soc. 2001, 10, 161–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danielson, P.B. The cytochrome p450 superfamily: Biochemistry, evolution and drug metabolism in humans. Curr. Drug Metab. 2002, 3, 561–597. [Google Scholar] [CrossRef]
- Isin, E.M.; Guengerich, F.P. Complex reactions catalyzed by cytochrome p450 enzymes. Biochim. Biophys. Acta 2007, 1770, 314–329. [Google Scholar] [CrossRef] [PubMed]
- Guengerich, F.P. Intersection of the roles of cytochrome p450 enzymes with xenobiotic and endogenous substrates: Relevance to toxicity and drug interactions. Chem. Res. Toxicol. 2017, 30, 2–12. [Google Scholar] [CrossRef]
- Panigrahy, D.; Kaipainen, A.; Greene, E.R.; Huang, S. Cytochrome p450-derived eicosanoids: The neglected pathway in cancer. Cancer Metastasis Rev. 2010, 29, 723–735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamieson, K.L.; Endo, T.; Darwesh, A.M.; Samokhvalov, V.; Seubert, J.M. Cytochrome p450-derived eicosanoids and heart function. Pharmacol. Ther. 2017, 179, 47–83. [Google Scholar] [CrossRef] [PubMed]
- White, R.E.; Coon, M.J. Oxygen activation by cytochrome p-450. Annu Rev. Biochem. 1980, 49, 315–356. [Google Scholar] [CrossRef]
- Modi, A.R.; Dawson, J.H. Oxidizing intermediates in p450 catalysis: A case for multiple oxidants. Adv. Exp. Med. Biol. 2015, 851, 63–81. [Google Scholar] [PubMed]
- Denisov, I.G.; Makris, T.M.; Sligar, S.G.; Schlichting, I. Structure and chemistry of cytochrome p450. Chem. Rev. 2005, 105, 2253–2277. [Google Scholar] [CrossRef]
- Zangar, R.C.; Davydov, D.R.; Verma, S. Mechanisms that regulate production of reactive oxygen species by cytochrome p450. Toxicol. Appl. Pharmacol. 2004, 199, 316–331. [Google Scholar] [CrossRef]
- Veith, A.; Moorthy, B. Role of cytochrome p450s in the generation and metabolism of reactive oxygen species. Curr. Opin. Toxicol. 2018, 7, 44–51. [Google Scholar] [CrossRef]
- Sweeney, R.M.; McAuley, D.F. Acute respiratory distress syndrome. Lancet 2016, 388, 2416–2430. [Google Scholar] [CrossRef] [Green Version]
- Buczynski, B.W.; Maduekwe, E.T.; O’Reilly, M.A. The role of hyperoxia in the pathogenesis of experimental bpd. Semin. Perinatol. 2013, 37, 69–78. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, S.; Sinha, K.; Sil, P.C. Cytochrome p450s: Mechanisms and biological implications in drug metabolism and its interaction with oxidative stress. Curr. Drug Metab. 2014, 15, 719–742. [Google Scholar] [CrossRef]
- Furge, L.L.; Guengerich, F.P. Cytochrome p450 enzymes in drug metabolism and chemical toxicology: An introduction. Biochem. Mol. Biol. Educ. 2006, 34, 66–74. [Google Scholar] [CrossRef]
- Baron, J.M.; Zwadlo-Klarwasser, G.; Jugert, F.; Hamann, W.; Rubben, A.; Mukhtar, H.; Merk, H.F. Cytochrome p450 1b1: A major p450 isoenzyme in human blood monocytes and macrophage subsets. Biochem. Pharmacol. 1998, 56, 1105–1110. [Google Scholar] [CrossRef]
- Chen, X.W.; Yu, T.J.; Zhang, J.; Li, Y.; Chen, H.L.; Yang, G.F.; Yu, W.; Liu, Y.Z.; Liu, X.X.; Duan, C.F.; et al. Cyp4a in tumor-associated macrophages promotes pre-metastatic niche formation and metastasis. Oncogene 2017, 36, 5045–5057. [Google Scholar] [CrossRef] [Green Version]
- Tian, L.X.; Tang, X.; Zhu, J.Y.; Luo, L.; Ma, X.Y.; Cheng, S.W.; Zhang, W.; Tang, W.Q.; Ma, W.; Yang, X.; et al. Cytochrome p450 1a1 enhances inflammatory responses and impedes phagocytosis of bacteria in macrophages during sepsis. Cell Commun. Signal. 2020, 18, 70. [Google Scholar] [CrossRef] [PubMed]
- Jung, S. Macrophages and monocytes in 2017: Macrophages and monocytes: Of tortoises and hares. Nat. Rev. Immunol. 2018, 18, 85–86. [Google Scholar] [CrossRef]
- Epelman, S.; Lavine, K.J.; Randolph, G.J. Origin and functions of tissue macrophages. Immunity 2014, 41, 21–35. [Google Scholar] [CrossRef] [Green Version]
- Davies, L.C.; Jenkins, S.J.; Allen, J.E.; Taylor, P.R. Tissue-resident macrophages. Nat. Immunol. 2013, 14, 986–995. [Google Scholar] [CrossRef]
- Lemke, G. How macrophages deal with death. Nat. Rev. Immunol. 2019, 19, 539–549. [Google Scholar] [CrossRef]
- Haas, A. The phagosome: Compartment with a license to kill. Traffic 2007, 8, 311–330. [Google Scholar] [CrossRef]
- Djaldetti, M.; Salman, H.; Bergman, M.; Djaldetti, R.; Bessler, H. Phagocytosis--the mighty weapon of the silent warriors. Microsc. Res. Tech. 2002, 57, 421–431. [Google Scholar] [CrossRef]
- Mitchell, G.; Chen, C.; Portnoy, D.A. Strategies used by bacteria to grow in macrophages. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [Green Version]
- Weiss, G.; Schaible, U.E. Macrophage defense mechanisms against intracellular bacteria. Immunol. Rev. 2015, 264, 182–203. [Google Scholar] [CrossRef] [Green Version]
- Del Cerro-Vadillo, E.; Madrazo-Toca, F.; Carrasco-Marin, E.; Fernandez-Prieto, L.; Beck, C.; Leyva-Cobian, F.; Saftig, P.; Alvarez-Dominguez, C. Cutting edge: A novel nonoxidative phagosomal mechanism exerted by cathepsin-d controls listeria monocytogenes intracellular growth. J. Immunol. 2006, 176, 1321–1325. [Google Scholar] [CrossRef] [Green Version]
- Aktan, F. Inos-mediated nitric oxide production and its regulation. Life Sci. 2004, 75, 639–653. [Google Scholar] [CrossRef]
- Utermohlen, O.; Karow, U.; Lohler, J.; Kronke, M. Severe impairment in early host defense against listeria monocytogenes in mice deficient in acid sphingomyelinase. J. Immunol. 2003, 170, 2621–2628. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Dominguez, C.; Carrasco-Marin, E.; Lopez-Mato, P.; Leyva-Cobian, F. The contribution of both oxygen and nitrogen intermediates to the intracellular killing mechanisms of c1q-opsonized listeria monocytogenes by the macrophage-like ic-21 cell line. Immunology 2000, 101, 83–89. [Google Scholar] [CrossRef]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef] [Green Version]
- Allan, E.R.; Tailor, P.; Balce, D.R.; Pirzadeh, P.; McKenna, N.T.; Renaux, B.; Warren, A.L.; Jirik, F.R.; Yates, R.M. Nadph oxidase modifies patterns of mhc class ii-restricted epitopic repertoires through redox control of antigen processing. J. Immunol. 2014, 192, 4989–5001. [Google Scholar] [CrossRef] [Green Version]
- Craig, M.; Slauch, J.M. Phagocytic superoxide specifically damages an extracytoplasmic target to inhibit or kill salmonella. PLoS ONE 2009, 4, e4975. [Google Scholar] [CrossRef] [Green Version]
- Wink, D.A.; Hines, H.B.; Cheng, R.Y.; Switzer, C.H.; Flores-Santana, W.; Vitek, M.P.; Ridnour, L.A.; Colton, C.A. Nitric oxide and redox mechanisms in the immune response. J. Leukoc. Biol. 2011, 89, 873–891. [Google Scholar] [CrossRef] [Green Version]
- Slauch, J.M. How does the oxidative burst of macrophages kill bacteria? Still an open question. Mol. Microbiol. 2011, 80, 580–583. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.H.; Yang, Y.H.; Chiang, B.L. Chronic granulomatous disease: A comprehensive review. Clin. Rev. Allergy Immunol. 2020. [Google Scholar] [CrossRef]
- Birmingham, C.L.; Canadien, V.; Kaniuk, N.A.; Steinberg, B.E.; Higgins, D.E.; Brumell, J.H. Listeriolysin o allows listeria monocytogenes replication in macrophage vacuoles. Nature 2008, 451, 350–354. [Google Scholar] [CrossRef]
- Mitchell, G.; Cheng, M.I.; Chen, C.; Nguyen, B.N.; Whiteley, A.T.; Kianian, S.; Cox, J.S.; Green, D.R.; McDonald, K.L.; Portnoy, D.A. Listeria monocytogenes triggers noncanonical autophagy upon phagocytosis, but avoids subsequent growth-restricting xenophagy. Proc. Natl. Acad. Sci. USA 2018, 115, E210–E217. [Google Scholar] [CrossRef] [Green Version]
- Tattoli, I.; Sorbara, M.T.; Yang, C.; Tooze, S.A.; Philpott, D.J.; Girardin, S.E. Listeria phospholipases subvert host autophagic defenses by stalling pre-autophagosomal structures. EMBO J. 2013, 32, 3066–3078. [Google Scholar] [CrossRef] [Green Version]
- Heckmann, B.L.; Green, D.R. Lc3-associated phagocytosis at a glance. J. Cell Sci. 2019, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herb, M.; Gluschko, A.; Schramm, M. Lc3-associated phagocytosis—The highway to hell for phagocytosed microbes. Semin. Cell Dev. Biol. 2020, 101, 68–76. [Google Scholar] [CrossRef]
- Sanjuan, M.A.; Dillon, C.P.; Tait, S.W.; Moshiach, S.; Dorsey, F.; Connell, S.; Komatsu, M.; Tanaka, K.; Cleveland, J.L.; Withoff, S.; et al. Toll-like receptor signalling in macrophages links the autophagy pathway to phagocytosis. Nature 2007, 450, 1253–1257. [Google Scholar] [CrossRef] [PubMed]
- Herb, M.; Gluschko, A.; Schramm, M. Lc3-associated phagocytosis initiated by integrin itgam-itgb2/mac-1 enhances immunity to listeria monocytogenes. Autophagy 2018, 14, 1462–1464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charbonneau, M.E.; Passalacqua, K.D.; Hagen, S.E.; Showalter, H.D.; Wobus, C.E.; O’Riordan, M.X.D. Perturbation of ubiquitin homeostasis promotes macrophage oxidative defenses. Sci. Rep. 2019, 9, 10245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noubade, R.; Wong, K.; Ota, N.; Rutz, S.; Eidenschenk, C.; Valdez, P.A.; Ding, J.; Peng, I.; Sebrell, A.; Caplazi, P.; et al. Nrros negatively regulates reactive oxygen species during host defence and autoimmunity. Nature 2014, 509, 235–239. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Sun, X.; Wang, P.; Zhang, S.; Wang, X.; Wu, H.; Hong, L.; Xie, C.; Li, X.; Zhao, H.; et al. Kinases mst1 and mst2 positively regulate phagocytic induction of reactive oxygen species and bactericidal activity. Nat. Immunol. 2015, 16, 1142–1152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moldovan, A.; Fraunholz, M.J. In or out: Phagosomal escape of staphylococcus aureus. Cell Microbiol. 2019, 21, e12997. [Google Scholar] [CrossRef] [Green Version]
- Kohler, J.; Breitbach, K.; Renner, C.; Heitsch, A.K.; Bast, A.; van Rooijen, N.; Vogelgesang, S.; Steinmetz, I. Nadph-oxidase but not inducible nitric oxide synthase contributes to resistance in a murine staphylococcus aureus newman pneumonia model. Microbes Infect. 2011, 13, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Abuaita, B.H.; Burkholder, K.M.; Boles, B.R.; O’Riordan, M.X. The endoplasmic reticulum stress sensor inositol-requiring enzyme 1alpha augments bacterial killing through sustained oxidant production. mBio 2015, 6, e00705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambeth, J.D.; Krause, K.H.; Clark, R.A. Nox enzymes as novel targets for drug development. Semin. Immunopathol. 2008, 30, 339–363. [Google Scholar] [CrossRef]
- Bloxham, D.P. The relationship of diphenyleneiodonium-induced hypoglycaemia to the specific covalent modification of nadh-ubiquinone oxidoreductase. Biochem. Soc. Trans. 1979, 7, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Abuaita, B.H.; Schultz, T.L.; O’Riordan, M.X. Mitochondria-derived vesicles deliver antimicrobial reactive oxygen species to control phagosome-localized staphylococcus aureus. Cell Host Microbe 2018, 24, 625–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, C.J.; Boyle, R.H.; Astin, J.W.; Flores, M.V.; Oehlers, S.H.; Sanderson, L.E.; Ellett, F.; Lieschke, G.J.; Crosier, K.E.; Crosier, P.S. Immunoresponsive gene 1 augments bactericidal activity of macrophage-lineage cells by regulating beta-oxidation-dependent mitochondrial ros production. Cell Metab. 2013, 18, 265–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundqvist, H.; Dahlgren, C. Isoluminol-enhanced chemiluminescence: A sensitive method to study the release of superoxide anion from human neutrophils. Free Radic. Biol. Med. 1996, 20, 785–792. [Google Scholar] [CrossRef]
- Queval, C.J.; Brosch, R.; Simeone, R. The macrophage: A disputed fortress in the battle against mycobacterium tuberculosis. Front. Microbiol. 2017, 8, 2284. [Google Scholar] [CrossRef]
- Koster, S.; Upadhyay, S.; Chandra, P.; Papavinasasundaram, K.; Yang, G.; Hassan, A.; Grigsby, S.J.; Mittal, E.; Park, H.S.; Jones, V.; et al. Mycobacterium tuberculosis is protected from nadph oxidase and lc3-associated phagocytosis by the lcp protein cpsa. Proc. Natl. Acad. Sci. USA 2017, 114, E8711–E8720. [Google Scholar] [CrossRef] [Green Version]
- Roca, F.J.; Whitworth, L.J.; Redmond, S.; Jones, A.A.; Ramakrishnan, L. Tnf induces pathogenic programmed macrophage necrosis in tuberculosis through a mitochondrial-lysosomal-endoplasmic reticulum circuit. Cell 2019, 178, 1344–1361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.S.; Jin, Y.B.; Kim, Y.S.; Kim, S.; Kim, J.K.; Lee, H.M.; Suh, H.W.; Choe, J.H.; Kim, Y.J.; Koo, B.S.; et al. Sirt3 promotes antimycobacterial defenses by coordinating mitochondrial and autophagic functions. Autophagy 2019, 15, 1356–1375. [Google Scholar] [CrossRef]
- Fabrega, A.; Vila, J. Salmonella enterica serovar typhimurium skills to succeed in the host: Virulence and regulation. Clin. Microbiol. Rev. 2013, 26, 308–341. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, S.; Lucchini, S.; Thompson, A.; Rhen, M.; Hinton, J.C. Unravelling the biology of macrophage infection by gene expression profiling of intracellular salmonella enterica. Mol. Microbiol. 2003, 47, 103–118. [Google Scholar] [CrossRef]
- Mastroeni, P.; Morgan, F.J.; McKinley, T.J.; Shawcroft, E.; Clare, S.; Maskell, D.J.; Grant, A.J. Enhanced virulence of salmonella enterica serovar typhimurium after passage through mice. Infect. Immun. 2011, 79, 636–643. [Google Scholar] [CrossRef] [Green Version]
- Mastroeni, P.; Vazquez-Torres, A.; Fang, F.C.; Xu, Y.; Khan, S.; Hormaeche, C.E.; Dougan, G. Antimicrobial actions of the nadph phagocyte oxidase and inducible nitric oxide synthase in experimental salmonellosis. Ii. Effects on microbial proliferation and host survival in vivo. J. Exp. Med. 2000, 192, 237–248. [Google Scholar] [CrossRef]
- Vazquez-Torres, A.; Xu, Y.; Jones-Carson, J.; Holden, D.W.; Lucia, S.M.; Dinauer, M.C.; Mastroeni, P.; Fang, F.C. Salmonella pathogenicity island 2-dependent evasion of the phagocyte nadph oxidase. Science 2000, 287, 1655–1658. [Google Scholar] [CrossRef]
- De Groote, M.A.; Ochsner, U.A.; Shiloh, M.U.; Nathan, C.; McCord, J.M.; Dinauer, M.C.; Libby, S.J.; Vazquez-Torres, A.; Xu, Y.; Fang, F.C. Periplasmic superoxide dismutase protects salmonella from products of phagocyte nadph-oxidase and nitric oxide synthase. Proc. Natl. Acad. Sci. USA 1997, 94, 13997–14001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhen, M. Salmonella and reactive oxygen species: A love-hate relationship. J. Innate Immun. 2019, 11, 216–226. [Google Scholar] [CrossRef] [PubMed]
- Burton, N.A.; Schurmann, N.; Casse, O.; Steeb, A.K.; Claudi, B.; Zankl, J.; Schmidt, A.; Bumann, D. Disparate impact of oxidative host defenses determines the fate of salmonella during systemic infection in mice. Cell Host Microbe 2014, 15, 72–83. [Google Scholar] [CrossRef] [Green Version]
- Fenlon, L.A.; Slauch, J.M. Phagocyte roulette in salmonella killing. Cell Host Microbe 2014, 15, 7–8. [Google Scholar] [CrossRef] [Green Version]
- Garaude, J.; Acin-Perez, R.; Martinez-Cano, S.; Enamorado, M.; Ugolini, M.; Nistal-Villan, E.; Hervas-Stubbs, S.; Pelegrin, P.; Sander, L.E.; Enriquez, J.A.; et al. Mitochondrial respiratory-chain adaptations in macrophages contribute to antibacterial host defense. Nat. Immunol. 2016, 17, 1037–1045. [Google Scholar] [CrossRef] [Green Version]
- Sena, L.A.; Li, S.; Jairaman, A.; Prakriya, M.; Ezponda, T.; Hildeman, D.A.; Wang, C.R.; Schumacker, P.T.; Licht, J.D.; Perlman, H.; et al. Mitochondria are required for antigen-specific t cell activation through reactive oxygen species signaling. Immunity 2013, 38, 225–236. [Google Scholar] [CrossRef] [Green Version]
- Sazanov, L.A. The mechanism of coupling between electron transfer and proton translocation in respiratory complex i. J. Bioenerg. Biomembr. 2014, 46, 247–253. [Google Scholar] [CrossRef]
- Orr, A.L.; Ashok, D.; Sarantos, M.R.; Shi, T.; Hughes, R.E.; Brand, M.D. Inhibitors of ros production by the ubiquinone-binding site of mitochondrial complex i identified by chemical screening. Free Radic. Biol. Med. 2013, 65, 1047–1059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Acin-Perez, R.; Iborra, S.; Marti-Mateos, Y.; Cook, E.C.L.; Conde-Garrosa, R.; Petcherski, A.; Munoz, M.D.M.; Martinez de Mena, R.; Krishnan, K.C.; Jimenez, C.; et al. Fgr kinase is required for proinflammatory macrophage activation during diet-induced obesity. Nat. Metab. 2020, 2, 974–988. [Google Scholar] [CrossRef]
- Zhou, R.; Yazdi, A.S.; Menu, P.; Tschopp, J. A role for mitochondria in nlrp3 inflammasome activation. Nature 2011, 469, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.P. Dubey, j.P. Toxoplasmosis of animals and humans. Parasites Vectors 2010, 3, 112. [Google Scholar] [CrossRef] [Green Version]
- Ybanez, R.H.D.; Ybanez, A.P.; Nishikawa, Y. Review on the current trends of toxoplasmosis serodiagnosis in humans. Front. Cell. Infect. Microbiol. 2020, 10, 204. [Google Scholar] [CrossRef]
- Park, J.; Hunter, C.A. The role of macrophages in protective and pathological responses to toxoplasma gondii. Parasite Immunol. 2020, 42, e12712. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.P.; Tomita, T.; Weiss, L.M.; Orlofsky, A. Proliferation of toxoplasma gondii in inflammatory macrophages in vivo is associated with diminished oxygen radical production in the host cell. Int. J. Parasitol. 2006, 36, 433–441. [Google Scholar] [CrossRef] [Green Version]
- Murray, H.W.; Rubin, B.Y.; Carriero, S.M.; Harris, A.M.; Jaffee, E.A. Human mononuclear phagocyte antiprotozoal mechanisms: Oxygen-dependent vs oxygen-independent activity against intracellular toxoplasma gondii. J. Immunol. 1985, 134, 1982–1988. [Google Scholar] [PubMed]
- Matta, S.K.; Patten, K.; Wang, Q.; Kim, B.H.; MacMicking, J.D.; Sibley, L.D. Nadph oxidase and guanylate binding protein 5 restrict survival of avirulent type iii strains of toxoplasma gondii in naive macrophages. mBio 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Lee, J.; Bae, S.J.; Kim, Y.; Park, B.J.; Choi, J.W.; Kwon, J.; Cha, G.H.; Yoo, H.J.; Jo, E.K.; et al. Nadph oxidase 4 is required for the generation of macrophage migration inhibitory factor and host defense against toxoplasma gondii infection. Sci. Rep. 2017, 7, 6361. [Google Scholar] [CrossRef] [Green Version]
- Laurindo, F.R.; Araujo, T.L.; Abrahao, T.B. Nox nadph oxidases and the endoplasmic reticulum. Antioxid. Redox Signal. 2014, 20, 2755–2775. [Google Scholar] [CrossRef] [Green Version]
- Sciarretta, S.; Zhai, P.; Shao, D.; Zablocki, D.; Nagarajan, N.; Terada, L.S.; Volpe, M.; Sadoshima, J. Activation of nadph oxidase 4 in the endoplasmic reticulum promotes cardiomyocyte autophagy and survival during energy stress through the protein kinase rna-activated-like endoplasmic reticulum kinase/eukaryotic initiation factor 2alpha/activating transcription factor 4 pathway. Circ. Res. 2013, 113, 1253–1264. [Google Scholar] [PubMed] [Green Version]
- Martinez, A.; Prolo, C.; Estrada, D.; Rios, N.; Alvarez, M.N.; Pineyro, M.D.; Robello, C.; Radi, R.; Piacenza, L. Cytosolic fe-superoxide dismutase safeguards trypanosoma cruzi from macrophage-derived superoxide radical. Proc. Natl. Acad. Sci. USA 2019, 116, 8879–8888. [Google Scholar] [CrossRef] [Green Version]
- Tomiotto-Pellissier, F.; Bortoleti, B.; Assolini, J.P.; Goncalves, M.D.; Carloto, A.C.M.; Miranda-Sapla, M.M.; Conchon-Costa, I.; Bordignon, J.; Pavanelli, W.R. Macrophage polarization in leishmaniasis: Broadening horizons. Front. Immunol. 2018, 9, 2529. [Google Scholar] [CrossRef] [Green Version]
- Peters, N.C.; Sacks, D.L. The impact of vector-mediated neutrophil recruitment on cutaneous leishmaniasis. Cell Microbiol. 2009, 11, 1290–1296. [Google Scholar] [CrossRef] [Green Version]
- Charmoy, M.; Auderset, F.; Allenbach, C.; Tacchini-Cottier, F. The prominent role of neutrophils during the initial phase of infection by leishmania parasites. J. Biomed. Biotechnol. 2010, 2010, 719361. [Google Scholar] [CrossRef] [Green Version]
- Scott, P.; Novais, F.O. Cutaneous leishmaniasis: Immune responses in protection and pathogenesis. Nat. Rev. Immunol. 2016, 16, 581–592. [Google Scholar] [CrossRef]
- Srivastava, S.; Shankar, P.; Mishra, J.; Singh, S. Possibilities and challenges for developing a successful vaccine for leishmaniasis. Parasites Vectors 2016, 9, 277. [Google Scholar] [CrossRef] [Green Version]
- Matte, C.; Casgrain, P.A.; Seguin, O.; Moradin, N.; Hong, W.J.; Descoteaux, A. Leishmania major promastigotes evade lc3-associated phagocytosis through the action of gp63. PLoS Pathog. 2016, 12, e1005690. [Google Scholar] [CrossRef]
- Alonso, D.; Serrano, E.; Bermejo, F.J.; Corral, R.S. Hif-1alpha-regulated mif activation and nox2-dependent ros generation promote leishmania amazonensis killing by macrophages under hypoxia. Cell. Immunol. 2019, 335, 15–21. [Google Scholar] [CrossRef] [PubMed]
- To, E.E.; Vlahos, R.; Luong, R.; Halls, M.L.; Reading, P.C.; King, P.T.; Chan, C.; Drummond, G.R.; Sobey, C.G.; Broughton, B.R.S.; et al. Endosomal nox2 oxidase exacerbates virus pathogenicity and is a target for antiviral therapy. Nat. Commun. 2017, 8, 69. [Google Scholar] [CrossRef]
- Boudreau, H.E.; Emerson, S.U.; Korzeniowska, A.; Jendrysik, M.A.; Leto, T.L. Hepatitis c virus (hcv) proteins induce nadph oxidase 4 expression in a transforming growth factor beta-dependent manner: A new contributor to hcv-induced oxidative stress. J. Virol. 2009, 83, 12934–12946. [Google Scholar] [CrossRef] [Green Version]
- Comstock, A.T.; Ganesan, S.; Chattoraj, A.; Faris, A.N.; Margolis, B.L.; Hershenson, M.B.; Sajjan, U.S. Rhinovirus-induced barrier dysfunction in polarized airway epithelial cells is mediated by nadph oxidase 1. J. Virol. 2011, 85, 6795–6808. [Google Scholar] [CrossRef] [Green Version]
- Vlahos, R.; Stambas, J.; Bozinovski, S.; Broughton, B.R.; Drummond, G.R.; Selemidis, S. Inhibition of nox2 oxidase activity ameliorates influenza a virus-induced lung inflammation. PLoS Pathog. 2011, 7, e1001271. [Google Scholar] [CrossRef] [Green Version]
- Lang, P.A.; Xu, H.C.; Grusdat, M.; McIlwain, D.R.; Pandyra, A.A.; Harris, I.S.; Shaabani, N.; Honke, N.; Maney, S.K.; Lang, E.; et al. Reactive oxygen species delay control of lymphocytic choriomeningitis virus. Cell Death Differ. 2013, 20, 649–658. [Google Scholar] [CrossRef] [Green Version]
- To, E.E.; Erlich, J.R.; Liong, F.; Luong, R.; Liong, S.; Esaq, F.; Oseghale, O.; Anthony, D.; McQualter, J.; Bozinovski, S.; et al. Mitochondrial reactive oxygen species contribute to pathological inflammation during influenza a virus infection in mice. Antioxid. Redox Signal. 2020, 32, 929–942. [Google Scholar] [CrossRef] [Green Version]
- Poetsch, A.R. The genomics of oxidative DNA damage, repair, and resulting mutagenesis. Comput. Struct. Biotechnol. J. 2020, 18, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.; Mizushima, N.; Virgin, H.W. Autophagy in immunity and inflammation. Nature 2011, 469, 323–335. [Google Scholar] [CrossRef]
- Dreux, M.; Chisari, F.V. Viruses and the autophagy machinery. Cell Cycle 2010, 9, 1295–1307. [Google Scholar] [CrossRef]
- Kyei, G.B.; Dinkins, C.; Davis, A.S.; Roberts, E.; Singh, S.B.; Dong, C.; Wu, L.; Kominami, E.; Ueno, T.; Yamamoto, A.; et al. Autophagy pathway intersects with hiv-1 biosynthesis and regulates viral yields in macrophages. J. Cell Biol. 2009, 186, 255–268. [Google Scholar] [CrossRef]
- Sir, D.; Ou, J.H. Autophagy in viral replication and pathogenesis. Mol. Cells 2010, 29, 1–7. [Google Scholar] [CrossRef]
- V’Kovski, P.; Kratzel, A.; Steiner, S.; Stalder, H.; Thiel, V. Coronavirus biology and replication: Implications for sars-cov-2. Nat. Rev. Microbiol. 2020, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Reggiori, F.; Monastyrska, I.; Verheije, M.H.; Cali, T.; Ulasli, M.; Bianchi, S.; Bernasconi, R.; de Haan, C.A.; Molinari, M. Coronaviruses hijack the lc3-i-positive edemosomes, er-derived vesicles exporting short-lived erad regulators, for replication. Cell Host Microbe 2010, 7, 500–508. [Google Scholar] [CrossRef] [Green Version]
- Cottam, E.M.; Maier, H.J.; Manifava, M.; Vaux, L.C.; Chandra-Schoenfelder, P.; Gerner, W.; Britton, P.; Ktistakis, N.T.; Wileman, T. Coronavirus nsp6 proteins generate autophagosomes from the endoplasmic reticulum via an omegasome intermediate. Autophagy 2011, 7, 1335–1347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and nf-kappab signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef] [Green Version]
- Brune, B.; Dehne, N.; Grossmann, N.; Jung, M.; Namgaladze, D.; Schmid, T.; von Knethen, A.; Weigert, A. Redox control of inflammation in macrophages. Antioxid. Redox Signal. 2013, 19, 595–637. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Yao, Y.; Qiu, X.; Wang, G.; Hu, Z.; Chen, S.; Wu, Z.; Yuan, N.; Gao, H.; Wang, J.; et al. Listeria hijacks host mitophagy through a novel mitophagy receptor to evade killing. Nat. Immunol. 2019, 20, 433–446. [Google Scholar] [CrossRef]
- Stavru, F.; Bouillaud, F.; Sartori, A.; Ricquier, D.; Cossart, P. Listeria monocytogenes transiently alters mitochondrial dynamics during infection. Proc. Natl. Acad. Sci. USA 2011, 108, 3612–3617. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Kong, L.; Li, X.; Wu, S.; Attri, K.S.; Li, Y.; Gong, W.; Zhao, B.; Li, L.; Herring, L.E.; et al. Listeria monocytogenes upregulates mitochondrial calcium signalling to inhibit lc3-associated phagocytosis as a survival strategy. Nat. Microbiol. 2021. [Google Scholar] [CrossRef]
- Lin, T.K.; Lin, K.J.; Lin, K.L.; Liou, C.W.; Chen, S.D.; Chuang, Y.C.; Wang, P.W.; Chuang, J.H.; Wang, T.J. When friendship turns sour: Effective communication between mitochondria and intracellular organelles in parkinson’s disease. Front. Cell Dev. Biol. 2020, 8, 607392. [Google Scholar] [CrossRef] [PubMed]
- Maubach, G.; Schmadicke, A.C.; Naumann, M. Nemo links nuclear factor-kappab to human diseases. Trends Mol. Med. 2017, 23, 1138–1155. [Google Scholar] [CrossRef]
- Hinz, M.; Scheidereit, C. The ikappab kinase complex in nf-kappab regulation and beyond. EMBO Rep. 2014, 15, 46–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, B.; Tannahill, G.M.; Murphy, M.P.; O’Neill, L.A. Metformin inhibits the production of reactive oxygen species from nadh:Ubiquinone oxidoreductase to limit induction of interleukin-1beta (il-1beta) and boosts interleukin-10 (il-10) in lipopolysaccharide (lps)-activated macrophages. J. Biol. Chem. 2015, 290, 20348–20359. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.P.; Smith, R.A. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 629–656. [Google Scholar] [CrossRef]
- Kelso, G.F.; Porteous, C.M.; Coulter, C.V.; Hughes, G.; Porteous, W.K.; Ledgerwood, E.C.; Smith, R.A.; Murphy, M.P. Selective targeting of a redox-active ubiquinone to mitochondria within cells: Antioxidant and antiapoptotic properties. J. Biol. Chem. 2001, 276, 4588–4596. [Google Scholar] [CrossRef] [Green Version]
- Rieber, N.; Hector, A.; Kuijpers, T.; Roos, D.; Hartl, D. Current concepts of hyperinflammation in chronic granulomatous disease. Clin. Dev. Immunol. 2012, 2012, 252460. [Google Scholar] [CrossRef]
- Deffert, C.; Carnesecchi, S.; Yuan, H.; Rougemont, A.L.; Kelkka, T.; Holmdahl, R.; Krause, K.H.; Schappi, M.G. Hyperinflammation of chronic granulomatous disease is abolished by nox2 reconstitution in macrophages and dendritic cells. J. Pathol. 2012, 228, 341–350. [Google Scholar] [CrossRef]
- Whitmore, L.C.; Hilkin, B.M.; Goss, K.L.; Wahle, E.M.; Colaizy, T.T.; Boggiatto, P.M.; Varga, S.M.; Miller, F.J.; Moreland, J.G. Nox2 protects against prolonged inflammation, lung injury, and mortality following systemic insults. J. Innate Immun. 2013, 5, 565–580. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.; Ma, H.Y.; Zhong, Z.; Dhar, D.; Liu, X.; Xu, J.; Koyama, Y.; Nishio, T.; Karin, D.; Karin, G.; et al. Nadph oxidase 1 in liver macrophages promotes inflammation and tumor development in mice. Gastroenterology 2019, 156, 1156–1172. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Iwata, K.; Zhu, K.; Matsumoto, M.; Matsumoto, K.; Asaoka, N.; Zhang, X.; Ibi, M.; Katsuyama, M.; Tsutsui, M.; et al. Nox1/nadph oxidase in bone marrow-derived cells modulates intestinal barrier function. Free Radic. Biol. Med. 2020, 147, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Estrada, E.Y.; Thompson, J.F.; Liu, W.; Rosenberg, G.A. Matrix metalloproteinase-mediated disruption of tight junction proteins in cerebral vessels is reversed by synthetic matrix metalloproteinase inhibitor in focal ischemia in rat. J. Cereb. Blood Flow Metab. Off. J. Int. Soc. Cereb. Blood Flow Metab. 2007, 27, 697–709. [Google Scholar] [CrossRef]
- Khokha, R.; Murthy, A.; Weiss, A. Metalloproteinases and their natural inhibitors in inflammation and immunity. Nat. Rev. Immunol. 2013, 13, 649–665. [Google Scholar] [CrossRef]
- Downs, K.P.; Nguyen, H.; Dorfleutner, A.; Stehlik, C. An overview of the non-canonical inflammasome. Mol. Asp. Med. 2020, 76, 100924. [Google Scholar] [CrossRef] [PubMed]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Dorfleutner, A.; Chu, L.; Stehlik, C. Inhibiting the inflammasome: One domain at a time. Immunol. Rev. 2015, 265, 205–216. [Google Scholar] [CrossRef] [Green Version]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proil-beta. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Davis, B.K.; Wen, H.; Ting, J.P. The inflammasome nlrs in immunity, inflammation, and associated diseases. Annu. Rev. Immunol. 2011, 29, 707–735. [Google Scholar] [CrossRef] [Green Version]
- Kerur, N.; Veettil, M.V.; Sharma-Walia, N.; Bottero, V.; Sadagopan, S.; Otageri, P.; Chandran, B. Ifi16 acts as a nuclear pathogen sensor to induce the inflammasome in response to kaposi sarcoma-associated herpesvirus infection. Cell Host Microbe 2011, 9, 363–375. [Google Scholar] [CrossRef] [Green Version]
- Matsuoka, Y.; Yamashita, A.; Matsuda, M.; Kawai, K.; Sawa, T.; Amaya, F. Nlrp2 inflammasome in dorsal root ganglion as a novel molecular platform that produces inflammatory pain hypersensitivity. Pain 2019, 160, 2149–2160. [Google Scholar] [CrossRef]
- Minkiewicz, J.; de Rivero Vaccari, J.P.; Keane, R.W. Human astrocytes express a novel nlrp2 inflammasome. Glia 2013, 61, 1113–1121. [Google Scholar] [CrossRef] [PubMed]
- Vladimer, G.I.; Weng, D.; Paquette, S.W.; Vanaja, S.K.; Rathinam, V.A.; Aune, M.H.; Conlon, J.E.; Burbage, J.J.; Proulx, M.K.; Liu, Q.; et al. The nlrp12 inflammasome recognizes yersinia pestis. Immunity 2012, 37, 96–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swanson, K.V.; Deng, M.; Ting, J.P. The nlrp3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Von Moltke, J.; Ayres, J.S.; Kofoed, E.M.; Chavarria-Smith, J.; Vance, R.E. Recognition of bacteria by inflammasomes. Annu. Rev. Immunol. 2013, 31, 73–106. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Van Bruggen, R.; Koker, M.Y.; Jansen, M.; van Houdt, M.; Roos, D.; Kuijpers, T.W.; van den Berg, T.K. Human nlrp3 inflammasome activation is nox1-4 independent. Blood 2010, 115, 5398–5400. [Google Scholar] [CrossRef] [PubMed]
- Van de Veerdonk, F.L.; Smeekens, S.P.; Joosten, L.A.; Kullberg, B.J.; Dinarello, C.A.; van der Meer, J.W.; Netea, M.G. Reactive oxygen species-independent activation of the il-1beta inflammasome in cells from patients with chronic granulomatous disease. Proc. Natl. Acad. Sci. USA 2010, 107, 3030–3033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meissner, F.; Seger, R.A.; Moshous, D.; Fischer, A.; Reichenbach, J.; Zychlinsky, A. Inflammasome activation in nadph oxidase defective mononuclear phagocytes from patients with chronic granulomatous disease. Blood 2010, 116, 1570–1573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dostert, C.; Petrilli, V.; Van Bruggen, R.; Steele, C.; Mossman, B.T.; Tschopp, J. Innate immune activation through nalp3 inflammasome sensing of asbestos and silica. Science 2008, 320, 674–677. [Google Scholar] [CrossRef] [Green Version]
- Dostert, C.; Guarda, G.; Romero, J.F.; Menu, P.; Gross, O.; Tardivel, A.; Suva, M.L.; Stehle, J.C.; Kopf, M.; Stamenkovic, I.; et al. Malarial hemozoin is a nalp3 inflammasome activating danger signal. PLoS ONE 2009, 4, e6510. [Google Scholar] [CrossRef]
- Wingler, K.; Hermans, J.J.; Schiffers, P.; Moens, A.; Paul, M.; Schmidt, H.H. Nox1, 2, 4, 5: Counting out oxidative stress. Br. J. Pharm. 2011, 164, 866–883. [Google Scholar] [CrossRef] [Green Version]
- Altenhofer, S.; Kleikers, P.W.; Radermacher, K.A.; Scheurer, P.; Rob Hermans, J.J.; Schiffers, P.; Ho, H.; Wingler, K.; Schmidt, H.H. The nox toolbox: Validating the role of nadph oxidases in physiology and disease. Cell Mol. Life Sci. 2012, 69, 2327–2343. [Google Scholar] [CrossRef] [Green Version]
- Latz, E. Nox-free inflammasome activation. Blood 2010, 116, 1393–1394. [Google Scholar] [CrossRef]
- Moon, J.S.; Nakahira, K.; Chung, K.P.; DeNicola, G.M.; Koo, M.J.; Pabon, M.A.; Rooney, K.T.; Yoon, J.H.; Ryter, S.W.; Stout-Delgado, H.; et al. Nox4-dependent fatty acid oxidation promotes nlrp3 inflammasome activation in macrophages. Nat. Med. 2016, 22, 1002–1012. [Google Scholar] [CrossRef] [PubMed]
- Naviaux, R.K. Oxidative shielding or oxidative stress? J. Pharmacol. Exp. Ther. 2012, 342, 608–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Go, Y.M.; Jones, D.P. Redox compartmentalization in eukaryotic cells. Biochim. Biophys. Acta 2008, 1780, 1273–1290. [Google Scholar] [CrossRef] [Green Version]
- Crane, D.D.; Bauler, T.J.; Wehrly, T.D.; Bosio, C.M. Mitochondrial ros potentiates indirect activation of the aim2 inflammasome. Front. Microbiol. 2014, 5, 438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markvicheva, K.N.; Bilan, D.S.; Mishina, N.M.; Gorokhovatsky, A.Y.; Vinokurov, L.M.; Lukyanov, S.; Belousov, V.V. A genetically encoded sensor for h2o2 with expanded dynamic range. Bioorgan. Med. Chem. 2011, 19, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Gutscher, M.; Sobotta, M.C.; Wabnitz, G.H.; Ballikaya, S.; Meyer, A.J.; Samstag, Y.; Dick, T.P. Proximity-based protein thiol oxidation by h2o2-scavenging peroxidases. J. Biol. Chem. 2009, 284, 31532–31540. [Google Scholar] [CrossRef] [Green Version]
- Bilan, D.S.; Pase, L.; Joosen, L.; Gorokhovatsky, A.Y.; Ermakova, Y.G.; Gadella, T.W.; Grabher, C.; Schultz, C.; Lukyanov, S.; Belousov, V.V. Hyper-3: A genetically encoded h(2)o(2) probe with improved performance for ratiometric and fluorescence lifetime imaging. ACS Chem. Biol. 2013, 8, 535–542. [Google Scholar] [CrossRef] [Green Version]
- Belousov, V.V.; Fradkov, A.F.; Lukyanov, K.A.; Staroverov, D.B.; Shakhbazov, K.S.; Terskikh, A.V.; Lukyanov, S. Genetically encoded fluorescent indicator for intracellular hydrogen peroxide. Nat. Methods 2006, 3, 281–286. [Google Scholar] [CrossRef]
- Hernández-Barrera, A.; Quinto, C.; Johnson, E.A.; Wu, H.-M.; Cheung, A.Y.; Cárdenas, L. Chapter fifteen—Using hyper as a molecular probe to visualize hydrogen peroxide in living plant cells: A method with virtually unlimited potential in plant biology. In Methods in Enzymology; Cadenas, E., Packer, L., Eds.; Academic Press: Cambridge, MA, USA, 2013; Volume 527, pp. 275–290. [Google Scholar]
- Ermakova, Y.G.; Bilan, D.S.; Matlashov, M.E.; Mishina, N.M.; Markvicheva, K.N.; Subach, O.M.; Subach, F.V.; Bogeski, I.; Hoth, M.; Enikolopov, G.; et al. Red fluorescent genetically encoded indicator for intracellular hydrogen peroxide. Nat. Commun. 2014, 5, 5222. [Google Scholar] [CrossRef]
- Caldefie-Chezet, F.; Walrand, S.; Moinard, C.; Tridon, A.; Chassagne, J.; Vasson, M.P. Is the neutrophil reactive oxygen species production measured by luminol and lucigenin chemiluminescence intra or extracellular? Comparison with dcfh-da flow cytometry and cytochrome c reduction. Clin. Chim. Acta Int. J. Clin. Chem. 2002, 319, 9–17. [Google Scholar] [CrossRef]
- Pavelkova, M.; Kubala, L. Luminol-, isoluminol- and lucigenin-enhanced chemiluminescence of rat blood phagocytes stimulated with different activators. Lumin. J. Biol. Chem. Lumin. 2004, 19, 37–42. [Google Scholar] [CrossRef]
- Ushijima, Y.; Totsune, H.; Nishida, A.; Nakano, M. Chemiluminescence from human polymorphonuclear leukocytes activated with opsonized zymosan. Free Radic. Biol. Med. 1997, 22, 401–409. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, H.; Kuppusamy, P.; Roubaud, V.; Zweier, J.L.; Trush, M.A. Validation of lucigenin (bis-n-methylacridinium) as a chemilumigenic probe for detecting superoxide anion radical production by enzymatic and cellular systems. J. Biol. Chem. 1998, 273, 2015–2023. [Google Scholar] [CrossRef] [Green Version]
- Afanas’ev, I.B.; Ostrachovitch, E.A.; Korkina, L.G. Lucigenin is a mediator of cytochrome c reduction but not of superoxide production. Arch. Biochem. Biophys. 1999, 366, 267–274. [Google Scholar] [CrossRef]
- Skatchkov, M.P.; Sperling, D.; Hink, U.; Mulsch, A.; Harrison, D.G.; Sindermann, I.; Meinertz, T.; Munzel, T. Validation of lucigenin as a chemiluminescent probe to monitor vascular superoxide as well as basal vascular nitric oxide production. Biochem. Biophys. Res. Commun. 1999, 254, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Hempel, S.L.; Buettner, G.R.; O’Malley, Y.Q.; Wessels, D.A.; Flaherty, D.M. Dihydrofluorescein diacetate is superior for detecting intracellular oxidants: Comparison with 2′,7′-dichlorodihydrofluorescein diacetate, 5(and 6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate, and dihydrorhodamine 123. Free Radic. Biol. Med. 1999, 27, 146–159. [Google Scholar] [CrossRef]
- Wang, Q.; Zou, M.H. Measurement of reactive oxygen species (ros) and mitochondrial ros in ampk knockout mice blood vessels. Methods Mol. Biol 2018, 1732, 507–517. [Google Scholar]
- Dahlgren, C.; Karlsson, A. Respiratory burst in human neutrophils. J. Immunol. Methods 1999, 232, 3–14. [Google Scholar] [CrossRef]
- Karakuzu, O.; Cruz, M.R.; Liu, Y.; Garsin, D.A. Amplex red assay for measuring hydrogen peroxide production from caenorhabditis elegans. Bio Protoc. 2019, 9, e3409. [Google Scholar] [CrossRef]
- Mohanty, J.G.; Jaffe, J.S.; Schulman, E.S.; Raible, D.G. A highly sensitive fluorescent micro-assay of h2o2 release from activated human leukocytes using a dihydroxyphenoxazine derivative. J. Immunol. Methods 1997, 202, 133–141. [Google Scholar] [CrossRef]
- Mak, T.W.; Grusdat, M.; Duncan, G.S.; Dostert, C.; Nonnenmacher, Y.; Cox, M.; Binsfeld, C.; Hao, Z.; Brustle, A.; Itsumi, M.; et al. Glutathione primes t cell metabolism for inflammation. Immunity 2017, 46, 675–689. [Google Scholar] [CrossRef] [Green Version]
- Mukhopadhyay, P.; Rajesh, M.; Hasko, G.; Hawkins, B.J.; Madesh, M.; Pacher, P. Simultaneous detection of apoptosis and mitochondrial superoxide production in live cells by flow cytometry and confocal microscopy. Nat. Protoc. 2007, 2, 2295–2301. [Google Scholar] [CrossRef]
- Robinson, K.M.; Janes, M.S.; Pehar, M.; Monette, J.S.; Ross, M.F.; Hagen, T.M.; Murphy, M.P.; Beckman, J.S. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc. Natl. Acad. Sci. USA 2006, 103, 15038–15043. [Google Scholar] [CrossRef] [Green Version]
- Akhtar, M.J.; Ahamed, M.; Alhadlaq, H.A.; Alshamsan, A. Mechanism of ros scavenging and antioxidant signalling by redox metallic and fullerene nanomaterials: Potential implications in ros associated degenerative disorders. Biochim. Biophys. Acta. Gen. Subj. 2017, 1861, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Gutteridge, J.M.; Halliwell, B. Antioxidants: Molecules, medicines, and myths. Biochem. Biophys. Res. Commun. 2010, 393, 561–564. [Google Scholar] [CrossRef]
- Patriarca, S.; Furfaro, A.L.; Domenicotti, C.; Odetti, P.; Cottalasso, D.; Marinari, U.M.; Pronzato, M.A.; Traverso, N. Supplementation with n-acetylcysteine and taurine failed to restore glutathione content in liver of streptozotocin-induced diabetics rats but protected from oxidative stress. Biochim. Biophys. Acta 2005, 1741, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Coles, L.D.; Kartha, R.V.; Nash, N.; Mishra, U.; Lund, T.C.; Cloyd, J.C. Intravenous administration of stable-labeled n-acetylcysteine demonstrates an indirect mechanism for boosting glutathione and improving redox status. J. Pharm. Sci. 2015, 104, 2619–2626. [Google Scholar] [CrossRef]
- Ezerina, D.; Takano, Y.; Hanaoka, K.; Urano, Y.; Dick, T.P. N-acetyl cysteine functions as a fast-acting antioxidant by triggering intracellular h2s and sulfane sulfur production. Cell Chem. Biol. 2018, 25, 447–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernardy, C.C.F.; Zarpelon, A.C.; Pinho-Ribeiro, F.A.; Calixto-Campos, C.; Carvalho, T.T.; Fattori, V.; Borghi, S.M.; Casagrande, R.; Verri, W.A., Jr. Tempol, a superoxide dismutase mimetic agent, inhibits superoxide anion-induced inflammatory pain in mice. Biomed. Res. Int. 2017, 2017, 9584819. [Google Scholar] [CrossRef] [PubMed]
- Rak, R.; Chao, D.L.; Pluta, R.M.; Mitchell, J.B.; Oldfield, E.H.; Watson, J.C. Neuroprotection by the stable nitroxide tempol during reperfusion in a rat model of transient focal ischemia. J. Neurosurg. 2000, 92, 646–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haj-Yehia, A.I.; Nassar, T.; Assaf, P.; Nassar, H.; Anggard, E.E. Effects of the superoxide dismutase-mimic compound tempol on oxidant stress-mediated endothelial dysfunction. Antioxid. Redox Signal. 1999, 1, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Manzano, V.M.; Munoz, J.C.; Jimenez, J.R.; Puyol, M.R.; Puyol, D.R.; Kitamura, M.; Cazana, F.J. Human renal mesangial cells are a target for the anti-inflammatory action of 9-cis retinoic acid. Br. J. Pharm. 2000, 131, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Krishna, C.M.; Liebmann, J.E.; Kaufman, D.; DeGraff, W.; Hahn, S.M.; McMurry, T.; Mitchell, J.B.; Russo, A. The catecholic metal sequestering agent 1,2-dihydroxybenzene-3,5-disulfonate confers protection against oxidative cell damage. Arch. Biochem. Biophys. 1992, 294, 98–106. [Google Scholar] [CrossRef]
- Hein, T.W.; Kuo, L. Ldls impair vasomotor function of the coronary microcirculation: Role of superoxide anions. Circ. Res. 1998, 83, 404–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dugas, A.J., Jr.; Castaneda-Acosta, J.; Bonin, G.C.; Price, K.L.; Fischer, N.H.; Winston, G.W. Evaluation of the total peroxyl radical-scavenging capacity of flavonoids: Structure-activity relationships. J. Nat. Prod. 2000, 63, 327–331. [Google Scholar] [CrossRef]
- Davies, M.J.; Forni, L.G.; Willson, R.L. Vitamin e analogue trolox c. E.S.R. And pulse-radiolysis studies of free-radical reactions. Biochem. J. 1988, 255, 513–522. [Google Scholar]
- Matsushita, T.; Fukuda, K.; Yamamoto, H.; Yamazaki, K.; Tomiyama, T.; Oh, M.; Hamanishi, C. Effect of ebselen, a scavenger of reactive oxygen species, on chondrocyte metabolism. Mod. Rheumatol. 2004, 14, 25–30. [Google Scholar] [CrossRef]
- Mugesh, G. Glutathione peroxidase activity of ebselen and its analogues: Some insights into the complex chemical mechanisms underlying the antioxidant activity. Curr. Chem. Biol. 2013, 7, 47–56. [Google Scholar] [CrossRef]
- Nakamura, Y.; Feng, Q.; Kumagai, T.; Torikai, K.; Ohigashi, H.; Osawa, T.; Noguchi, N.; Niki, E.; Uchida, K. Ebselen, a glutathione peroxidase mimetic seleno-organic compound, as a multifunctional antioxidant. Implication for inflammation-associated carcinogenesis. J. Biol. Chem. 2002, 277, 2687–2694. [Google Scholar] [CrossRef] [Green Version]
- Frei, B.; England, L.; Ames, B.N. Ascorbate is an outstanding antioxidant in human blood plasma. Proc. Natl. Acad. Sci. USA 1989, 86, 6377–6381. [Google Scholar] [CrossRef] [Green Version]
- Retsky, K.L.; Freeman, M.W.; Frei, B. Ascorbic acid oxidation product(s) protect human low density lipoprotein against atherogenic modification. Anti- rather than prooxidant activity of vitamin c in the presence of transition metal ions. J. Biol. Chem. 1993, 268, 1304–1309. [Google Scholar] [CrossRef]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta 2012, 1826, 443–457. [Google Scholar] [CrossRef] [Green Version]
- Padh, H. Vitamin c: Newer insights into its biochemical functions. Nutr. Rev. 1991, 49, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.J.; Johnson, D.; Jarvis, S.M. Vitamin c transport systems of mammalian cells. Mol. Membr. Biol. 2001, 18, 87–95. [Google Scholar] [CrossRef]
- Hollander-Czytko, H.; Grabowski, J.; Sandorf, I.; Weckermann, K.; Weiler, E.W. Tocopherol content and activities of tyrosine aminotransferase and cystine lyase in arabidopsis under stress conditions. J. Plant Physiol. 2005, 162, 767–770. [Google Scholar] [CrossRef]
- Ni, Y.; Eng, C. Vitamin e protects against lipid peroxidation and rescues tumorigenic phenotypes in cowden/cowden-like patient-derived lymphoblast cells with germline sdhx variants. Clin. Cancer Res. 2012, 18, 4954–4961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newaz, M.A.; Nawal, N.N. Effect of alpha-tocopherol on lipid peroxidation and total antioxidant status in spontaneously hypertensive rats. Am. J. Hypertens. 1998, 11, 1480–1485. [Google Scholar] [CrossRef] [Green Version]
- Smith, R.A.; Porteous, C.M.; Coulter, C.V.; Murphy, M.P. Selective targeting of an antioxidant to mitochondria. Eur. J. Biochem. 1999, 263, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, G.; Szyndralewiez, C.; Molango, S.; Carnesecchi, S.; Heitz, F.; Wiesel, P.; Wood, J.M. Therapeutic potential of nadph oxidase 1/4 inhibitors. Br. J. Pharm. 2017, 174, 1647–1669. [Google Scholar] [CrossRef] [Green Version]
- Altenhofer, S.; Radermacher, K.A.; Kleikers, P.W.; Wingler, K.; Schmidt, H.H. Evolution of nadph oxidase inhibitors: Selectivity and mechanisms for target engagement. Antioxid. Redox Signal. 2015, 23, 406–427. [Google Scholar] [CrossRef]
- Wind, S.; Beuerlein, K.; Eucker, T.; Muller, H.; Scheurer, P.; Armitage, M.E.; Ho, H.; Schmidt, H.H.; Wingler, K. Comparative pharmacology of chemically distinct nadph oxidase inhibitors. Br. J. Pharm. 2010, 161, 885–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trevelin, S.C.; Dos Santos, C.X.; Ferreira, R.G.; de Sa Lima, L.; Silva, R.L.; Scavone, C.; Curi, R.; Alves-Filho, J.C.; Cunha, T.M.; Roxo-Junior, P.; et al. Apocynin and nox2 regulate nf-kappab by modifying thioredoxin-1 redox-state. Sci. Rep. 2016, 6, 34581. [Google Scholar] [CrossRef] [Green Version]
- Heumuller, S.; Wind, S.; Barbosa-Sicard, E.; Schmidt, H.H.; Busse, R.; Schroder, K.; Brandes, R.P. Apocynin is not an inhibitor of vascular nadph oxidases but an antioxidant. Hypertension 2008, 51(2), 211–217. [Google Scholar] [CrossRef]
- Aldieri, E.; Riganti, C.; Polimeni, M.; Gazzano, E.; Lussiana, C.; Campia, I.; Ghigo, D. Classical inhibitors of nox nad(p)h oxidases are not specific. Curr. Drug Metab. 2008, 9, 686–696. [Google Scholar] [CrossRef]
- Mora-Pale, M.; Weiwer, M.; Yu, J.; Linhardt, R.J.; Dordick, J.S. Inhibition of human vascular nadph oxidase by apocynin derived oligophenols. Bioorgan. Med. Chem. 2009, 17, 5146–5152. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, B.V.; Tew, D.G.; Jones, O.T.; England, P.J. Studies on the inhibitory mechanism of iodonium compounds with special reference to neutrophil nadph oxidase. Biochem. J. 1993, 290 Pt 1, 41–49. [Google Scholar] [CrossRef]
- Geyer, O.; Podos, S.M.; Mittag, T. Nitric oxide synthase activity in tissues of the bovine eye. Graefe. Arch. Clin. Exp. Ophthalmol. Albrecht von Graefes Arch. Klin. Exp. Ophthalmol. 1997, 235, 786–793. [Google Scholar] [CrossRef]
- Stuehr, D.J.; Fasehun, O.A.; Kwon, N.S.; Gross, S.S.; Gonzalez, J.A.; Levi, R.; Nathan, C.F. Inhibition of macrophage and endothelial cell nitric oxide synthase by diphenyleneiodonium and its analogs. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1991, 5, 98–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tazzeo, T.; Worek, F.; Janssen, L. The nadph oxidase inhibitor diphenyleneiodonium is also a potent inhibitor of cholinesterases and the internal ca(2+) pump. Br. J. Pharm. 2009, 158, 790–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leusen, J.H.; Fluiter, K.; Hilarius, P.M.; Roos, D.; Verhoeven, A.J.; Bolscher, B.G. Interactions between the cytosolic components p47phox and p67phox of the human neutrophil nadph oxidase that are not required for activation in the cell-free system. J. Biol. Chem. 1995, 270, 11216–11221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ten Freyhaus, H.; Huntgeburth, M.; Wingler, K.; Schnitker, J.; Baumer, A.T.; Vantler, M.; Bekhite, M.M.; Wartenberg, M.; Sauer, H.; Rosenkranz, S. Novel nox inhibitor vas2870 attenuates pdgf-dependent smooth muscle cell chemotaxis, but not proliferation. Cardiovasc. Res. 2006, 71, 331–341. [Google Scholar] [CrossRef]
- Laleu, B.; Gaggini, F.; Orchard, M.; Fioraso-Cartier, L.; Cagnon, L.; Houngninou-Molango, S.; Gradia, A.; Duboux, G.; Merlot, C.; Heitz, F.; et al. First in class, potent, and orally bioavailable nadph oxidase isoform 4 (nox4) inhibitors for the treatment of idiopathic pulmonary fibrosis. J. Med. Chem. 2010, 53, 7715–7730. [Google Scholar] [CrossRef]
- Strengert, M.; Jennings, R.; Davanture, S.; Hayes, P.; Gabriel, G.; Knaus, U.G. Mucosal reactive oxygen species are required for antiviral response: Role of duox in influenza a virus infection. Antioxid. Redox Signal. 2014, 20, 2695–2709. [Google Scholar] [CrossRef]
- Sedeek, M.; Callera, G.; Montezano, A.; Gutsol, A.; Heitz, F.; Szyndralewiez, C.; Page, P.; Kennedy, C.R.; Burns, K.D.; Touyz, R.M.; et al. Critical role of nox4-based nadph oxidase in glucose-induced oxidative stress in the kidney: Implications in type 2 diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2010, 299, F1348–F1358. [Google Scholar] [CrossRef] [PubMed]
- Gaggini, F.; Laleu, B.; Orchard, M.; Fioraso-Cartier, L.; Cagnon, L.; Houngninou-Molango, S.; Gradia, A.; Duboux, G.; Merlot, C.; Heitz, F.; et al. Design, synthesis and biological activity of original pyrazolo-pyrido-diazepine, -pyrazine and -oxazine dione derivatives as novel dual nox4/nox1 inhibitors. Bioorgan. Med. Chem. 2011, 19, 6989–6999. [Google Scholar] [CrossRef] [PubMed]
- Aoyama, T.; Paik, Y.H.; Watanabe, S.; Laleu, B.; Gaggini, F.; Fioraso-Cartier, L.; Molango, S.; Heitz, F.; Merlot, C.; Szyndralewiez, C.; et al. Nicotinamide adenine dinucleotide phosphate oxidase in experimental liver fibrosis: Gkt137831 as a novel potential therapeutic agent. Hepatology 2012, 56, 2316–2327. [Google Scholar] [CrossRef] [Green Version]
- Schildknecht, S.; Weber, A.; Gerding, H.R.; Pape, R.; Robotta, M.; Drescher, M.; Marquardt, A.; Daiber, A.; Ferger, B.; Leist, M. The nox1/4 inhibitor gkt136901 as selective and direct scavenger of peroxynitrite. Curr. Med. Chem. 2014, 21, 365–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorin, Y.; Cavaglieri, R.C.; Khazim, K.; Lee, D.Y.; Bruno, F.; Thakur, S.; Fanti, P.; Szyndralewiez, C.; Barnes, J.L.; Block, K.; et al. Targeting nadph oxidase with a novel dual nox1/nox4 inhibitor attenuates renal pathology in type 1 diabetes. Am. J. Physiol. Ren. Physiol. 2015, 308, F1276–F1287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carnesecchi, S.; Deffert, C.; Pagano, A.; Garrido-Urbani, S.; Metrailler-Ruchonnet, I.; Schappi, M.; Donati, Y.; Matthay, M.A.; Krause, K.H.; Barazzone Argiroffo, C. Nadph oxidase-1 plays a crucial role in hyperoxia-induced acute lung injury in mice. Am. J. Respir. Crit. Care Med. 2009, 180, 972–981. [Google Scholar] [CrossRef]
- Green, D.E.; Murphy, T.C.; Kang, B.Y.; Kleinhenz, J.M.; Szyndralewiez, C.; Page, P.; Sutliff, R.L.; Hart, C.M. The nox4 inhibitor gkt137831 attenuates hypoxia-induced pulmonary vascular cell proliferation. Am. J. Respir. Cell Mol. Biol. 2012, 47, 718–726. [Google Scholar] [CrossRef] [Green Version]
- Bettaieb, A.; Jiang, J.X.; Sasaki, Y.; Chao, T.I.; Kiss, Z.; Chen, X.; Tian, J.; Katsuyama, M.; Yabe-Nishimura, C.; Xi, Y.; et al. Hepatocyte nicotinamide adenine dinucleotide phosphate reduced oxidase 4 regulates stress signaling, fibrosis, and insulin sensitivity during development of steatohepatitis in mice. Gastroenterology 2015, 149, 468–480. [Google Scholar] [CrossRef] [Green Version]
- Heinz, S.; Freyberger, A.; Lawrenz, B.; Schladt, L.; Schmuck, G.; Ellinger-Ziegelbauer, H. Mechanistic investigations of the mitochondrial complex i inhibitor rotenone in the context of pharmacological and safety evaluation. Sci. Rep. 2017, 7, 45465. [Google Scholar] [CrossRef] [Green Version]
- Stowe, D.F.; Camara, A.K. Mitochondrial reactive oxygen species production in excitable cells: Modulators of mitochondrial and cell function. Antioxid. Redox Signal. 2009, 11, 1373–1414. [Google Scholar] [CrossRef] [Green Version]
- Scialo, F.; Fernandez-Ayala, D.J.; Sanz, A. Role of mitochondrial reverse electron transport in ros signaling: Potential roles in health and disease. Front. Physiol. 2017, 8, 428. [Google Scholar] [CrossRef]
- Palmer, G.; Horgan, D.J.; Tisdale, H.; Singer, T.P.; Beinert, H. Studies on the respiratory chain-linked reduced nicotinamide adenine dinucleotide dehydrogenase. Xiv. Location of the sites of inhibition of rotenone, barbiturates, and piericidin by means of electron paramagnetic resonance spectroscopy. J. Biol. Chem. 1968, 243, 844–847. [Google Scholar] [CrossRef]
- Lambert, A.J.; Brand, M.D. Inhibitors of the quinone-binding site allow rapid superoxide production from mitochondrial nadh:Ubiquinone oxidoreductase (complex i). J. Biol. Chem. 2004, 279, 39414–39420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panov, A.; Dikalov, S.; Shalbuyeva, N.; Taylor, G.; Sherer, T.; Greenamyre, J.T. Rotenone model of parkinson disease: Multiple brain mitochondria dysfunctions after short term systemic rotenone intoxication. J. Biol. Chem. 2005, 280, 42026–42035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- St-Pierre, J.; Buckingham, J.A.; Roebuck, S.J.; Brand, M.D. Topology of superoxide production from different sites in the mitochondrial electron transport chain. J. Biol. Chem. 2002, 277, 44784–44790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bleier, L.; Drose, S. Superoxide generation by complex iii: From mechanistic rationales to functional consequences. Biochim. Biophys. Acta 2013, 1827, 1320–1331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.; Vazquez, E.J.; Moghaddas, S.; Hoppel, C.L.; Lesnefsky, E.J. Production of reactive oxygen species by mitochondria: Central role of complex iii. J. Biol. Chem. 2003, 278, 36027–36031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller, F.; Crofts, A.R.; Kramer, D.M. Multiple q-cycle bypass reactions at the qo site of the cytochrome bc1 complex. Biochemistry 2002, 41, 7866–7874. [Google Scholar] [CrossRef] [PubMed]
- Starkov, A.A.; Fiskum, G. Myxothiazol induces h(2)o(2) production from mitochondrial respiratory chain. Biochem. Biophys. Res. Commun. 2001, 281, 645–650. [Google Scholar] [CrossRef] [PubMed]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herb, M.; Schramm, M. Functions of ROS in Macrophages and Antimicrobial Immunity. Antioxidants 2021, 10, 313. https://doi.org/10.3390/antiox10020313
Herb M, Schramm M. Functions of ROS in Macrophages and Antimicrobial Immunity. Antioxidants. 2021; 10(2):313. https://doi.org/10.3390/antiox10020313
Chicago/Turabian StyleHerb, Marc, and Michael Schramm. 2021. "Functions of ROS in Macrophages and Antimicrobial Immunity" Antioxidants 10, no. 2: 313. https://doi.org/10.3390/antiox10020313
APA StyleHerb, M., & Schramm, M. (2021). Functions of ROS in Macrophages and Antimicrobial Immunity. Antioxidants, 10(2), 313. https://doi.org/10.3390/antiox10020313