GABA Regulates Phenolics Accumulation in Soybean Sprouts under NaCl Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Experimental Design
2.3. Sprout Length, Fresh Weight, and Dry Weight Determination
2.4. Phenolics Content Assay
2.4.1. Extraction of Free Phenolics
2.4.2. Extraction of Bound Phenolics
2.4.3. Determination of Total Phenolic Content
2.4.4. Determination of Free and Bound Phenolic Acid Content
2.4.5. Determination of Isoflavones Content
2.5. Determination of Key Enzymes Activity for Phenolics Synthesis
2.6. Gene Expression
2.7. Antioxidant Capacity Determination
2.7.1. DPPH Free-Radical Scavenging Ability
2.7.2. ABTS Free-Radical Scavenging Activity
2.8. Antioxidant Enzyme Activity
2.9. Data Processing and Statistical Analysis
3. Results
3.1. Effects of GABA on Length, Fresh Weight, and Dry Weight of Sprouts under NaCl Stress
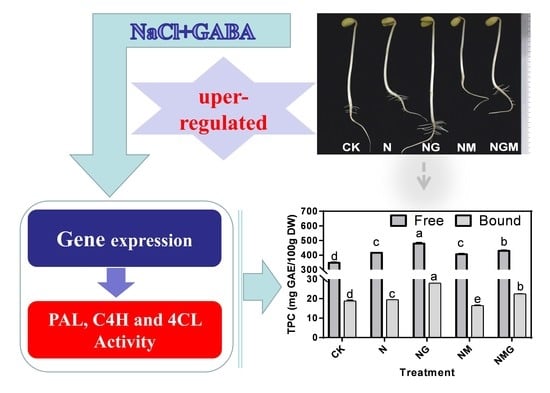
3.2. Effects of GABA on Phenolic Acid and Isoflavone Content in Soybean Sprouts under NaCl Stress
3.3. Effects of GABA on Key Enzymes Activity and mRNA Levels in Soybean Sprouts under NaCl Stress
3.4. Effects of GABA on Free-Radical Scavenging Ability and Antioxidant Enzyme Activity in Soybean Sprouts under NaCl Stress
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nwokolo, E. Soybean (Glycine max (L.) Merr.). In Food and Feed from Legumes and Oilseeds; Nwokolo, E., Smartt, J., Eds.; Springer: Boston, MA, USA, 1996; pp. 90–102. [Google Scholar]
- Duranti, M. Grain legume proteins and nutraceutical properties. Fitoterapia 2006, 77, 67–82. [Google Scholar] [CrossRef]
- Cantliffe, D.J.; Sung, Y.; Nascimento, W.M. Lettuce seed germination. In Horticultural Reviews; Jules, J., Ed.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 1999; pp. 229–275. [Google Scholar]
- Halmer, P.; Bewley, J.D.; Thorpe, T.A. An enzyme to degrade lettuce endosperm cell walls. Appearance of a mannanase following phytochrome- and gibberellin-induced germination. Planta 1976, 130, 189–196. [Google Scholar] [CrossRef]
- Kumari, S.; Chang, S.K.C. Effect of cooking on isoflavones, phenolic acids, and antioxidant activity in sprouts of prosoy soybean (Glycine max). J. Food Sci. 2016, 81, C1679–C1691. [Google Scholar] [CrossRef]
- Vidal-Valverde, C.; Frias, J.; Sierra, I.; Blázquez, I.; Lambein, F.; Kuo, Y.-H. New functional foods by germination: Effect on the nutritive value of beans, lentils and peas. Eur. Food Res. Technol. 2002, 215, 472–477. [Google Scholar] [CrossRef]
- Kulbat, K. The role of phenolic compounds in plant resistance. Biotechnol. Food Sci. 2016, 80, 97–108. [Google Scholar]
- Yang, R.; Guo, Q.; Gu, Z. GABA shunt and polyamine degradation pathway on gamma-aminobutyric acid accumulation in germinating fava bean (Vicia faba (L.)) under hypoxia. Food Chem. 2013, 136, 152–159. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, P.; Wang, M.; Sun, M.; Gu, Z.; Yang, R. GABA mediates phenolic compounds accumulation and the antioxidant system enhancement in germinated hulless barley under NaCl stress. Food Chem. 2019, 270, 593–601. [Google Scholar] [CrossRef]
- Beuve, N.; Rispail, N.; Laine, P.; Cliquet, J.-B.; Ourry, A.; Le Deunff, E. Putative role of γ -aminobutyric acid (GABA) as a long-distance signal in up-regulation of nitrate uptake in Brassica napus (L.). Plant Cell Environ. 2004, 27, 1035–1046. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Li, Z.; Yu, G. The dominant glutamic acid metabolic flux to produce γ-amino butyric acid over proline in nicotiana tabacum leaves under water stress relates to its significant role in antioxidant activity. J. Integr. Plant Biol. 2011, 53, 608–618. [Google Scholar] [CrossRef]
- Shi, S.Q.; Shi, Z.; Jiang, Z.P.; Li-Wang, Q.I.; Sun, X.M.; Chun-Xiu, L.I.; Liu, J.F.; Xiao, W.F.; Zhang, S.G. Effects of exogenous GABA on gene expression of Caragana intermedia roots under NaCl stress: Regulatory roles for H2O2 and ethylene production. Plant Cell Environ. 2010, 33, 149–162. [Google Scholar] [CrossRef]
- Ma, Y.; Wang, P.; Chen, Z.; Gu, Z.; Yang, R. NaCl stress on physio-biochemical metabolism and antioxidant capacity in germinated hulless barley (Hordeum vulgare (L.)). J. Sci. Food Agric. 2019, 99, 1755–1764. [Google Scholar] [CrossRef]
- Chen, Z.; Yu, L.; Wang, X.; Gu, Z.; Beta, T. Changes of phenolic profiles and antioxidant activity in canaryseed (Phalaris canariensis (L.)) during germination. Food Chem. 2016, 194, 608–618. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299C, 152–178. [Google Scholar]
- Jiao, C.; Yang, R.; Zhou, Y.; Gu, Z. Nitric oxide mediates isoflavone accumulation and the antioxidant system enhancement in soybean sprouts. Food Chem. 2016, 204, 373–380. [Google Scholar] [CrossRef]
- Assis, J.S.; Maldonado, R.; Munoz, T.; Escribano, M.; Merodio, C. Effect of high carbon dioxide concentration on PAL activity and phenolic contents in ripening cherimoya fruit. Postharvest Biol. Technol. 2001, 23, 33–39. [Google Scholar] [CrossRef] [Green Version]
- Lamb, C.J.; Rubery, P.H. A spectrophotometric assay for trans-cinnamic acid 4-hydroxylase activity. Anal. Biochem. 1975, 68, 554–561. [Google Scholar] [CrossRef]
- Han, C.; Li, J.; Jin, P.; Li, X.; Wang, L.; Zheng, Y. The effect of temperature on phenolic content in wounded carrots. Food Chem. 2017, 215, 116–123. [Google Scholar] [CrossRef]
- Chisari, M.; Barbagallo, R.N.; Spagna, G. Characterization and Role of Polyphenol Oxidase and Peroxidase in Browning of Fresh-Cut Melon. J. Agric. Food Chem. 2008, 56, 132–138. [Google Scholar] [CrossRef]
- Wang, Y.S.; Tian, S.P.; Yong, X. Effects of high oxygen concentration on pro- and anti-oxidant enzymes in peach fruits during postharvest periods. Food Chem. 2005, 91, 99–104. [Google Scholar] [CrossRef]
- Swigonska, S.; Amarowicz, R.; Król, A.; Mostek, A.; Badowiec, A.; Weidner, S. Influence of abiotic stress during soybean germination followed by recovery on the phenolic compounds of radicles and their antioxidant capacity. Acta Soc. Bot. Pol. 2014, 83, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Liu, J.; Wang, Y.; Abozeid, A.; Tang, Z.H. The different resistance of two astragalus plants to UV-B stress is tightly associated with the organ-specific isoflavone metabolism. Photochem. Photobiol. 2017, 94, 115–125. [Google Scholar] [CrossRef]
- Zhu, D.; Hettiarachchy, N.S.; Horax, R.; Chen, P. Isoflavone contents in germinated soybean seeds. Plant Foods Hum. Nutr. 2005, 60, 147–151. [Google Scholar] [CrossRef]
- Cekic, F. Exogenous GABA stimulates endogenous GABA and phenolic acid contents in tomato plants under salt stress. Celal Bayar Üniversitesi Fen Bilimleri Derg. 2018, 14, 61–64. [Google Scholar] [CrossRef] [Green Version]
- Cho, D.H.; Lim, S.T. Changes in phenolic acid composition and associate enzyme activity in shoot and kernel fractions of brown rice during germination. Food Chem. 2018, 256, 163–170. [Google Scholar] [CrossRef]
- Ma, D.; Li, Y.; Zhang, J.; Chenyang, W.; Qin, H.; Ding, H.; Xie, Y.; Guo, T. Accumulation of phenolic compounds and expression profiles of phenolic acid biosynthesis-related genes in developing grains of white, purple, and red wheat. Front. Plant Sci. 2016, 7, 528. [Google Scholar] [CrossRef]
- Gharibi, S.; Saeidi, G.; Goli, S.A.H. Effect of drought stress on total phenolic, lipid peroxidation, and antioxidant activity of achillea species. Appl. Biochem. Biotechnol. 2016, 178, 796–809. [Google Scholar] [CrossRef]
- Rezaei-Chiyaneh, E.; Seyyedi, S.M.; Ebrahimian, E.; Moghaddam, S.S.; Damalas, C.A. Exogenous application of gamma-aminobutyric acid (GABA) alleviates the effect of water deficit stress in black cumin (Nigella sativa (L.)). Ind. Crop. Prod. 2018, 112, 741–748. [Google Scholar] [CrossRef]



| Gene | Primer Name | Primer Sequences (5’→3’) |
|---|---|---|
| PAL | Sense | CTACCATCACCAATGGGAGCC |
| Ant-sense | CTCCCCAGTTTAACGGATCACT | |
| C4H | Sense | TGGCCTGCTAATGGGTATTGT |
| Ant-sense | ACACAAATACTGGCTCTGCT | |
| 4CL | Sense | AGTCGCAGCCTTTCCATCAA |
| Ant-sense | GACGATGTAGCGGATGTCGT | |
| EF1b | Sense | CCACTGCTGAAGAAGATGATGATG |
| Ant-sense | AAGGACAGAAGACTTGCCACTC |
| Phenolic acid | Treatment | Content (µg/g DW) | ||
|---|---|---|---|---|
| Free | Bound | Total | ||
| p-hydroxybenzoic acid | CK | 17.46 ± 0.13 d | 3.06 ± 0.28 b | 20.52 ± 0.21 d |
| N | 28.23 ± 0.45 b | 3.41 ± 0.14 b | 31.64 ± 0.31 b | |
| NG | 37.15 ± 4.67 a | 4.67 ± 0.17 a | 41.82 ± 4.84 a | |
| NM | 21.31 ± 1.01 cd | 1.73 ± 0.33 d | 23.04 ± 1.34 c | |
| NMG | 26.43 ± 0.11 bc | 2.50 ± 0.27 c | 28.93 ± 0.16 b | |
| Vanillic acid | CK | 70.74 ± 0.05 d | 55.59 ± 0.64 b | 126.33 ± 0.67 d |
| N | 129.08 ± 0.15 b | 62.77 ± 2.18 a | 191.84 ± 2.03 ab | |
| NG | 149.21 ± 19.13 a | 66.03 ± 2.08 a | 215.24 ± 21.21 a | |
| NM | 90.32 ± 0.27 c | 23.28 ± 4.68 d | 113.60 ± 4.94 c | |
| NMG | 99.25 ± 0.93 c | 42.27 ± 0.17 c | 141.52 ± 1.11 b | |
| Syringic acid | CK | 241.59 ± 3.31 c | 223.09 ± 7.93 c | 464.68 ± 11.33 d |
| N | 265.03 ± 0.68 b | 295.37 ± 5.52 b | 560.40 ± 4.84 c | |
| NG | 379.63 ± 0.81 a | 337.20 ± 0.44 a | 716.84 ± 1.25 a | |
| NM | 199.06 ± 1.31 d | 106.79 ± 1.48 d | 305.85 ± 0.17 e | |
| NMG | 386.42 ± 10.73 a | 224.66 ± 5.35 c | 611.08 ± 10.38 b | |
| p-coumaric acid | CK | 791.14 ± 2.04 c | 117.75 ± 1.14 c | 908.89 ± 2.27 c |
| N | 1043.82 ± 19.17 b | 134.85 ± 4.80 b | 1178.68 ± 14.37 b | |
| NG | 1253.58 ± 4.12 a | 160.73 ± 0.14 a | 1414.31 ± 4.26 a | |
| NM | 726.63 ± 72.17 c | 83.77 ± 0.60 d | 810.40 ± 71.57 d | |
| NMG | 893.92 ± 16.75 bc | 169.08 ± 6.21 a | 1063 ± 16 bc | |
| Ferulic acid | CK | 197.89 ± 4.21 b | 18.01 ± 1.13 c | 215.90 ± 4.54 b |
| N | 267.82 ± 0.32 a | 23.01 ± 2.29 b | 290.83 ± 1.97 ab | |
| NG | 283.14 ± 0.90 a | 27.22 ± 1.04 a | 310.35 ± 0.14 a | |
| NM | 181.80 ± 17.39 b | 14.13 ± 1.15 d | 195.93 ± 18.54 c | |
| NMG | 271.67 ± 8.20 a | 20.44 ± 0.40 bc | 292.10 ± 8.60 ab | |
| Erucic acid | CK | 45.81 ± 0.41 c | 12.55 ± 0.78 b | 58.36 ± 1.07 d |
| N | 73.91 ± 5.31 b | 13.18 ± 1.18 a | 87.09 ± 4.13 b | |
| NG | 153.91 ± 0.91 a | 13.89 ± 0.38 a | 167.80 ± 0.53 a | |
| NM | 39.28 ± 0.35 c | 4.50 ± 0.26 d | 43.78 ± 0.62 e | |
| NMG | 71.85 ± 2.40 b | 7.31 ± 0.28 c | 79.16 ± 2.12 c | |
| Total | CK | 1364.63 ± 5.83 c | 430.05 ± 9.06 c | 1794.69 ± 14.89 c |
| N | 1807.89 ± 14.82 b | 532.60 ± 9.16 b | 2340.49 ± 5.66 b | |
| NG | 2256.62 ± 20.69 a | 609.74 ± 0.24 a | 2866.36 ± 20.94 a | |
| NM | 1258.40 ± 92.51 c | 234.20 ± 4.34 d | 1492.59 ± 96.85 d | |
| NMG | 1749.54 ± 136.64 b | 465.81 ± 12.74 c | 2215.35 ± 149.38 b | |
| Isoflavone (µg/g DW) | Treatment | ||||
|---|---|---|---|---|---|
| CK | N | NG | NM | NMG | |
| Daidzin | 481.44 ± 2.41 a | 430.85 ± 38.65 bc | 415.46 ± 2.39 c | 407.32 ± 11.47 c | 473.09 ± 11.17 ab |
| Glycitin | 27.33 ± 5.83 a | 26.72 ± 1.94 a | 33.32 ± 0.26 a | 29.50 ± 0.48 a | 31.48 ± 1.21 a |
| Genistin | 282.31 ± 5.54 bc | 284.67 ± 4.75 b | 294.21 ± 1.53 a | 265.90 ± 14.47 c | 311.22 ± 3.18 a |
| Malonyldaidzin | 2635.59 ± 17.69 a | 2620.03 ± 57.84 a | 2706.77 ± 25.32 a | 2207.63 ± 0.33 b | 2715.73 ± 148.84 a |
| Malonylglycitin | 322.59 ± 13.17 a | 268.01 ± 9.89 b | 262.00 ± 4.34 b | 231.27 ± 4.13 c | 273.04 ± 11.21 b |
| Malonylgenistin | 1681.32 ± 44.13 c | 2670.88 ± 44.91 b | 2860.55 ± 0.88 a | 2858.78 ± 48.41 a | 2717.28 ± 17.80 a |
| Daidzein | 61.35 ± 0.13 c | 105.01 ± 6.66 a | 78.15 ± 0.26 b | 77.56 ± 0.82 b | 111.98 ± 4.22 a |
| Genistein | ND | 40.92 ± 0.63 b | 38.10 ± 0.09 c | 36.81 ± 0.37 c | 42.65 ± 0.91 a |
| Total | 5436.92 ± 76.99 d | 6447.08 ± 161.30 b | 6689.56 ± 34.88 a | 6114.75 ± 17.30 c | 6676.46 ± 151.26 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Xie, C.; Wang, P.; Gu, Z.; Yang, R. GABA Regulates Phenolics Accumulation in Soybean Sprouts under NaCl Stress. Antioxidants 2021, 10, 990. https://doi.org/10.3390/antiox10060990
Zhao Y, Xie C, Wang P, Gu Z, Yang R. GABA Regulates Phenolics Accumulation in Soybean Sprouts under NaCl Stress. Antioxidants. 2021; 10(6):990. https://doi.org/10.3390/antiox10060990
Chicago/Turabian StyleZhao, Yunyun, Chong Xie, Pei Wang, Zhenxin Gu, and Runqiang Yang. 2021. "GABA Regulates Phenolics Accumulation in Soybean Sprouts under NaCl Stress" Antioxidants 10, no. 6: 990. https://doi.org/10.3390/antiox10060990
APA StyleZhao, Y., Xie, C., Wang, P., Gu, Z., & Yang, R. (2021). GABA Regulates Phenolics Accumulation in Soybean Sprouts under NaCl Stress. Antioxidants, 10(6), 990. https://doi.org/10.3390/antiox10060990