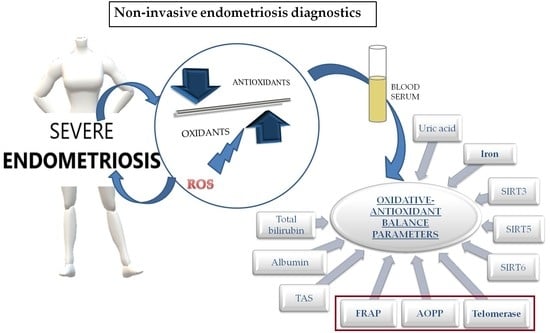
Is There a Balance in Oxidative-Antioxidant Status in Blood Serum of Patients with Advanced Endometriosis?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Total Antioxidant Capacity Concentration
2.3. Low-Molecular-Weight Antioxidants and Iron Measurements
2.4. Sirtuins and Telomerase Concentrations
2.5. Oxidative Protein Damage Measurements
2.6. Statistical Analysis
3. Results
3.1. The Concentrations of Total Antioxidant Capacity
3.2. The Concentrations of Low-Molecular-Weight Antioxidants and Iron
3.3. The Concentrations of Sirtuins and Telomerase
3.4. The Concentrations of Advanced Protein Oxidation Products
3.5. ROC Curve Analysis
4. Discussion
Strengths and Limitations
- To our knowledge, the concentrations of serum SIRT3, SIRT5, SIRT6 and telomerase in patients with endometriosis were analyzed for the first time.
- The three blood serum oxidative stress markers selected by us, FRAP, telomerase and AOPP, seem to be the most promising parameters for advanced endometriosis detection.
- The analysis of 11 oxidative stress biomarkers’ expression and the determination of their importance in endometriosis are key to understanding the mechanisms involved in the development of the disease.
- The results obtained for the endometriosis group were compared to those determined for healthy women who were our control group, which is more appropriate from the point of view of drawing constructive conclusions than comparing to a group of patients without endometriosis but suffering from other gynecological diseases.
- Serum oxidative stress biomarkers may reflect an oxidative state that may be caused by diseases other than endometriosis.
- Some parameters of oxidative stress are not stable and may fluctuate under the influence of many factors, including hormones and the day of the menstrual cycle.
- Limitations are also due to the relatively small group size and the collection of venous blood samples regardless of the day of the menstrual cycle.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Giudice, L.C.; Kao, L.C. Endometriosis. Lancet 2004, 364, 1789–1799. [Google Scholar] [CrossRef]
- Scutiero, G.; Iannone, P.; Bernardi, G.; Bonaccorsi, G.; Spadaro, S.; Volta, C.A.; Greco, P.; Nappi, L. Oxidative stress and endometriosis: A systematic review of the literature. Oxid. Med. Cell. Longev. 2017, 2017, 7265238. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Agarwal, A.; Krajcir, N.; Alvarez, J.G. Role of oxidative stress in endometriosis. Reprod. Biomed. Online 2006, 13, 126–134. [Google Scholar] [CrossRef]
- Van Langendonckt, A.; Casanas-Roux, F.; Donnez, J. Oxidative stress and peritoneal endometriosis. Fertil. Steril. 2002, 77, 861–870. [Google Scholar] [CrossRef]
- Jackson, L.W.; Schisterman, E.F.; Dey-Rao, R.; Browne, R.; Armstrong, D. Oxidative stress and endometriosis. Hum. Reprod. 2005, 20, 2014–2020. [Google Scholar] [CrossRef] [Green Version]
- Kelton, J.G.; Ulan, R.; Stiller, C.; Holmes, E. Comparison of chemical composition of peritoneal fluid and serum. A method for monitoring dialysis patients and a tool for assessing binding to serum proteins in vivo. Ann. Intern. Med. 1978, 89, 67–70. [Google Scholar] [CrossRef]
- Bokor, A.; Debrock, S.; Drijkoningen, M.; Goossens, W.; Fülöp, V.; D’Hooghe, T. Quantity and quality of retrograde menstruation: A case control study. Reprod. Biol. Endocrinol. 2009, 7, 123. [Google Scholar] [CrossRef] [Green Version]
- Musante, L.; Bruschi, M.; Candiano, G.; Petretto, A.; Dimasi, N.; del Boccio, P.; Urbani, A.; Rialdi, G.; Ghiggeri, G.M. Characterization of oxidation end product of plasma albumin “in vivo”. Biochem. Biophys. Res. Commun. 2006, 349, 668–673. [Google Scholar] [CrossRef]
- Belinskaia, D.A.; Voronina, P.A.; Shmurak, V.I.; Vovk, M.A.; Batalova, A.A.; Jenkins, R.O.; Goncharov, N.V. The universal soldier: Enzymatic and non-enzymatic antioxidant functions of serum albumin. Antioxidants 2020, 9, 966. [Google Scholar] [CrossRef] [PubMed]
- Ghiselli, A.; Serafini, M.; Natella, F.; Scaccini, C. Total antioxidant capacity as a tool to assess redox status: Critical view and experimental data. Free Radic. Biol. Med. 2000, 29, 1106–1114. [Google Scholar] [CrossRef]
- Young, I.S.; Woodside, J.V. Antioxidants in health and disease. J. Clin. Pathol. 2001, 54, 176–186. [Google Scholar] [CrossRef] [Green Version]
- Rubio, C.P.; Hernández-Ruiz, J.; Martinez-Subiela, S.; Tvarijonaviciute, A.; Ceron, J.J. Spectrophotometric assays for total antioxidant capacity (TAC) in dog serum: An update. BMC Vet. Res. 2016, 12, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ialongo, C. Preanalytic of total antioxidant capacity assays performed in serum, plasma, urine and saliva. Clin. Biochem. 2017, 50, 356–363. [Google Scholar] [CrossRef]
- Grune, T.; Schröder, P.; Biesalski, H. Low molecular weight antioxidants. In Reactions, Processes. The Handbook of Environmental Chemistry; Springer: Berlin/Heidelberg, Germany, 2005; Volume 2, pp. 77–90. [Google Scholar]
- Oettl, K.; Stauber, R.E. Physiological and pathological changes in the redox state of human serum albumin critically influence its binding properties. Br. J. Pharmacol. 2007, 151, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Ekarattanawong, S.; Tanprasertkul, C.; Somprasit, C.; Chamod, P.; Tiengtip, R.; Bhamarapravatana, K.; Suwannarurk, K. Possibility of using superoxide dismutase and glutathione peroxidase as endometriosis biomarkers. Int. J. Womens Health 2017, 9, 711–716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alizadeh, M.; Mahjoub, S.; Esmaelzadeh, S.; Hajian, K.; Basirat, Z.; Ghasemi, M. Evaluation of oxidative stress in endometriosis: A case-control study. Casp. J. Intern. Med. 2015, 6, 25–29. [Google Scholar]
- Carvalho, L.F.P.; Samadder, A.N.; Agarwal, A.; Fernandes, L.F.C.; Abrão, M.S. Oxidative stress biomarkers in patients with endometriosis: Systematic review. Arch. Gynecol. Obstet. 2012, 286, 1033–1040. [Google Scholar] [CrossRef]
- Donnez, J.; Binda, M.M.; Donnez, O.; Dolmans, M.M. Oxidative stress in the pelvic cavity and its role in the pathogenesis of endometriosis. Fertil. Steril. 2016, 106, 1011–1017. [Google Scholar] [CrossRef] [Green Version]
- Halliwell, B.; Gutteridge, J.M.C. The definition and measurement of antioxidants in biological systems. Free Radic. Biol. Med. 1995, 18, 125–126. [Google Scholar] [CrossRef]
- Kratz, E.M.; Sołkiewicz, K.; Kubis-Kubiak, A.; Piwowar, A. Sirtuins as important factors in pathological states and the role of their molecular activity modulators. Int. J. Mol. Sci. 2021, 22, 630. [Google Scholar] [CrossRef]
- Kratz, E.M.; Kokot, I.; Dymicka-Piekarska, V.; Piwowar, A. Sirtuins—The new important players in women’s gynecological health. Antioxidants 2021, 10, 84. [Google Scholar] [CrossRef]
- Kratz, E.M.; Sołkiewicz, K.; Kaczmarek, A.; Piwowar, A. Sirtuins: Enzymes with multidirectional catalytic activity. Postepy Hig. Med. Dosw. 2021, 75, 152–174. [Google Scholar] [CrossRef]
- Santos, L.; Escande, C.; Denicola, A. Potential modulation of sirtuins by oxidative stress. Oxid. Med. Cell. Longev. 2016, 2016, 9831825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michan, S.; Sinclair, D. Sirtuins in mammals: Insights into their biological function. Biochem. J. 2007, 404, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Lombard, D.B.; Zwaans, B.M.M. SIRT3: As simple as it seems? Gerontology 2013, 60, 56–64. [Google Scholar] [CrossRef] [Green Version]
- González-Fernández, R.; Martín-Ramírez, R.; Rotoli, D.; Hernández, J.; Naftolin, F.; Martín-Vasallo, P.; Palumbo, A.; Ávila, J. Granulosa-lutein cell sirtuin gene expression profiles differ between normal donors and infertile women. Int. J. Mol. Sci. 2020, 21, 295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valentijn, A.J.; Saretzki, G.; Tempest, N.; Critchley, H.O.D.; Hapangama, D.K. Human endometrial epithelial telomerase is important for epithelial proliferation and glandular formation with potential implications in endometriosis. Hum. Reprod. 2015, 30, 2816–2828. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Liu, J.; Qiu, Z.; Chen, D.; Luo, C.; Liu, X.; Hua, R.; Zhu, X.; Lin, Y.; Li, L.; et al. Advanced oxidation protein products from the follicular microenvironment and their role in infertile women with endometriosis. Exp. Ther. Med. 2018, 15, 479–486. [Google Scholar] [CrossRef]
- Colombo, G.; Clerici, M.; Giustarini, D.; Portinaro, N.; Badalamenti, S.; Rossi, R.; Milzani, A.; Dalle-Donne, I. A central role for intermolecular dityrosine cross-linking of fibrinogen in high molecular weight advanced oxidation protein product (AOPP) formation. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 1–12. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Gausson, V.; Nguyen, A.T.; Touam, M.; Drüeke, T.; Santangelo, F.; Descamps-Latscha, B. AOPP-induced activation of human neutrophil and monocyte oxidative metabolism: A potential target for N-acetylcysteine treatment in dialysis patients. Kidney Int. 2003, 64, 82–91. [Google Scholar] [CrossRef] [Green Version]
- Kokot, I.; Piwowar, A.; Jędryka, M.; Sołkiewicz, K.; Kratz, E.M. Diagnostic significance of selected serum inflammatory markers in women with advanced endometriosis. Int. J. Mol. Sci. 2021, 22, 2295. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Witko-Sarsat, V.V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef] [Green Version]
- Bossuyt, X. Clinical performance characteristics of a laboratory test. A practical approach in the autoimmune laboratory. Autoimmun. Rev. 2009, 8, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, E.M.; Giorgi, V.S.I.; Rodrigues, J.K.; de Andrade, A.Z.; Junior, A.A.J.; Navarro, P.A. Systemic oxidative stress as a possible mechanism underlying the pathogenesis of mild endometriosis-related infertility. Reprod. Biomed. Online 2019, 39, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Turgut, A.; Özler, A.; Görük, N.Y.; Tunç, S.Y.; Evliyaoǧlu, O.; Gül, T. Copper, ceruloplasmin and oxidative stress in patients with advanced-stage endometriosis. Eur. Rev. Med. Pharmacol. Sci. 2013, 17, 1472–1478. [Google Scholar]
- Jana, S.K.; Dutta, M.; Joshi, M.; Srivastava, S.; Chakravarty, B.; Chaudhury, K. 1H NMR based targeted metabolite profiling for understanding the complex relationship connecting oxidative stress with endometriosis. Biomed Res. Int. 2013, 2013. [Google Scholar] [CrossRef]
- Said, T.M.; Kattal, N.; Sharma, R.K.; Sikka, S.C.; Thomas, A.J.; Mascha, E.; Agarwal, A. Enhanced chemiluminescence assay vs colorimetric assay for measurement of the total antioxidant capacity of human seminal plasma. J. Androl. 2003, 24, 676–680. [Google Scholar] [CrossRef]
- Jansen, E.H.; Ruskovska, T. Comparative analysis of serum (anti)oxidative status. Parameters in healthy persons. Int. J. Mol. Sci. 2013, 14, 6106–6115. [Google Scholar] [CrossRef] [Green Version]
- Cao, G.; Prior, R.L. Comparison of different analytical methods for assessing total antioxidant capacity of human serum. Clin. Chem. 1998, 44, 1309–1315. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Srinivasa Rao, P.V.L.N.; Kiranmayi, V.S.; Swathi, P.; Jeyseelan, L.; Suchitra, M.M.; Bitla, A.R. Comparison of two analytical methods used for the measurement of total antioxidant status. J. Antioxid. Act. 2015, 1, 22–28. [Google Scholar] [CrossRef]
- Roche, M.; Rondeau, P.; Singh, N.R.; Tarnus, E.; Bourdon, E. The antioxidant properties of serum albumin. FEBS Lett. 2008, 582, 1783–1787. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.; Rahim, A.; Afzal, M.; Naveed, A.K.; Ayub, S.; Jahan, S. Serum albumin and C3 complement levels in endometriosis. J. Coll. Physicians Surg. Pakistan 2019, 29, 702–705. [Google Scholar] [CrossRef]
- Miller, N.J.; Rice-Evans, C.; Davies, M.J.; Gopinathan, V.; Milner, A. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin. Sci. 1993, 84, 407–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, A.M.N.; Hider, R.C. Influence of non-enzymatic post-translation modifications on the ability of human serum albumin to bind iron. Implications for non-transferrin-bound iron speciation. Biochim. Biophys. Acta Proteins Proteom. 2009, 1794, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Gutteridge, J.M.C. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 1984, 219, 1–14. [Google Scholar] [CrossRef]
- Chmaj-Wierzchowska, K.; Kampioni, M.; Wilczak, M.; Opala, T. Do inflammatory factors play a significant role in etiopathogenesis of endometrial cysts? Part 1. Ann. Agric. Environ. Med. 2013, 20, 854–858. [Google Scholar] [PubMed]
- Tatone, C.; di Emidio, G.; Barbonetti, A.; Carta, G.; Luciano, A.M.; Falone, S.; Amicarelli, F.; Di Emidio, G.; Barbonetti, A.; Carta, G.; et al. Sirtuins in gamete biology and reproductive physiology: Emerging roles and therapeutic potential in female and male infertility. Hum. Reprod. Update 2018, 24, 267–289. [Google Scholar] [CrossRef]
- Shi, T.; Wang, F.; Stieren, E.; Tong, Q. SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J. Biol. Chem. 2005, 280, 13560–13567. [Google Scholar] [CrossRef] [Green Version]
- Cervellati, C.; Bergamini, C.M. Oxidative damage and the pathogenesis of menopause related disturbances and diseases. Clin. Chem. Lab. Med. 2016, 54, 739–753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santulli, P.; Chouzenoux, S.; Fiorese, M.; Marcellin, L.; Lemarechal, H.; Millischer, A.-E.E.; Dé Ric Batteux, F.; Borderie, D.; Chapron, C.; Batteux, F.; et al. Protein oxidative stress markers in peritoneal fluids of women with deep infiltrating endometriosis are increased. Hum. Reprod. 2015, 30, 49–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, T.; Su, Z.; Li, Y.; Zhang, X.; You, Q. Chitinase-3 like-protein-1 function and its role in diseases. Signal Transduct. Target. Ther. 2020, 5, 201. [Google Scholar] [CrossRef] [PubMed]
- Bozkus, F.; Guler, S.; Sımsek, S. Serum telomerase levels and COPD exacerbations. Respir. Care 2016, 61, 359–365. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.Q.; Bonnefoi, H.; Pelte, M.F.; Lyautey, J.; Lederrey, C.; Movarekhi, S.; Schaeffer, P.; Mulcahy, H.E.; Meyer, P.; Stroun, M.; et al. Telomerase RNA as a detection marker in the serum of breast cancer patients. Clin. Cancer Res. 2000, 6, 3823–3826. [Google Scholar]
- Varma, R.; Rollason, T.; Gupta, J.K.; Maher, E.R. Endometriosis and the neoplastic process. Reproduction 2004, 127, 293–304. [Google Scholar] [CrossRef] [Green Version]
- Zafrakas, M.; Tarlatzis, B.C.; Streichert, T.; Pournaropoulos, F.; Wölfle, U.; Smeets, S.J.; Wittek, B.; Grimbizis, G.; Brakenhoff, R.H.; Pantel, K.; et al. Genome-wide microarray gene expression, array-CGH analysis, and telomerase activity in advanced ovarian endometriosis: A high degree of differentiation rather than malignant potential. Int. J. Mol. Med. 2008, 21, 335–344. [Google Scholar] [CrossRef]
- Sofiyeva, N.; Ekizoglu, S.; Gezer, A.; Yilmaz, H.; Kolomuc Gayretli, T.; Buyru, N.; Oral, E. Does telomerase activity have an effect on infertility in patients with endometriosis? Eur. J. Obstet. Gynecol. Reprod. Biol. 2017, 213, 116–122. [Google Scholar] [CrossRef]
- Liu, J.; Wen, S.; Lin, Y.; Yang, X.; Liu, Z.; Quan, S.; Song, Y. Advanced oxidation protein products change biological behaviors of rat endometrial epithelial cells by activating ERK/P38 signaling pathways. Biol. Open 2020, 9, bio048876. [Google Scholar] [CrossRef] [PubMed]
- Amreen, S.; Kumar, P.; Gupta, P.; Rao, P. Evaluation of oxidative stress and severity of endometriosis. J. Hum. Reprod. Sci. 2019, 12, 40–46. [Google Scholar] [CrossRef] [PubMed]



| E n = 43 | NE n = 35 | Control n = 18 | |
|---|---|---|---|
| Median (IQR) | Median (IQR) | Median (IQR) | |
| BMI [kg/m2] | 24 (22–26) | 26 (24–28) | 24 (21–26) |
| Age [years] | 34 (30–41) | 40 (33–42) | 39 (35–41) |
| Reason for surgery [n, %] | Moderate stage (III E) 20, 46.5% Severe stage (IV E) 23, 53.5% | Leiomyoma 13, 37.0% Benign ovarian cyst 3, 8.5% Cervical intraepithelial neoplasia grade 3 12, 34.0% Ovarian teratoma 3, 8.5% Uterine polyp 1, 3.0% Borderline cystadenoma mucinosum 1, 3.0% BRCA1 mutation 2, 6.0% | Not applicable |
| E n = 43 | III E n = 20 | IV E n = 23 | NE n = 35 | Control n = 18 | |
|---|---|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |
| TAC | |||||
| TAS [mmol/L] | 1.52 ± 0.23 | 1.53 ± 0.19 | 1.52 ± 0.27 | 1.61 ± 0.25 | 1.60 ± 0.22 |
| FRAP [mmol/L] | 1.08 ± 0.22 p = 0.015 * | 1.09 ± 0.27 | 1.07 ± 0.18 p = 0.017 * | 1.14 ± 0.28 p = 0.017 * | 0.95 ± 0.22 |
| Low-molecular-weight antioxidants | |||||
| ALBUMIN [g/dL] | 4.45 ± 0.66 | 4.68 ± 0.66 | 4.25 ± 0.60 | 4.29 ± 0.49 | 4.26 ± 0.19 |
| T-BIL [mg/dL] | 0.56 ± 0.33 | 0.54 ± 0.24 | 0.58 ± 0.40 | 0.68 ± 0.41 | 0.59 ± 0.24 |
| URIC ACID [mg/dL] | 4.78 ± 0.91 | 4.86 ± 1.13 | 4.72 ± 0.68 | 4.75 ± 1.40 | 4.72 ± 0.95 |
| Iron | |||||
| IRON [μg/dL] | 101.74 ± 62.80 | 122.70 ± 63.17 | 83.52 ± 57.76 p = 0.025 * p = 0.013 ** | 111.91 ± 76.12 | 105.89 ± 38.24 |
| SIRTs and TE | |||||
| SIRT3 [ng/mL] | 12.09 ± 7.06 | 12.30 ± 6.84 | 11.94 ± 7.40 | 11.00 ± 6.23 | 10.74 ± 6.11 |
| SIRT5 [ng/mL] | 8.09 ± 4.38 | 8.39 ± 4.34 | 7.83 ± 4.54 | 9.46 ± 5.45 | 7.98 ± 5.98 |
| SIRT6 [ng/mL] | 12.32 ± 16.84 | 8.51 ± 15.90 | 14.76 ± 17.71 | 14.54 ± 17.66 | 7.17 ± 10.40 |
| TE [ng/mL] | 1.61 ± 1.88 p = 0.004 * | 1.01 ± 0.60 p = 0.021 * | 2.00 ± 2.32 p = 0.017 * | 1.54 ± 2.75 | 0.43 ± 0.52 |
| AOPP | |||||
| AOPP [µmol/L] | 235.09 ± 143.11 p < 0.001 * | 225.63 ± 156.64 p = 0.005 * | 242.55 ± 135.40 p < 0.001 * | 196.27 ± 180.94 p = 0.028 * | 105.16 ± 49.24 |
| Parameter | TAS | FRAP | ALB | T-BIL | UA | IRON | SIRT3 | SIRT5 | SIRT6 | TE |
|---|---|---|---|---|---|---|---|---|---|---|
| FRAP | r = 0.576 p < 0.001 | |||||||||
| ALB | r = −0.180 p = 0.038 | r = −0.339 p < 0.001 | ||||||||
| T-BIL | NS | NS | r = 0.393 p < 0.001 | |||||||
| UA | r = 0.264 p = 0.002 | r = 0.590 p < 0.001 | NS | NS | ||||||
| IRON | NS | NS | r = 0.344 p < 0.001 | r = 0.405 p < 0.001 | r = 0.176 p = 0.039 | |||||
| SIRT3 | NS | r = −0.305 p < 0.001 | r = 0.298 p < 0.001 | NS | NS | r = 0.184 p = 0.043 | ||||
| SIRT5 | NS | r = −0.229 p = 0.019 | NS | NS | r = −0.199 p = 0.041 | r = 0.192 p = 0.050 | r = 0.845 p < 0.001 | |||
| SIRT6 | NS | NS | NS | NS | r = −0.233 p = 0.042 | NS | r = 0.770 p < 0.001 | r = 0.863 p < 0.001 | ||
| TE | NS | r = 0.431 p < 0.001 | r = −0.323 p = 0.004 | NS | r = 0.356 p = 0.001 | NS | r = −0.227 p = 0.049 | NS | NS | |
| AOPP | r = −0.211 p = 0.026 | NS | NS | NS | r = 0.378 p < 0.001 | r = 0.235 p = 0.012 | NS | NS | NS | r = 0.363 p = 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kokot, I.; Piwowar, A.; Jędryka, M.; Kratz, E.M. Is There a Balance in Oxidative-Antioxidant Status in Blood Serum of Patients with Advanced Endometriosis? Antioxidants 2021, 10, 1097. https://doi.org/10.3390/antiox10071097
Kokot I, Piwowar A, Jędryka M, Kratz EM. Is There a Balance in Oxidative-Antioxidant Status in Blood Serum of Patients with Advanced Endometriosis? Antioxidants. 2021; 10(7):1097. https://doi.org/10.3390/antiox10071097
Chicago/Turabian StyleKokot, Izabela, Agnieszka Piwowar, Marcin Jędryka, and Ewa Maria Kratz. 2021. "Is There a Balance in Oxidative-Antioxidant Status in Blood Serum of Patients with Advanced Endometriosis?" Antioxidants 10, no. 7: 1097. https://doi.org/10.3390/antiox10071097
APA StyleKokot, I., Piwowar, A., Jędryka, M., & Kratz, E. M. (2021). Is There a Balance in Oxidative-Antioxidant Status in Blood Serum of Patients with Advanced Endometriosis? Antioxidants, 10(7), 1097. https://doi.org/10.3390/antiox10071097