Effects of a 12-Month Treatment with Glucagon-like Peptide-1 Receptor Agonists, Sodium-Glucose Cotransporter-2 Inhibitors, and Their Combination on Oxidant and Antioxidant Biomarkers in Patients with Type 2 Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Laboratory Assays
2.2.1. TBARS
2.2.2. MDA
2.2.3. ABTS
2.2.4. TAC
2.2.5. RP
2.3. Statistical Analysis
3. Results
3.1. Glycemic Control after Intervention
3.2. TBARS
3.3. MDA
3.4. ABTS
3.5. TAC
3.6. RP
3.7. Association of Glycemic Control with Changes of Oxidative Stress Markers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- MacDonald, M.R.; Petrie, M.C.; Varyani, F.; Ostergren, J.; Michelson, E.L.; Young, J.B.; Solomon, S.D.; Granger, C.B.; Swedberg, K.; Yusuf, S.; et al. CHARM Investigators. Impact of diabetes on outcomes in patients with low and preserved ejection fraction heart failure: An analysis of the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) programme. Eur. Heart J. 2008, 29, 1377–1385. [Google Scholar] [CrossRef] [Green Version]
- Seferović, P.M.; Petrie, M.C.; Filippatos, G.S.; Anker, S.D.; Rosano, G.; Bauersachs, J.; Paulus, W.J.; Komajda, M.; Cosentino, F.; De Boer, R.A.; et al. Type 2 diabetes mellitus and heart failure: A position statement from the Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 853–872. [Google Scholar] [CrossRef]
- MacDonald, M.R.; Petrie, M.C.; Hawkins, N.M.; Petrie, J.R.; Fisher, M.; McKelvie, R.; Aquilar, D.; Krum, H.; McMurray, J.J. Diabetes, left ventricular systolic dysfunction, and chronic heart failure. Eur. Heart J. 2008, 29, 1224–1240. [Google Scholar] [CrossRef]
- Vaur, L.; Gueret, P.; Lievre, M.; Chabaud, S.; Passa, P. DIABHYCAR Study Group. Development of congestive heart failure in type 2 diabetic patients with microalbuminuria or proteinuria: Observations from the DIABHYCAR (type 2 DIABetes, Hypertension, CArdiovascular Events and Ramipril) study. Diabetes Care 2003, 26, 855–886. [Google Scholar] [CrossRef] [Green Version]
- Seferovic, P.M.; Paulus, W.J. Clinical diabetic cardiomyopathy: A two-faced disease with restrictive and dilated phenotypes. Eur. Heart J. 2015, 36, 1718–1727. [Google Scholar] [CrossRef] [PubMed]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulrich, F.; Ning, X.; Huige, L. Roles of vascular oxidative stress and nitric oxide in the pathogenesis of atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar]
- Fridlyand, L.E.; Philipson, L.H. Does the glucose-dependent insulin secretion mechanism itself cause oxidative stress in pancreatic beta-cells? Diabetes 2004, 53, 1942–1948. [Google Scholar] [CrossRef] [Green Version]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; E Grobbee, D.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef] [Green Version]
- DeFronzo, R.A. Combination therapy with GLP-1 receptor agonist and SGLT2 inhibitor. Diabetes Obes. Metab. 2017, 19, 1353–1362. [Google Scholar] [CrossRef]
- Zelniker, T.A.; Braunwald, E. Mechanisms of Cardiorenal Effects of Sodium-Glucose Cotransporter 2 Inhibitors: JACC State-of-the-Art Review. J. Am. Coll Cardiol. 2020, 75, 422–434. [Google Scholar] [CrossRef]
- Nagahisa, T.; Saisho, Y. Cardiorenal Protection: Potential of SGLT2 Inhibitors and GLP-1 Receptor Agonists in the Treatment of Type 2 Diabetes. Diabetes Ther. 2019, 10, 1733–1752. [Google Scholar] [CrossRef]
- Salonen, J.T.; Nyyssonen, K.; Salonen, R.; Porkkala-Sarataho, E.; Tuomainen, T.P.; Diczfalusy, U.; Bjorkhem, I. Lipoprotein oxidation and progression of carotid atherosclerosis. Circulation 1997, 95, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Kolyva, A.S.; Zolota, V.; Mpatsoulis, D.; Skroubis, G.; Solomou, E.E.; Habeos, I.G.; Assimakopoulos, S.F.; Goutzourelas, N.; Kouretas, D.; Gogos, C.A. The role of obesity in the immune response during sepsis. Nutr. Diabetes 2014, 4, e137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusirisin, W.; Jaikang, C.; Chaiyasut, C.; Narongchai, P. Effect of polyphenolic compounds from Solanum torvum on plasma lipid peroxidation, superoxide anion and cytochrome P450 2E1 in human liver microsomes. Med. Chem. 2009, 5, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Narasimhan, S.; Gokulakrishnan, K.; Sampathkumar, R.; Farooq, S.; Ravikumar, R.; Mohan, V.; Balasubramanyam, M. Oxidative stress is independently associated with non-alcoholic fatty liver disease (NAFLD) in subjects with and without type 2 diabetes. Clin. Biochem. 2010, 43, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Faris, M.E.A.-I.E.; Hussein, R.N.; Al-Kurd, R.A.; Al-Fararjeh, M.A.; Bustanji, Y.K.; Mohammad, M.K. Impact of Ramadan intermittent fasting on oxidative stress measured by urinary 15–isoprostane. J. Nutr. Metab. 2012, 2012, 802924. [Google Scholar] [CrossRef] [Green Version]
- Walter, M.F.; Jacob, R.F.; Jeffers, B.; Ghadanfar, M.M.; Preston, G.M.; Buch, J.; Mason, R.P. Serum levels of thiobarbituric acid reactive substances predict cardiovascular events in patients with stable coronary artery disease: A longitudinal analysis of the PREVENT study. J. Am. Coll. Cardiol. 2004, 44, 1996–2002. [Google Scholar] [CrossRef] [Green Version]
- Chrysohoou, C.; Panagiotakos, D.B.; Pitsavos, C.; Skoumas, I.; Papademetriou, L.; Economou, M.; Stefanadis, C. The implication of obesity on total antioxidant capacity in apparentlyhealthy men and women: The ATTICA study. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 590–597. [Google Scholar] [CrossRef]
- Weber, D.; Davies, M.; Grune, T. Determination of protein carbonyls in plasma, cell extracts, tissue homogenates, isolated proteins: Focus on sample preparation and derivatization conditions. Redox Biol. 2015, 5, 367–380. [Google Scholar] [CrossRef] [Green Version]
- Janaszewska, A.; Bartosz, G. Assay of total antioxidant capacity: Comparison of four methods as applied to human blood plasma. Scand. J. Clin. Lab. Investig. 2002, 62, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmi de Toledo, F.; Grundler, F.; Goutzourelas, N.; Tekos, F.; Vassi, E.; Mesnage, R.; Kouretas, D. Influence of Long-Term Fasting on Blood Redox Status in Humans. Antioxidants 2020, 9, 496. [Google Scholar] [CrossRef] [PubMed]
- Spanidis, Y.; Goutzourelas, N.; Stagos, D.; Kolyva, A.S.; Gogos, C.A.; Bar-Or, D.; Kouretas, D. Assessment of Oxidative Stress in Septic and Obese Patients Using Markers of Oxidation-reduction Potential. In Vivo 2015, 29, 595–600. [Google Scholar] [PubMed]
- Hjort, L.; Jørgensen, S.W.; Gillberg, L.; Hall, E.; Brøns, C.; Frystyk, J.; Vaag, A.A.; Ling, C. 36 h fasting of young men influences adipose tissue DNA methylation of LEP and ADIPOQ in a birth weight-dependent manner. Clin. Epigenet. 2017, 9, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teruya, T.; Chaleckis, R.; Takada, J.; Yanagida, M.; Kondoh, H. Diverse metabolic reactions activated during 58-hr fasting are revealed by non-targeted metabolomic analysis of human blood. Sci. Rep. 2019, 9, 854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saleh, S.; Hanna, G.; El-Nabi, S.H.; El-Domiaty, H.; Shabaan, A.; Ewida, S.F. Dapagliflozin, a sodium glucose cotransporter 2 inhibitors, protects cardiovascular function in type-2 diabetic murine model. J. Genet. 2020, 99, 46. [Google Scholar] [CrossRef]
- Abdel-Wahab, A.F.; Bamagous, G.A.; Al-Harizy, R.M.; ElSawy, N.A.; Shahzad, N.; Ibrahim, I.A.; Al Ghamdi, S.S. Renal protective effect of SGLT2 inhibitor dapagliflozin alone and in combination with irbesartan in a rat model of diabetic nephropathy. Biomed. Pharmacother. 2018, 103, 59–66. [Google Scholar] [CrossRef]
- Ho, E.; Galougahi, K.K.; Liu, C.C.; Bhindi, R.; Figtree, G.A. Biological markers of oxidative stress: Applications to cardiovascular research and practice. Redox Biol. 2013, 1, 483–491. [Google Scholar] [CrossRef] [Green Version]
- Yen, G.C.; Duh, P.D. Scavenging effect of methanolic extracts of peanut hulls on free-radical and active-oxygen species. J. Agric. Food Chem. 1994, 42, 629–632. [Google Scholar] [CrossRef]
- El-Haskoury, R.; Al-Waili, N.; El-Hilaly, J.; Al-Waili; Lyoussi, B. Antioxidant, hypoglycemic, and hepatoprotective effect of aqueous and ethyl acetate extract of carob honey in streptozotocin-induced diabetic rats. Vet. World 2019, 12, 1916–1923. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Pavlidis, G.; Thymis, J.; Birba, D.; Kalogeris, A.; Kousathana, F.; Kountouri, A.; Balampanis, K.; Parissis, J.; Andreadou, I.; et al. Effects of Glucagon-Like Peptide-1 Receptor Agonists, Sodium-Glucose Cotransporter-2 Inhibitors, and Their Combination on Endothelial Glycocalyx, Arterial Function, and Myocardial Work Index in Patients With Type 2 Diabetes Mellitus After 12-Month Treatment. J. Am. Heart Assoc. 2020, 9, e015716. [Google Scholar] [PubMed] [Green Version]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts) Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [PubMed]
- Hanefeld, M.; Monnier, L.; Schnell, O.; Owens, D. Early Treatment with Basal Insulin Glargine in People with Type 2 Diabetes: Lessons from ORIGIN and Other Cardiovascular Trials. Diabetes Ther. 2016, 7, 187–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerstein, H.C.; Jung, H.; Rydén, L.; Diaz, R.; Gilbert, R.E.; Yusuf, S. ORIGIN Investigators. Effect of Basal Insulin Glargine on First and Recurrent Episodes of Heart Failure Hospitalization: The ORIGIN Trial (Outcome Reduction With Initial Glargine Intervention). Circulation 2018, 137, 88–90. [Google Scholar] [CrossRef]
- Davies, M.J.; D’Alessio, D.A.; Fradkin, J.; Kernan, W.N.; Mathieu, C.; Mingrone, G.; Rossing, P.; Tsapas, A.; Wexler, D.J.; Buse, J.B. Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2018, 41, 2669–2701. [Google Scholar] [CrossRef] [Green Version]
- Keles, M.S.; Taysi, S.; Sen, N.; Aksoy, H.; Akcay, F. Effect of corticosteroid therapy on serum and CSF malondialdehyde and antioxidant proteins in multiple sclerosis. Can. J. Neurol Sci. 2001, 2, 141–143. [Google Scholar] [CrossRef] [Green Version]
- Lambadiari, V.; Pavlidis, G.; Kousathana, F.; Varoudi, M.; Vlastos, D.; Maratou, E.; Georgiou, D.; Andreadou, I.; Parissis, J.; Triantafyllidi, H.; et al. Effects of 6-month treatment with the glucagon like peptide-1 analogue liraglutide on arterial stiffness, left ventricular myocardial deformation and oxidative stress in subjects with newly diagnosed type 2 diabetes. Cardiovasc. Diabetol. 2018, 17, 8. [Google Scholar] [CrossRef] [Green Version]
- Cano, A.; Acosta, Μ.; Arnao, M.B. A method to measure antioxidant activity in organic media: Application to lipophilic vitamins. Redox Rep. 2000, 5, 365–370. [Google Scholar] [CrossRef] [Green Version]
- Zimmet, P.; Alberti, K.G.; Magliano, D.J.; Bennett, P.H. Diabetes mellitus statistics on prevalence and mortality: Facts and fallacies. Nat. Rev. Endocrinol. 2016, 12, 616–622. [Google Scholar] [CrossRef]
- Pickering, R.J.; Rosado, C.J.; Sharma, A.; Buksh, S.; Tate, M.; de Haan, J.B. Recent novel approaches to limit oxidative stress and inflammation in diabetic complications. Clin. Transl. Immunol. 2018, 7, e1016. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Pi, J.; Bai, Y.; Zhang, Q.; Wong, V.; Floering, L.M.; Daniel, K.; Reece, J.M.; Deeney, J.T.; Andersen, M.E.; Corkey, B.E.; et al. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes 2007, 56, 1783–1791. [Google Scholar] [CrossRef] [Green Version]
- Oguntibeju, O.O. Type 2 diabetes mellitus, oxidative stress and inflammation: Examining the links. Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 45–63. [Google Scholar]
- Wang, Z.Q.; Jing, L.L.; Yan, J.C.; Sun, Z.; Bao, Z.Y.; Shao, C.; Pang, Q.W.; Geng, Y.; Zhang, L.L.; Li, L.H. Role of AGEs in the progression and regression of atherosclerotic plaques. Glycoconj. J. 2018, 35, 443–450. [Google Scholar] [CrossRef]
- Aghadavod, E.; Khodadadi, S.; Baradaran, A.; Nasri, P.; Bahmani, M.; Rafieian-Kopaei, M. Role of oxidative stress and inflammatory factors in diabetic kidney disease. Iran. J. Kidney Dis. 2016, 10, 337–343. [Google Scholar] [PubMed]
- Yang, X.; Li, Y.; Li, Y.; Ren, X.; Zhang, X.; Hu, D.; Gao, Y.; Xing, Y.; Shang, H. Oxidative stress-mediated atherosclerosis: Mechanisms and therapies. Front. Physiol. 2017, 8, 600. [Google Scholar] [CrossRef] [Green Version]
- Kattoor, A.J.; Pothineni, N.V.K.; Palagiri, D.; Mehta, J.L. Oxidative stress in atherosclerosis. Curr. Atheroscler. Rep. 2017, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Nakagawa, Y.; Murakami, C.; Aoki, N.; Kim-Mitsuyama, S.; Miyazaki, H. Protein tyrosine phosphatase PTPepsilonM negatively regulates PDGF beta-receptor signaling induced by high glucose and PDGF in vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 2010, 299, C1144–C1152. [Google Scholar] [CrossRef]
- Oelze, M.; Kröller-Schön, S.; Welschof, P.; Jansen, T.; Hausding, M.; Mikhed, Y.; Stamm, P.; Mader, M.; Zinßius, E.; Agdauletova, S.; et al. The sodium-glucose co-transporter 2 inhibitor empagliflozin improves diabetes-induced vascular dysfunction in the streptozotocin diabetes rat model by interfering with oxidative stress and glucotoxicity. PLoS ONE 2014, 9, e112394. [Google Scholar] [CrossRef] [PubMed]
- Terami, N.; Ogawa, D.; Tachibana, H.; Hatanaka, T.; Wada, J.; Nakatsuka, A.; Eguchi, J.; Horiguchi, C.S.; Nishii, N.; Yamada, H.; et al. Long-term treatment with the sodium glucose cotransporter 2 inhibitor, dapagliflozin, ameliorates glucose homeostasis and diabetic nephropathy in db/db mice. PLoS ONE 2014, 9, e100777. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Atkin, S.L.; Butler, A.E.; Sahebkar, A. Sodium–glucose cotransporter inhibitors and oxidative stress: An update. J. Cell Physiol. 2019, 234, 3231–3237. [Google Scholar] [CrossRef] [PubMed]
- Zaibi, N.; Li, P.; Xu, S.Z. Protective effects of dapagliflozin against oxidative stress-induced cell injury in human proximal tubular cells. PLoS ONE 2021, 16, e0247234. [Google Scholar] [CrossRef] [PubMed]
- Tahara, A.; Kurosaki, E.; Yokono, M.; Yamajuku, D.; Kihara, R.; Hayashizaki, Y.; Takasu, T.; Imamura, M.; Li, Q.; Tomiyama, H.; et al. Effects of sodium-glucose cotransporter 2 selective inhibitor ipragliflozin on hyperglycaemia, oxidative stress, inflammation and liver injury in streptozotocin-induced type 1 diabetic rats. J. Pharm. Pharmacol. 2014, 66, 975–987. [Google Scholar] [CrossRef]
- Tahara, A.; Takasu, T. SGLT2 inhibitor ipragliflozin alone and combined with pioglitazone prevents progression of nonalcoholic steatohepatitis in a type 2 diabetes rodent model. Physiol. Rep. 2019, 7, e14286. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Nagata, N.; Chen, G.; Nagashimada, M.; Zhuge, F.; Ni, Y.; Sakai, Y.; Kaneko, S.; Ota, T. Empagliflozin reverses obesity and insulin resistance through fat browning and alternative macrophage activation in mice fed a high-fat diet. BMJ Open Diabetes Res. Care. 2019, 7, e000783. [Google Scholar] [CrossRef]
- Xu, L.; Nagata, N.; Nagashimada, M.; Zhuge, F.; Ni, Y.; Chen, G.; Mayoux, E.; Kaneko, S.; Ota, T. SGLT2 Inhibition by Empagliflozin Promotes Fat Utilization and Browning and Attenuates Inflammation and Insulin Resistance by Polarizing M2 Macrophages in Diet-induced Obese Mice. EBioMedicine 2017, 20, 137–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusaka, H.; Koibuchi, N.; Hasegawa, Y.; Ogawa, H.; Kim-Mitsuyama, S. Empagliflozin lessened cardiac injury and reduced visceral adipocyte hypertrophy in prediabetic rats with metabolic syndrome. Cardiovasc. Diabetol. 2016, 15, 157. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.K.; Kannan, K.; Lim, G. Ketosis (acetoacetate) can generate oxygen radicals and cause increased lipid peroxidation and growth inhibition in human endothelial cells. Free Radic. Biol. Med. 1998, 25, 1083–1088. [Google Scholar] [CrossRef]
- Lee, D.M.; Hoffman, W.H.; Carl, G.F.; Khichi, M.; Cornwell, P.E. Lipid peroxidation and antioxidant vitamins prior to, during, and after correction of diabetic ketoacidosis. J. Diabetes Complicat. 2002, 16, 294–300. [Google Scholar] [CrossRef]
- Bray, J.J.H.; Foster-Davies, H.; Stephens, J.W. A systematic review examining the effects of sodium-glucose cotransporter-2 inhibitors (SGLT2is) on biomarkers of inflammation and oxidative stress. Diabetes Res. Clin. Pract. 2020, 168, 108368. [Google Scholar] [CrossRef]
- Iannantuoni, F.; de Marañon, A.M.; Diaz-Morales, N.; Falcon, R.; Bañuls, C.; Abad-Jimenez, Z.; Victor, V.M.; Hernandez-Mijares, A.; Rovira-Llopis, S. The SGLT2 Inhibitor Empagliflozin Ameliorates the Inflammatory Profile in Type 2 Diabetic Patients and Promotes an Antioxidant Response in Leukocytes. J. Clin. Med. 2019, 8, 1814. [Google Scholar] [CrossRef] [Green Version]
- Osorio, H.; Coronel, I.; Arellano, A.; Pacheco, U.; Bautista, R.; Franco, M.; Escalante, B. Sodium-glucose cotransporter inhibition prevents oxidative stress in the kidney of diabetic rats. Oxid. Med. Cell Longev. 2012, 2012, 542042. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Zhang, J.; Xue, M.; Li, X.; Han, F.; Liu, X.; Xu, L.; Lu, Y.; Cheng, Y.; Li, T.; et al. SGLT2 inhibition with empagliflozin attenuates myocardial oxidative stress and fibrosis in diabetic mice heart. Cardiovasc. Diabetol. 2019, 18, 15. [Google Scholar] [CrossRef] [PubMed]
- Krasner, N.M.; Ido, Y.; Ruderman, N.B.; Cacicedo, J.M. Glucagon-like Peptide-1 (GLP-1) analog liraglutide inhibits endothelial cell inflammation through a calcium and AMPK dependent mechanism. PLoS ONE 2014, 9, e97554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, G.; Zhang, D.; Yu, H.; Zhang, P.; Wang, Y.; Zheng, A.; Qin, S. Cardioprotective effects of exenatide against oxidative stress-induced injury. Int. J. Mol. Med. 2013, 32, 1011–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, H.; Morii, T.; Fujishima, H.; Sato, T.; Shimizu, T.; Hosoba, M.; Yamada, Y. The protective roles of GLP-1R signaling in diabetic nephropathy: Possible mechanism and therapeutic potential. Kidney Int. 2014, 85, 579–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, C.G.; Luo, Y.; Wang, H.; Li, J.-Y.; Yang, J.; Liu, Y.-X.; Qu, H.-Q.; Wang, B.-L.; Zhu, M. Liraglutide Ameliorates Lipotoxicity-Induced Oxidative Stress by Activating the NRF2 Pathway in HepG2 Cells. Horm. Metab. Res. 2020, 52, 532–539. [Google Scholar] [CrossRef]
- Duparc, T.; Briand, F.; Trenteseaux, C.; Merian, J.; Combes, G.; Najib, S.; Sulpice, T.; Martinez, L.O. Liraglutide improves hepatic steatosis and metabolic dysfunctions in a 3-week dietary mouse model of nonalcoholic steatohepatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2019, 317, G508–G517. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.; Joharapurkar, A.; Kshirsagar, S.; Sutariya, B.; Patel, M.; Patel, H.; Pandey, D.; Patel, D.; Ranvir, R.; Kadam, S. Coagonist of GLP-1 and Glucagon Receptor Ameliorates Development of Non-Alcoholic Fatty Liver Disease. Cardiovasc. Hematol. Agents Med. Chem. 2018, 16, 35–43. [Google Scholar] [CrossRef]
- Krause, G.C.; Lima, K.G.; Dias, H.B.; Da Silva, E.F.G.; Haute, G.V.; Basso, B.S.; Gassen, R.B.; Marczak, E.S.; Nunes, R.S.B.; De Oliveira, J.R. Liraglutide, a glucagon-like peptide-1 analog, induce autophagy and senescence in HepG2 cells. Eur. J. Pharmacol. 2017, 809, 32–41. [Google Scholar] [CrossRef]
- Oeseburg, H.; de Boer, R.A.; Buikema, H.; van der Harst, P.; van Gilst, W.H.; Sillje, H.H.W. Glucagon-like peptide 1 prevents reactive oxygen species-induced endothelial cell senescence through the activation of protein kinase A. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1407–1414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, S.; Gross, C.M.; Rafikov, R.; Kumar, S.; Fineman, J.R.; Ludewig, B.; Jonigk, D.; Black, S.M. Nitration of tyrosine 247 inhibits protein kinase G-1α activity by attenuating cyclic guanosine monophosphate binding. J. Biol. Chem. 2014, 289, 7948–7961. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| All Patients (n = 160) | Insulin (n = 40) | GLP-1RA (n = 40) | SGLT-2i (n = 40) | GLP-1RA+SGLT-2i (n = 40) | p | |
|---|---|---|---|---|---|---|
| Age, years | 58 ± 10 | 57 ± 10 | 57 ± 9 | 58 ± 10 | 58 ± 9 | 0.518 |
| BMI, Kg/m2 | 30 ± 3 | 29.7 ± 3 | 30 ± 4 | 29.8 ± 3 | 30.5 ± 3 | 0.652 |
| CAD, n (%) | 54 (34) | 14 (35) | 13 (32.5) | 13 (32.5) | 14 (35) | 0.869 |
| Creatinine, mg/dL | 1.1 ± 0.3 | 1.0 ± 0.3 | 1.1 ± 0.2 | 1.1 ± 0.2 | 1.1 ± 0.3 | 0.833 |
| Duration of diabetes, years | 6.5 (2–10) | 6.7 (1–9) | 5.9 (1–8) | 6.6 (1–11) | 6.8 (2–12) | 0.446 |
| eGFR, mL/min per 1.73 m2 | 85 ± 10 | 86 ± 9 | 85 ± 8 | 85 ± 10 | 83 ± 11 | 0.315 |
| Fasting plasma glucose, mg/dL | 152 ± 42 | 158 ± 48 | 152 ± 45 | 145 ± 34 | 151 ± 40 | 0.474 |
| Sex (male/female), n (%) | 115/45 (72/28) | 28/12 (70/30) | 27/13 (67.5/32.5) | 30/10 (75/25) | 30/10 (75/25) | 0.151 |
| TC, mg/dL | 172 ± 42 | 178 ± 43 | 172 ± 38 | 163 ± 37 | 175 ± 50 | 0.237 |
| LDL-C, mg/dL | 99 ± 26 | 104 ± 27 | 101 ± 26 | 92 ± 21 | 100 ± 30 | 0.361 |
| HDL-C, mg/dL | 42 ± 12 | 46 ± 12 | 41 ± 11 | 39 ± 9 | 43 ± 14 | 0.545 |
| TG, mg/dL | 155 ± 44 | 143 ± 42 | 158 ± 41 | 157 ± 44 | 161 ± 48 | 0.198 |
| Risk factors, n (%) | ||||||
| Current smoking | 64 (40) | 15 (37.5) | 17 (42.5) | 16 (40) | 16 (40) | 0.837 |
| Dyslipidemia | 160 (100) | 40 (100) | 40 (100) | 40 (100) | 40 (100) | 1.000 |
| Family history CAD | 51 (32) | 12 (30) | 11 (27.5) | 15 (37.5) | 13 (32.5) | 0.392 |
| Hypertension | 97 (61) | 24 (60) | 24 (60) | 24 (60) | 25 (62.5) | 0.789 |
| Cardiovascular medications, n (%) | ||||||
| ACEI or ARB | 80 (50) | 20 (50) | 19 (47.5) | 21 (52.5) | 20 (50) | 0.734 |
| Aldosterone antagonists | 7 (4) | 1 (2.5) | 2 (5) | 1 (2.5) | 3 (7.5) | 0.827 |
| Antiplatelet | 57 (36) | 14 (35) | 13 (32.5) | 15 (37.5) | 15 (37.5) | 0.898 |
| Beta blockers | 78 (49) | 18 (45) | 19 (47.5) | 21 (52.5) | 20 (50) | 0.753 |
| Calcium channel blocker | 40 (25) | 10 (25) | 9 (22.5) | 10 (25) | 11 (27.5) | 0.512 |
| Diuretics | 28 (17.5) | 6 (15) | 5 (12.5) | 8 (20) | 9 (22.5) | 0.969 |
| Fibrate | 10 (6) | 2 (5) | 2 (5) | 3 (7.5) | 3 (7.5) | 0.787 |
| Statins | 160 (100) | 40 (100) | 40 (100) | 40 (100) | 40 (100) | 1.000 |
| Antidiabetic medications, n (%) | ||||||
| Metformin | 109 (68) | 29 (72.5) | 25 (62.5) | 27 (67.5) | 28 (70) | 0.192 |
| Variables | All Participants (n = 160) | Insulin (n = 40) | GLP1-RA (n = 40) | SGLT2i (n = 40) | GLP1-RA +SGLT2i (n = 40) |
|---|---|---|---|---|---|
| TBARS, μmol/L | |||||
| Baseline | 8.27 ± 2.67 | 8.81 ± 1.22 | 8.23 ± 2.91 | 7.76 ± 2.69 | 8.24 ± 2.38 |
| 4 mo | 7.94 ± 2.81 † | 8.11 ± 1.18 | 8.10 ± 2.74 | 7.79 ± 2.58 | 7.67 ± 1.99 † |
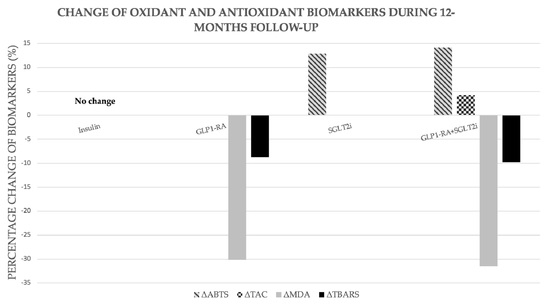
| Δ% | −3.99 | −5.84 | −1.57 | 0.38 | −6.91 |
| 12 mo | 7.91 ± 2.54 †† | 8.45 ± 1.01 | 7.06 ± 2.50 ††,* | 8.01 ± 2.37 | 7.43 ± 1.94 †††,** |
| Δ% | −4.35 | −0.5 | −8.76 | 3.22 | −9.83 |
| MDA, nM/L | |||||
| Baseline | 1.73 ± 0.39 | 1.48 ± 0.34 | 1.99 ± 0.46 | 1.87 ± 0.48 | 1.59 ± 0.27 |
| 4 mo | 1.61 ± 0.36 † | 1.56 ± 0.41 | 1.83 ± 0.39 | 1.83 ± 0.37 | 1.24 ± 0.32 ††,* |
| Δ% | −6.93 | 5.40 | −8.04 | −2.13 | −22.01 |
| 12 mo | 1.45 ± 0.28 †† | 1.55 ± 0.26 | 1.39 ± 0.31 †††,** | 1.80 ± 0.40 | 1.09 ± 0.25 †††,*** |
| Δ% | −16.18 | 4.72 | −30.15 | −3.74 | −31.44 |
| ABTS, mmol/L | |||||
| Baseline | 16.73 ± 4.10 | 17.18 ± 2.35 | 17.12 ± 3.02 | 16.16 ± 4.16 | 17.49 ± 4.93 |
| 4 mo | 17.49 ± 4.08 | 17.90 ± 2.96 | 17.31 ± 3.59 | 16.46 ± 4.74 | 18.27 ± 4.11 † |
| Δ% | 4.72 | 4.19 | 3.14 | 1.85 | 7.55 |
| 12 mo | 18.08 ± 4.37 † | 17.60 ± 1.99 | 16.53 ± 3.41 | 18.24 ± 4.93 ††,** | 19.06 ± 4.71 ††,** |
| Δ% | 10.34 | 2.44 | −3.44 | 12.87 | 14.13 |
| TAC, mmol/L | |||||
| Baseline | 0.89 ± 0.14 | 0.92 ± 0.16 | 0.84 ± 0.17 | 0.87 ± 0.12 | 0.90 ± 0.14 |
| 4 mo | 0.88 ± 0.16 | 0.89 ± 0.18 | 0.82 ± 0.17 | 0.88 ± 0.16 | 0.90 ± 0.13 |
| Δ% | −1.12 | −3.37 | −2.38 | 1.14 | −0.63 |
| 12 mo | 0.88 ± 0.17 | 0.91 ± 0.16 | 0.84 ± 0.16 | 0.86 ± 0.17 | 0.94 ± 0.17 †,* |
| Δ% | −1.12 | −1.08 | −0.45 | −1.14 | 4.25 |
| RP, μmol/mL | |||||
| Baseline | 1.16 ± 0.14 | 1.18 ± 0.11 | 1.18 ± 0.14 | 1.16 ± 0.13 | 1.12 ± 0.18 |
| 4 mo | 1.14 ± 0.13 | 1.17 ± 0.14 | 1.17 ± 0.13 | 1.15 ± 0.12 | 1.10 ± 0.10 |
| Δ% | −1.72 | −1.68 | −0.84 | −1.72 | −1.80 |
| 12 mo | 1.12 ± 0.17 | 1.14 ± 0.13 | 1.14 ± 0.15 | 1.14 ± 0.14 | 1.09 ± 0.15 |
| Δ% | −2.97 | −3.36 | −3.38 | −2.69 | −2.1 |
| HbA1c, % | |||||
| Baseline | 8.1 ± 1.1 | 8.2 ± 1.2 | 8 ± 1.1 | 7.8 ± 0.9 | 8.2 ± 1.2 |
| 4 mo | 6.9 ± 1.1 ††† | 7 ± 1.1 †† | 6.7 ± 1 † | 7 ± 1 ††† | 6.7 ± 0.8 ††† |
| Δ% | −17.4 | −17.1 | −19.4 | −11.4 | −22.4 |
| 12 mo | 6.8 ± 1.1 ††† | 7.1 ± 1.2 †† | 6.7 ± 0.9 † | 7.1 ± 1.1 †† | 6.4 ± 0.8 †† |
| Δ% | −19.1 | −15.5 | −19.5 | −9.8 | −28.1 |
| Fasting Glucose, mg/dl | |||||
| Baseline | 152 ± 42 | 158 ± 48 | 152 ± 45 | 145 ± 34 | 151 ± 40 |
| 4 mo | 127 ± 30 ††† | 134 ± 44 ††† | 122 ± 27 † | 126 ± 20 † | 125 ± 29 † |
| Δ% | −19.73 | −17.94 | −24.61 | −15.10 | −20.88 |
| 12 mo | 120 ± 31 †† | 121 ± 40 † | 118 ± 24 † | 124 ± 30 † | 116 ± 30 † |
| Δ% | −26.71 | −30.65 | −28.80 | −16.9 | −30.2 |
| BMI, kg/m2 | |||||
| Baseline | 30 ± 3 | 29.7 ± 3 | 30 ± 4 | 29.8 ± 3 | 30.5 ± 3 |
| 4 mo | 28.52 ± 3 ††† | 28.85 ± 3 | 28 ± 3 ††† | 28.82 ± 2 ††† | 28.54 ± 3 ††† |
| Δ% | −5.31 | −3.12 | −7.10 | −3.51 | −7.04 |
| 12 mo | 28.24 ± 3 ††† | 29.62 ± 3 | 27.61 ± 3 †† | 28.44 ± 2 | 27.31 ± 4 ††† |
| Δ% | −6.40 | −0.32 | −8.71 | −4.93 | −11.71 |
| TC, mg/dL | |||||
| Baseline | 172 ± 42 | 178 ± 43 | 172 ± 38 | 163 ± 37 | 175 ± 50 |
| 4 mo | 152 ± 44 †† | 160 ± 48 † | 154 ± 47 | 140 ± 30 † | 154 ± 51 † |
| Δ% | −13.22 | −11.31 | −11.71 | −16.43 | −13.64 |
| 12 mo | 147 ± 31 †† | 154 ± 25 † | 145 ± 28 † | 147 ± 32 | 144 ± 35 †† |
| Δ% | −17 | −15.50 | −18.63 | −10.92 | −21.51 |
| LDL-C, mg/dL | |||||
| Baseline | 99 ± 26 | 104 ± 27 | 101 ± 26 | 92 ± 21 | 100 ± 30 |
| 4 mo | 85 ± 31 †† | 90 ± 35 | 91 ± 33 | 78 ± 24 † | 79 ± 29 † |
| Δ% | −16.50 | −15.53 | −11 | −17.91 | −26.62 |
| 12 mo | 80 ± 24 †† | 82 ± 23 | 80 ± 21 † | 81 ± 25 | 75 ± 24 † |
| Δ% | −23.73 | −26.80 | −26.32 | −13.51 | −33.44 |
| HDL-C, mg/dL | |||||
| Baseline | 42 ± 12 | 46 ± 12 | 41 ± 11 | 39 ± 9 | 43 ± 14 |
| 4 mo | 43 ± 11 | 46 ± 13 | 39 ± 7 | 41 ± 10 | 45 ± 12 |
| Δ% | 2.30 | 0.42 | 5.11 | 4.83 | 4.41 |
| 12 mo | 44 ± 10 | 46 ± 7 | 42 ± 11 | 43 ± 8 | 46 ± 13 |
| Δ% | 4.5 | 0.6 | 2.4 | 9.3 | 6.5 |
| TG, mg/dL | |||||
| Baseline | 155 ± 44 | 143 ± 42 | 158 ± 41 | 157 ± 44 | 161 ± 48 |
| 4 mo | 139 ± 37 † | 123 ± 28 † | 140 ± 38 | 146 ± 43 † | 147 ± 40 |
| Δ% | −11.51 | −16.20- | −12.93 | −7.52 | −10.93 |
| 12 mo | 122 ± 27 †† | 118 ± 31 | 124 ± 22 † | 125 ± 29 † | 123 ± 24 † |
| Δ% | −27 | −21.12 | −27.40 | −25.63 | −30.92 |
| ΔGlu (4 Months) | ΔGlu (12 Months) | ΔHbA1C (4 Months) | ΔHbA1C (12 Months) | |
|---|---|---|---|---|
| ΔABTS (4 months) | r = −0.07 p = 0.802 | N/A | r = −0.262 p = 0.038 | N/A |
| ΔABTS (12 months) | N/A | r = −0.296 p = 0.041 | N/A | r = −0.138 p = 0.265 |
| ΔTBARS (4 months) | r = 0.231 p = 0.049 | N/A | r = 0.159 p = 0.163 | N/A |
| ΔTBARS (12 months) | N/A | r = 0.233 p = 0.091 | N/A | r = 0.280 p = 0.045 |
| ΔTAC (4 months) | r = −0.053 p = 0.601 | N/A | r = −0.150 p = 0.213 | N/A |
| ΔTAC (12 months) | N/A | r = −0.112 p = 0.386 | N/A | r = −0.104 p = 0.401 |
| ΔMDA (4 months) | r = −0.180 p = 0.286 | N/A | r = −0.164 p = 0.195 | N/A |
| ΔMDA (12 months) | N/A | r = −0.181 p = 0.295 | N/A | r = −0.171 p = 0.314 |
| ΔRP (4 months) | r = −0.119 p = 0.318 | N/A | r = −0.062 p = 0.602 | N/A |
| ΔRP (12 months) | N/A | r = −0.136 p = 0.248 | N/A | r = −0.063 p = 0.595 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lambadiari, V.; Thymis, J.; Kouretas, D.; Skaperda, Z.; Tekos, F.; Kousathana, F.; Kountouri, A.; Balampanis, K.; Parissis, J.; Andreadou, I.; et al. Effects of a 12-Month Treatment with Glucagon-like Peptide-1 Receptor Agonists, Sodium-Glucose Cotransporter-2 Inhibitors, and Their Combination on Oxidant and Antioxidant Biomarkers in Patients with Type 2 Diabetes. Antioxidants 2021, 10, 1379. https://doi.org/10.3390/antiox10091379
Lambadiari V, Thymis J, Kouretas D, Skaperda Z, Tekos F, Kousathana F, Kountouri A, Balampanis K, Parissis J, Andreadou I, et al. Effects of a 12-Month Treatment with Glucagon-like Peptide-1 Receptor Agonists, Sodium-Glucose Cotransporter-2 Inhibitors, and Their Combination on Oxidant and Antioxidant Biomarkers in Patients with Type 2 Diabetes. Antioxidants. 2021; 10(9):1379. https://doi.org/10.3390/antiox10091379
Chicago/Turabian StyleLambadiari, Vaia, John Thymis, Dimitris Kouretas, Zoi Skaperda, Fotios Tekos, Foteini Kousathana, Aikaterini Kountouri, Konstantinos Balampanis, John Parissis, Ioanna Andreadou, and et al. 2021. "Effects of a 12-Month Treatment with Glucagon-like Peptide-1 Receptor Agonists, Sodium-Glucose Cotransporter-2 Inhibitors, and Their Combination on Oxidant and Antioxidant Biomarkers in Patients with Type 2 Diabetes" Antioxidants 10, no. 9: 1379. https://doi.org/10.3390/antiox10091379
APA StyleLambadiari, V., Thymis, J., Kouretas, D., Skaperda, Z., Tekos, F., Kousathana, F., Kountouri, A., Balampanis, K., Parissis, J., Andreadou, I., Tsoumani, M., Chania, C., Katogiannis, K., Dimitriadis, G., Bamias, A., & Ikonomidis, I. (2021). Effects of a 12-Month Treatment with Glucagon-like Peptide-1 Receptor Agonists, Sodium-Glucose Cotransporter-2 Inhibitors, and Their Combination on Oxidant and Antioxidant Biomarkers in Patients with Type 2 Diabetes. Antioxidants, 10(9), 1379. https://doi.org/10.3390/antiox10091379