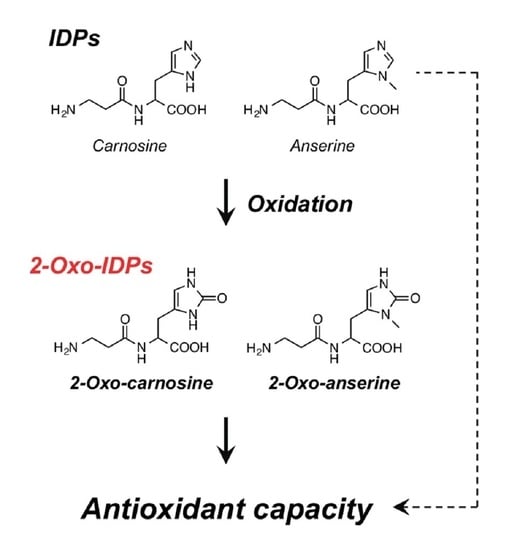
2-Oxo-Imidazole-Containing Dipeptides Play a Key Role in the Antioxidant Capacity of Imidazole-Containing Dipeptides
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Measurement of Antioxidant Capacity
2.3. pKa Determination by Potentiometric Titration
2.4. HPLC Analysis
2.5. Quantitative HPLC-ESI-MS/MS Analysis
2.6. Purification of 2-Oxo-IDP-Free IDPs
2.7. Statistical Analysis
3. Results
3.1. Comparison of Antioxidant Capacity of 2-Oxo-IDPs with IDPs
3.2. Reactivity of 2-Oxo-IDPs and IDPs to Endogenous Radicals
3.3. Antioxidant Capacity of Commercial IDPs
3.4. Contaminated 2-Oxo-IDPs in Commercial IDPs Standards
3.5. Preparation of Purified IDPs and Analysis of Antioxidant Capacity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gulewitsch, W.; Amiradžibi, S. Ueber das Carnosin, eine neue organische Base des Fleischextractes. Ber. Dtsch. Chem. Ges. 1900, 33, 1902–1903. [Google Scholar] [CrossRef] [Green Version]
- Jukic, I.; Kolobaric, N.; Stupin, A.; Matic, A.; Kozina, N.; Mihaljevic, Z.; Mihalj, M.; Susnjara, P.; Stupin, M.; Curic, Z.B.; et al. Carnosine, Small but Mighty-Prospect of Use as Functional Ingredient for Functional Food Formulation. Antioxidants 2021, 10, 1037. [Google Scholar] [CrossRef] [PubMed]
- Boldyrev, A.A.; Aldini, G.; Derave, W. Physiology and pathophysiology of carnosine. Physiol. Rev. 2013, 93, 1803–1845. [Google Scholar] [CrossRef] [PubMed]
- Ihara, H.; Kakihana, Y.; Yamakage, A.; Kai, K.; Shibata, T.; Nishida, M.; Yamada, K.I.; Uchida, K. 2-Oxo-histidine-containing dipeptides are functional oxidation products. J. Biol. Chem. 2019, 294, 1279–1289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drozak, J.; Piecuch, M.; Poleszak, O.; Kozlowski, P.; Chrobok, L.; Baelde, H.J.; de Heer, E. UPF0586 Protein C9orf41 Homolog Is Anserine-producing Methyltransferase. J. Biol. Chem. 2015, 290, 17190–17205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drozak, J.; Veiga-da-Cunha, M.; Vertommen, D.; Stroobant, V.; Van Schaftingen, E. Molecular identification of carnosine synthase as ATP-grasp domain-containing protein 1 (ATPGD1). J. Biol. Chem. 2010, 285, 9346–9356. [Google Scholar] [CrossRef] [Green Version]
- Teufel, M.; Saudek, V.; Ledig, J.P.; Bernhardt, A.; Boularand, S.; Carreau, A.; Cairns, N.J.; Carter, C.; Cowley, D.J.; Duverger, D.; et al. Sequence identification and characterization of human carnosinase and a closely related non-specific dipeptidase. J. Biol. Chem. 2003, 278, 6521–6531. [Google Scholar] [CrossRef] [Green Version]
- Severin, C.E.; Boldyrev, A.A.; Dupin, A.M. Biological role of histidine dipeptides in excitable tissues. Vopr. Med. Khim. 1984, 30, 32–36. [Google Scholar]
- Klebanov, G.I.; Teselkin Yu, O.; Babenkova, I.V.; Popov, I.N.; Levin, G.; Tyulina, O.V.; Boldyrev, A.A.; Vladimirov Yu, A. Evidence for a direct interaction of superoxide anion radical with carnosine. Biochem. Mol. Biol. Int. 1997, 43, 99–106. [Google Scholar] [CrossRef]
- Tamba, M.; Torreggiani, A. Hydroxyl radical scavenging by carnosine and Cu(II)-carnosine complexes: A pulse-radiolysis and spectroscopic study. Int. J. Radiat. Biol. 1999, 75, 1177–1188. [Google Scholar] [CrossRef]
- Nicoletti, V.G.; Santoro, A.M.; Grasso, G.; Vagliasindi, L.I.; Giuffrida, M.L.; Cuppari, C.; Purrello, V.S.; Stella, A.M.; Rizzarelli, E. Carnosine interaction with nitric oxide and astroglial cell protection. J. Neurosci. Res. 2007, 85, 2239–2245. [Google Scholar] [CrossRef]
- Fontana, M.; Pinnen, F.; Lucente, G.; Pecci, L. Prevention of peroxynitrite-dependent damage by carnosine and related sulphonamido pseudodipeptides. Cell. Mol. Life Sci. 2002, 59, 546–551. [Google Scholar] [CrossRef]
- Pattison, D.I.; Davies, M.J. Evidence for rapid inter- and intramolecular chlorine transfer reactions of histamine and carnosine chloramines: Implications for the prevention of hypochlorous-acid-mediated damage. Biochemistry 2006, 45, 8152–8162. [Google Scholar] [CrossRef]
- Yanai, N.; Shiotani, S.; Hagiwara, S.; Nabetani, H.; Nakajima, M. Antioxidant combination inhibits reactive oxygen species mediated damage. Biosci. Biotechnol. Biochem. 2008, 72, 3100–3106. [Google Scholar] [CrossRef] [Green Version]
- Abdelkader, H.; Longman, M.; Alany, R.G.; Pierscionek, B. On the Anticataractogenic Effects of L-Carnosine: Is It Best Described as an Antioxidant, Metal-Chelating Agent or Glycation Inhibitor? Oxid. Med. Cell. Longev. 2016, 2016, 3240261. [Google Scholar] [CrossRef] [Green Version]
- Kakihana, Y.; Kasamatsu, S.; Uchida, K.; Ihara, H. Distribution and quantitative analysis of homoanserine and its 2-oxo derivative in mouse tissues. Free Radic. Res. 2021, in press. [Google Scholar] [CrossRef] [PubMed]
- Abramovic, H.; Grobin, B.; Poklar, N.U.; Cigic, B. The Methodology Applied in DPPH, ABTS and Folin-Ciocalteau Assays Has a Large Influence on the Determined Antioxidant Potential. Acta Chim. Slov. 2017, 64, 491–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, F.; Xu, T.; Lu, B.; Liu, R. Guidelines for antioxidant assays for food components. Food Front. 2020, 1, 60–69. [Google Scholar] [CrossRef] [Green Version]
- Canabady-Rochelle, L.L.; Harscoat-Schiavo, C.; Kessler, V.; Aymes, A.; Fournier, F.; Girardet, J.M. Determination of reducing power and metal chelating ability of antioxidant peptides: Revisited methods. Food Chem. 2015, 183, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.A.; Sawa, T.; Ihara, H.; Kasamatsu, S.; Yoshitake, J.; Rahaman, M.M.; Okamoto, T.; Fujii, S.; Akaike, T. Regulation by mitochondrial superoxide and NADPH oxidase of cellular formation of nitrated cyclic GMP: Potential implications for ROS signalling. Biochem. J. 2012, 441, 719–730. [Google Scholar] [CrossRef] [Green Version]
- Koppenol, W.H.; Kissner, R.; Beckman, J.S. Syntheses of peroxynitrite: To go with the flow or on solid grounds? Methods Enzymol. 1996, 269, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Navarro, R.R.; Ichikawa, H.; Tatsumi, K. Fenton-based treatment of electroless copper plating waste for organics mineralization and CuO recovery. Green Chem. 2019, 21, 2273–2278. [Google Scholar] [CrossRef]
- Qiang, Z.; Adams, C. Potentiometric determination of acid dissociation constants (pKa) for human and veterinary antibiotics. Water Res. 2004, 38, 2874–2890. [Google Scholar] [CrossRef]
- Zhao, J.; Posa, D.K.; Kumar, V.; Hoetker, D.; Kumar, A.; Ganesan, S.; Riggs, D.W.; Bhatnagar, A.; Wempe, M.F.; Baba, S.P. Carnosine protects cardiac myocytes against lipid peroxidation products. Amino Acids 2019, 51, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Watanabe, R.; Kato, T.; Seko, T.; Matsuda, T.; Omura, Y.; Shigemura, Y.; Kawabata, Y.; Maegawa, T. Isolation of balenine from opah (Lampris megalopsis) muscle and comparison of antioxidant and iron-chelating activities with other major imidazole dipeptides. Food Chem. 2021, 364, 130343. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Ozyurek, M.; Guclu, K.; Capanoglu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef]
- Apak, R.; Ozyurek, M.; Guclu, K.; Capanoglu, E. Antioxidant Activity/Capacity Measurement. 2. Hydrogen Atom Transfer (HAT)-Based, Mixed-Mode (Electron Transfer (ET)/HAT), and Lipid Peroxidation Assays. J. Agric. Food Chem. 2016, 64, 1028–1045. [Google Scholar] [CrossRef]
- Koskenkorva-Frank, T.S.; Weiss, G.; Koppenol, W.H.; Burckhardt, S. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: Insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radic. Biol. Med. 2013, 65, 1174–1194. [Google Scholar] [CrossRef]
- Dobbie, H.; Kermack, W.O. Complex-formation between polypeptides and metals. 2. The reaction between cupric ions and some dipeptides. Biochem. J. 1955, 59, 246–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenz, G.R.; Martell, A.E. Metal Complexes of Carnosine. Biochemistry 1964, 3, 750–753. [Google Scholar] [CrossRef]
- Torreggiani, A.; Tamba, M.; Fini, G. Binding of copper(II) to carnosine: Raman and IR spectroscopic study. Biopolymers 2000, 57, 149–159. [Google Scholar] [CrossRef]
- Torreggiani, A.; Bonora, S.; Fini, G. Raman and IR spectroscopic investigation of zinc(II)-carnosine complexes. Biopolymers 2000, 57, 352–364. [Google Scholar] [CrossRef]
- Marusic, N.; Aristoy, M.C.; Toldra, F. Nutritional pork meat compounds as affected by ham dry-curing. Meat Sci. 2013, 93, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Kohen, R.; Yamamoto, Y.; Cundy, K.C.; Ames, B.N. Antioxidant activity of carnosine, homocarnosine, and anserine present in muscle and brain. Proc. Natl. Acad. Sci. USA 1988, 85, 3175–3179. [Google Scholar] [CrossRef] [Green Version]
- Peters, V.; Calabrese, V.; Forsberg, E.; Volk, N.; Fleming, T.; Baelde, H.; Weigand, T.; Thiel, C.; Trovato, A.; Scuto, M.; et al. Protective Actions of Anserine Under Diabetic Conditions. Int. J. Mol. Sci. 2018, 19, 2751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, G. Important roles of dietary taurine, creatine, carnosine, anserine and 4-hydroxyproline in human nutrition and health. Amino Acids 2020, 52, 329–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aruoma, O.I.; Laughton, M.J.; Halliwell, B. Carnosine, homocarnosine and anserine: Could they act as antioxidants in vivo? Biochem. J. 1989, 264, 863–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; Dickinson, L.C.; Yang, L.; Decker, E.A. Identification of hydrazine in commercial preparations of carnosine and its influence on carnosine’s antioxidative properties. Anal. Biochem. 1998, 261, 79–86. [Google Scholar] [CrossRef]
- Fedorova, T.N.; Devyatov, A.A.; Berezhnoi, D.S.; Stvolinskii, S.L.; Morozova, M.P.; Gavrilova, S.A.; Tutelyan, V.A. Oxidative Status in Different Areas of the Cerebral Cortex of Wistar Rats during Focal Ischemia and Its Modulation with Carnosine. Bull. Exp. Biol. Med. 2018, 165, 746–750. [Google Scholar] [CrossRef]
- Kaneko, J.; Enya, A.; Enomoto, K.; Ding, Q.; Hisatsune, T. Anserine (beta-alanyl-3-methyl-L-histidine) improves neurovascular-unit dysfunction and spatial memory in aged AbetaPPswe/PSEN1dE9 Alzheimer’s-model mice. Sci. Rep. 2017, 7, 12571. [Google Scholar] [CrossRef] [Green Version]
- Boldyrev, A.; Fedorova, T.; Stepanova, M.; Dobrotvorskaya, I.; Kozlova, E.; Boldanova, N.; Bagyeva, G.; Ivanova-Smolenskaya, I.; Illarioshkin, S. Carnosine increases efficiency of DOPA therapy of Parkinson’s disease: A pilot study. Rejuvenation Res. 2008, 11, 821–827. [Google Scholar] [CrossRef]
- Elbarbary, N.S.; Ismail, E.A.R.; El-Naggar, A.R.; Hamouda, M.H.; El-Hamamsy, M. The effect of 12 weeks carnosine supplementation on renal functional integrity and oxidative stress in pediatric patients with diabetic nephropathy: A randomized placebo-controlled trial. Pediatr. Diabetes 2018, 19, 470–477. [Google Scholar] [CrossRef]
- Houjeghani, S.; Kheirouri, S.; Faraji, E.; Jafarabadi, M.A. L-Carnosine supplementation attenuated fasting glucose, triglycerides, advanced glycation end products, and tumor necrosis factor-alpha levels in patients with type 2 diabetes: A double-blind placebo-controlled randomized clinical trial. Nutr. Res. 2018, 49, 96–106. [Google Scholar] [CrossRef] [PubMed]
- Masuoka, N.; Lei, C.; Li, H.; Inamura, N.; Shiotani, S.; Yanai, N.; Sato, K.; Sakurai, K.; Hisatsune, T. Anserine, HClO-scavenger, protected against cognitive decline in individuals with mild cognitive impairment. Aging 2021, 13, 1729–1741. [Google Scholar] [CrossRef] [PubMed]
- Masuoka, N.; Yoshimine, C.; Hori, M.; Tanaka, M.; Asada, T.; Abe, K.; Hisatsune, T. Effects of Anserine/Carnosine Supplementation on Mild Cognitive Impairment with APOE4. Nutrients 2019, 11, 1626. [Google Scholar] [CrossRef] [Green Version]
- Chan, W.K.M.; Decker, E.A.; Lee, J.B.; Butterfield, D.A. EPR Spin-Trapping Studies of the Hydroxyl Radical Scavenging Activity of Carnosine and Related Dipeptides. J. Agric. Food Chem. 1994, 42, 1407–1410. [Google Scholar] [CrossRef]
- Uchida, K.; Kawakishi, S. Ascorbate-mediated specific oxidation of the imidazole ring in a histidine derivative. Bioorg. Chem. 1989, 17, 330–343. [Google Scholar] [CrossRef]
- Uchida, K.; Kawakishi, S. 2-Oxo-histidine as a novel biological marker for oxidatively modified proteins. FEBS Lett. 1993, 332, 208–210. [Google Scholar] [CrossRef] [Green Version]
- Schoneich, C.; Williams, T.D. Cu(II)-catalyzed oxidation of beta-amyloid peptide targets His13 and His14 over His6: Detection of 2-Oxo-histidine by HPLC-MS/MS. Chem. Res. Toxicol. 2002, 15, 717–722. [Google Scholar] [CrossRef]
- Srikanth, R.; Wilson, J.; Vachet, R.W. Correct identification of oxidized histidine residues using electron-transfer dissociation. J. Mass Spectrom. 2009, 44, 755–762. [Google Scholar] [CrossRef] [Green Version]
- Amano, M.; Kobayashi, N.; Yabuta, M.; Uchiyama, S.; Fukui, K. Detection of histidine oxidation in a monoclonal immunoglobulin gamma (IgG) 1 antibody. Anal. Chem. 2014, 86, 7536–7543. [Google Scholar] [CrossRef]
- Miyahara, Y.; Shintani, K.; Hayashihara-Kakuhou, K.; Zukawa, T.; Morita, Y.; Nakazawa, T.; Yoshida, T.; Ohkubo, T.; Uchiyama, S. Effect of UVC Irradiation on the Oxidation of Histidine in Monoclonal Antibodies. Sci. Rep. 2020, 10, 6333. [Google Scholar] [CrossRef] [PubMed]
- Pfister, F.; Riedl, E.; Wang, Q.; vom Hagen, F.; Deinzer, M.; Harmsen, M.C.; Molema, G.; Yard, B.; Feng, Y.; Hammes, H.P. Oral carnosine supplementation prevents vascular damage in experimental diabetic retinopathy. Cell. Physiol. Biochem. 2011, 28, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Jamshidzadeh, A.; Heidari, R.; Latifpour, Z.; Ommati, M.M.; Abdoli, N.; Mousavi, S.; Azarpira, N.; Zarei, A.; Zarei, M.; Asadi, B.; et al. Carnosine ameliorates liver fibrosis and hyperammonemia in cirrhotic rats. Clin. Res. Hepatol. Gastroenterol. 2017, 41, 424–434. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.H.; Liu, T.C.; Yin, M.C. Beneficial effects of histidine and carnosine on ethanol-induced chronic liver injury. Food Chem. Toxicol. 2008, 46, 1503–1509. [Google Scholar] [CrossRef]
- Sakae, K.; Yanagisawa, H. Oral treatment of pressure ulcers with polaprezinc (zinc L-carnosine complex): 8-week open-label trial. Biol. Trace Elem. Res. 2014, 158, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Tanigawa, K.; Kaneko, J.; Totsuka, M.; Katakura, Y.; Imabayashi, E.; Matsuda, H.; Hisatsune, T. Anserine/Carnosine Supplementation Preserves Blood Flow in the Prefrontal Brain of Elderly People Carrying APOE e4. Aging Dis. 2018, 9, 334–345. [Google Scholar] [CrossRef] [Green Version]
- Hisatsune, T.; Kaneko, J.; Kurashige, H.; Cao, Y.; Satsu, H.; Totsuka, M.; Katakura, Y.; Imabayashi, E.; Matsuda, H. Effect of Anserine/Carnosine Supplementation on Verbal Episodic Memory in Elderly People. J. Alzheimers Dis. 2016, 50, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Rokicki, J.; Li, L.; Imabayashi, E.; Kaneko, J.; Hisatsune, T.; Matsuda, H. Daily Carnosine and Anserine Supplementation Alters Verbal Episodic Memory and Resting State Network Connectivity in Healthy Elderly Adults. Front. Aging Neurosci. 2015, 7, 219. [Google Scholar] [CrossRef] [Green Version]
- Szczesniak, D.; Budzen, S.; Kopec, W.; Rymaszewska, J. Anserine and carnosine supplementation in the elderly: Effects on cognitive functioning and physical capacity. Arch. Gerontol. Geriatr. 2014, 59, 485–490. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasamatsu, S.; Komae, S.; Matsukura, K.; Kakihana, Y.; Uchida, K.; Ihara, H. 2-Oxo-Imidazole-Containing Dipeptides Play a Key Role in the Antioxidant Capacity of Imidazole-Containing Dipeptides. Antioxidants 2021, 10, 1434. https://doi.org/10.3390/antiox10091434
Kasamatsu S, Komae S, Matsukura K, Kakihana Y, Uchida K, Ihara H. 2-Oxo-Imidazole-Containing Dipeptides Play a Key Role in the Antioxidant Capacity of Imidazole-Containing Dipeptides. Antioxidants. 2021; 10(9):1434. https://doi.org/10.3390/antiox10091434
Chicago/Turabian StyleKasamatsu, Shingo, Somei Komae, Kana Matsukura, Yuki Kakihana, Koji Uchida, and Hideshi Ihara. 2021. "2-Oxo-Imidazole-Containing Dipeptides Play a Key Role in the Antioxidant Capacity of Imidazole-Containing Dipeptides" Antioxidants 10, no. 9: 1434. https://doi.org/10.3390/antiox10091434
APA StyleKasamatsu, S., Komae, S., Matsukura, K., Kakihana, Y., Uchida, K., & Ihara, H. (2021). 2-Oxo-Imidazole-Containing Dipeptides Play a Key Role in the Antioxidant Capacity of Imidazole-Containing Dipeptides. Antioxidants, 10(9), 1434. https://doi.org/10.3390/antiox10091434