Synergy of the Inhibitory Action of Polyphenols Plus Vitamin C on Amyloid Fibril Formation: Case Study of Human Stefin B
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Protein Expression and Purification
2.3. ThT Fluorescence
2.4. Transmission Electron Microscopy (TEM)
3. Results
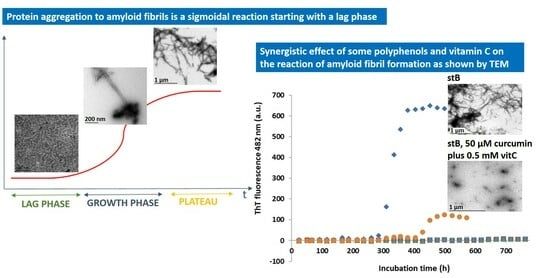
3.1. Kinetics of the Fibrillation by stB in Presence of Curcumin and Vitamin C and Morphologic Characterization of the Fibrils at the Plateau Phase
3.2. Kinetics of the Fibrillation Reaction by stB in Presence of Resveratrol and Vitamin C and Morphology of the Fibrils at the Plateau Phase
3.3. Kinetics of the Fibrillation by stB in Presence of Quercetin and Vitamin C and Morphologic Characterization of the Fibrils at the Plateau
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dobson, C.M. Getting out of shape. Nature 2002, 418, 729–730. [Google Scholar] [CrossRef]
- Zerovnik, E.; Pompe-Novak, M.; Skarabot, M.; Ravnikar, M.; Musevic, I.; Turk, V. Human stefin B readily forms amyloid fibrils in vitro. Biochim. Biophys. Acta 2002, 1594, 1–5. [Google Scholar] [CrossRef]
- Zerovnik, E.; Turk, V.; Waltho, J.P. Amyloid fibril formation by human stefin B: Influence of the initial pH-induced intermediate state. Biochem. Soc. Trans. 2002, 30, 543–547. [Google Scholar] [CrossRef]
- Zerovnik, E.; Skarabot, M.; Skerget, K.; Giannini, S.; Stoka, V.; Jenko-Kokalj, S.; Staniforth, R.A. Amyloid fibril formation by human stefin B: Influence of pH and TFE on fibril growth and morphology. Amyloid 2007, 14, 237–247. [Google Scholar] [CrossRef]
- Taler-Vercic, A.; Kirsipuu, T.; Friedemann, M.; Noormagi, A.; Polajnar, M.; Smirnova, J.; Znidaric, M.T.; Zganec, M.; Skarabot, M.; Vilfan, A.; et al. The role of initial oligomers in amyloid fibril formation by human stefin B. Int. J. Mol. Sci. 2013, 14, 18362–18384. [Google Scholar] [CrossRef] [PubMed]
- Jenko Kokalj, S.; Guncar, G.; Stern, I.; Morgan, G.; Rabzelj, S.; Kenig, M.; Staniforth, R.A.; Waltho, J.P.; Zerovnik, E.; Turk, D. Essential role of proline isomerization in stefin B tetramer formation. J. Mol. Biol 2007, 366, 1569–1579. [Google Scholar] [CrossRef]
- Hasanbasic, S.; Taler-Vercic, A.; Puizdar, V.; Stoka, V.; Tusek Znidaric, M.; Vilfan, A.; Berbic, S.; Zerovnik, E. Prolines Affect the Nucleation Phase of Amyloid Fibrillation Reaction; Mutational Analysis of Human Stefin B. ACS Chem. Neurosci. 2019, 10, 2730–2740. [Google Scholar] [CrossRef]
- Ono, K.; Hasegawa, K.; Naiki, H.; Yamada, M. Curcumin has potent anti-amyloidogenic effects for Alzheimer’s beta-amyloid fibrils in vitro. J. Neurosci. Res. 2004, 75, 742–750. [Google Scholar] [CrossRef]
- Porat, Y.; Abramowitz, A.; Gazit, E. Inhibition of amyloid fibril formation by polyphenols: Structural similarity and aromatic interactions as a common inhibition mechanism. Chem. Biol. Drug Des. 2006, 67, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.P.; Mohamed, T.; Teckwani, K.; Tin, G. Curcumin Binding to Beta Amyloid: A Computational Study. Chem. Biol. Drug Des. 2015, 86, 813–820. [Google Scholar] [CrossRef]
- Yang, F.; Lim, G.; Begum, A.; Ubeda, O.; Simmons, M.; Ambegaokar, S.; Chen, P.; Kayed, R.; Glabe, C.; Frautschy, S.; et al. Curcumin Inhibits Formation of Amyloid Oligomers and Fibrils, Binds Plaques, and Reduces Amyloid in Vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef]
- Reddy, P.H.; Manczak, M.; Yin, X.; Grady, M.C.; Mitchell, A.; Tonk, S.; Kuruva, C.S.; Bhatti, J.S.; Kandimalla, R.; Vijayan, M.; et al. Protective Effects of Indian Spice Curcumin Against Amyloid-β in Alzheimer’s Disease. J. Alzheimers Dis. 2018, 61, 843–866. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Kotia, V.; Ghosh, D.; Mohite, G.M.; Kumar, A.; Maji, S.K. Curcumin Modulates α-Synuclein Aggregation and Toxicity. ACS Chem. Neurosci. 2013, 4, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.F.; Yu, K.H.; Jheng, C.P.; Chung, R.; Lee, C.I. Curcumin reduces amyloid fibrillation of prion protein and decreases reactive oxidative stress. Pathogens 2013, 2, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.H.; Lim, S.S.; Yuen, K.H.; Lee, C.Y. Curcumin and a hemi-analogue with improved blood-brain barrier permeability protect against amyloid-beta toxicity in Caenorhabditis elegans via SKN-1/Nrf activation. J. Pharm. Pharmacol. 2019, 71, 860–868. [Google Scholar] [CrossRef]
- Tsai, Y.-M.; Chien, C.-F.; Lin, L.-C.; Tsai, T.-H. Curcumin and its nano-formulation: The kinetics of tissue distribution & blood-brain-barrier penetration. Int. J. Pharmaceut. 2011, 416, 331–338. [Google Scholar]
- Lo Cascio, F.; Puangmalai, N.; Ellsworth, A.; Bucchieri, F.; Pace, A.; Palumbo Piccionello, A.; Kayed, R. Toxic Tau Oligomers Modulated by Novel Curcumin Derivatives. Sci. Rep. 2019, 9, 19011. [Google Scholar] [CrossRef]
- Caruana, M.; Neuner, J.; Hogen, T.; Schmidt, F.; Kamp, F.; Scerri, C.; Giese, A.; Vassallo, N. Polyphenolic compounds are novel protective agents against lipid membrane damage by alpha-synuclein aggregates in vitro. Biochim. Biophys. Acta 2012, 1818, 2502–2510. [Google Scholar] [CrossRef]
- Scarmeas, N.; Stern, Y.; Mayeux, R.; Luchsinger, J.A. Mediterranean diet, Alzheimer disease, and vascular mediation. Arch. Neurol. 2006, 63, 1709–1717. [Google Scholar] [CrossRef] [PubMed]
- Tangney, C.C.; Kwasny, M.J.; Li, H.; Wilson, R.S.; Evans, D.A.; Morris, M.C. Adherence to a Mediterranean-type dietary pattern and cognitive decline in a community population. Am. J. Clin. Nutr. 2011, 93, 601–607. [Google Scholar] [CrossRef]
- Quideau, S. Plant “polyphenolic” small molecules can induce a calorie restriction-mimetic life-span extension by activating sirtuins: Will “polyphenols” someday be used as chemotherapeutic drugs in Western medicine? Chembiochem 2004, 5, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Viviane, L.; Ndam, N.; Jan, S.; Paul, F.M.; Hermann, S. Natural polyphenols binding to amyloid: A broad class of compounds to treat different human amyloid diseases. Mol. Nutr. Food Res. 2015, 59, 8–20. [Google Scholar] [CrossRef]
- Shariatizi, S.; Meratan, A.A.; Ghasemi, A.; Nemat-Gorgani, M. Inhibition of amyloid fibrillation and cytotoxicity of lysozyme fibrillation products by polyphenols. Int. J. Biol. Macromol. 2015, 80, 95–106. [Google Scholar] [CrossRef]
- Leri, M.; Chaudhary, H.; Iashchishyn, I.A.; Pansieri, J.; Svedružić, Ž.M.; Gómez Alcalde, S.; Musteikyte, G.; Smirnovas, V.; Stefani, M.; Bucciantini, M.; et al. Natural Compound from Olive Oil Inhibits S100A9 Amyloid Formation and Cytotoxicity: Implications for Preventing Alzheimer’s Disease. ACS Chem. Neurosci. 2021, 12, 1905–1918. [Google Scholar] [CrossRef]
- Kook, S.Y.; Lee, K.M.; Kim, Y.; Cha, M.Y.; Kang, S.; Baik, S.H.; Lee, H.; Park, R.; Mook-Jung, I. High-dose of vitamin C supplementation reduces amyloid plaque burden and ameliorates pathological changes in the brain of 5XFAD mice. Cell Death Dis. 2014, 5, e1083. [Google Scholar] [CrossRef]
- Jahić, A.; Žnidarič, M.T.; Pintar, S.; Berbić, S.; Žerovnik, E. The effect of three polyphenols and some other antioxidant substances on amyloid fibril formation by Human cystatin C. Neurochem. Int. 2020, 140, 104806. [Google Scholar] [CrossRef]
- Hasanbasic, S.; Jahic, A.; Berbic, S.; Znidaric, M.T.; Zerovnik, E. Inhibition of Protein Aggregation by Several Antioxidants. Oxid. Med. Cell. Longev. 2018, 2018, 8613209. [Google Scholar] [CrossRef]
- Zerovnik, E.; Skerget, K.; Tusek-Znidaric, M.; Loeschner, C.; Brazier, M.W.; Brown, D.R. High affinity copper binding by stefin B (cystatin B) and its role in the inhibition of amyloid fibrillation. FEBS J. 2006, 273, 4250–4263. [Google Scholar] [CrossRef]
- Rabzelj, S.; Turk, V.; Zerovnik, E. In vitro study of stability and amyloid-fibril formation of two mutants of human stefin B (cystatin B) occurring in patients with EPM1. Protein Sci. 2005, 14, 2713–2722. [Google Scholar] [CrossRef]
- Ladiwala, A.R.; Lin, J.C.; Bale, S.S.; Marcelino-Cruz, A.M.; Bhattacharya, M.; Dordick, J.S.; Tessier, P.M. Resveratrol selectively remodels soluble oligomers and fibrils of amyloid Abeta into off-pathway conformers. J. Biol. Chem. 2010, 285, 24228–24237. [Google Scholar] [CrossRef] [PubMed]
- Ladiwala, A.R.; Dordick, J.S.; Tessier, P.M. Aromatic small molecules remodel toxic soluble oligomers of amyloid beta through three independent pathways. J. Biol. Chem. 2011, 286, 3209–3218. [Google Scholar] [CrossRef] [PubMed]
- Ceru, S.; Konjar, S.; Maher, K.; Repnik, U.; Krizaj, I.; Bencina, M.; Renko, M.; Nepveu, A.; Zerovnik, E.; Turk, B.; et al. Stefin B interacts with histones and cathepsin L in the nucleus. J. Biol. Chem. 2010, 285, 10078–10086. [Google Scholar] [CrossRef]
- Cipollini, E.; Riccio, M.; Di Giaimo, R.; Dal Piaz, F.; Pulice, G.; Catania, S.; Caldarelli, I.; Dembic, M.; Santi, S.; Melli, M. Cystatin B and its EPM1 mutants are polymeric and aggregate prone in vivo. Biochim. Biophys. Acta 2008, 1783, 312–322. [Google Scholar] [CrossRef]
- Di Giaimo, R.; Riccio, M.; Santi, S.; Galeotti, C.; Ambrosetti, D.C.; Melli, M. New insights into the molecular basis of progressive myoclonus epilepsy: A multiprotein complex with cystatin B. Hum. Mol. Genet. 2002, 11, 2941–2950. [Google Scholar] [CrossRef]
- Skerget, K.; Taler-Vercic, A.; Bavdek, A.; Hodnik, V.; Ceru, S.; Tusek-Znidaric, M.; Kumm, T.; Pitsi, D.; Pompe-Novak, M.; Palumaa, P.; et al. Interaction between oligomers of stefin B and amyloid-beta in vitro and in cells. J. Biol. Chem. 2010, 285, 3201–3210. [Google Scholar] [CrossRef]
- Žerovnik, E. Putative alternative functions of human stefin B (cystatin B): Binding to amyloid-beta, membranes, and copper. J. Mol. Recognit. 2017, 30, e2562. [Google Scholar] [CrossRef] [PubMed]
- Žerovnik, E. Co-chaperoning by amyloid-forming proteins: Cystatins vs. crystallins. Eur. Biophys. J. EBJ 2017, 46, 789–793. [Google Scholar] [CrossRef] [PubMed]
- Penna, E.; Cerciello, A.; Chambery, A.; Russo, R.; Cernilogar, F.M.; Pedone, E.M.; Perrone-Capano, C.; Cappello, S.; Di Giaimo, R.; Crispino, M. Cystatin B Involvement in Synapse Physiology of Rodent Brains and Human Cerebral Organoids. Front. Mol. Neurosci. 2019, 12, 195. [Google Scholar] [CrossRef]
- Di Matteo, F.; Pipicelli, F.; Kyrousi, C.; Tovecci, I.; Penna, E.; Crispino, M.; Chambery, A.; Russo, R.; Ayo-Martin, A.C.; Giordano, M.; et al. Cystatin B is essential for proliferation and interneuron migration in individuals with EPM1 epilepsy. EMBO Mol. Med. 2020, 12, e11419. [Google Scholar] [CrossRef]
- Lalioti, M.D.; Mirotsou, M.; Buresi, C.; Peitsch, M.C.; Rossier, C.; Ouazzani, R.; Baldy-Moulinier, M.; Bottani, A.; Malafosse, A.; Antonarakis, S.E. Identification of mutations in cystatin B, the gene responsible for the Unverricht-Lundborg type of progressive myoclonus epilepsy (EPM1). Am. J. Hum. Genet. 1997, 60, 342–351. [Google Scholar]
- Pennacchio, L.A.; Lehesjoki, A.E.; Stone, N.E.; Willour, V.L.; Virtaneva, K.; Miao, J.; D’Amato, E.; Ramirez, L.; Faham, M.; Koskiniemi, M.; et al. Mutations in the gene encoding cystatin B in progressive myoclonus epilepsy (EPM1). Science 1996, 271, 1731–1734. [Google Scholar] [CrossRef] [PubMed]
- Lalioti, M.D.; Scott, H.S.; Buresi, C.; Rossier, C.; Bottani, A.; Morris, M.A.; Malafosse, A.; Antonarakis, S.E. Dodecamer repeat expansion in cystatin B gene in progressive myoclonus epilepsy. Nature 1997, 386, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Joensuu, T.; Lehesjoki, A.E.; Kopra, O. Molecular background of EPM1-Unverricht-Lundborg disease. Epilepsia 2008, 49, 557–563. [Google Scholar] [CrossRef]
- Polajnar, M.; Ceru, S.; Kopitar-Jerala, N.; Zerovnik, E. Human stefin B normal and patho-physiological role: Molecular and cellular aspects of amyloid-type aggregation of certain EPM1 mutants. Front. Mol. Neurosci. 2012, 5, 88. [Google Scholar] [CrossRef] [PubMed]
- Polajnar, M.; Zerovnik, E. Impaired autophagy: A link between neurodegenerative and neuropsychiatric diseases. J. Cell. Mol. Med. 2014, 18, 1705–1711. [Google Scholar] [CrossRef]
- Polajnar, M.; Zerovnik, E. Impaired autophagy: A link between neurodegenerative diseases and progressive myoclonus epilepsies. Trends Mol. Med. 2011, 17, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Palsdottir, A.; Snorradottir, A.O.; Thorsteinsson, L. Hereditary cystatin C amyloid angiopathy: Genetic, clinical, and pathological aspects. Brain Pathol. 2006, 16, 55–59. [Google Scholar]
- Mi, W.; Jung, S.S.; Yu, H.; Schmidt, S.D.; Nixon, R.A.; Mathews, P.M.; Tagliavini, F.; Levy, E. Complexes of amyloid-beta and cystatin C in the human central nervous system. J. Alzheimer’s Dis. JAD 2009, 18, 273–280. [Google Scholar] [CrossRef]
- Mi, W.; Pawlik, M.; Sastre, M.; Jung, S.S.; Radvinsky, D.S.; Klein, A.M.; Sommer, J.; Schmidt, S.D.; Nixon, R.A.; Mathews, P.M.; et al. Cystatin C inhibits amyloid-beta deposition in Alzheimer’s disease mouse models. Nat. Genet. 2007, 39, 1440–1442. [Google Scholar] [CrossRef]
- Sastre, M.; Calero, M.; Pawlik, M.; Mathews, P.M.; Kumar, A.; Danilov, V.; Schmidt, S.D.; Nixon, R.A.; Frangione, B.; Levy, E. Binding of cystatin C to Alzheimer’s amyloid beta inhibits in vitro amyloid fibril formation. Neurobiol. Aging 2004, 25, 1033–1043. [Google Scholar] [CrossRef]
- Perez-Gonzalez, R.; Sahoo, S.; Gauthier, S.A.; Kim, Y.; Li, M.; Kumar, A.; Pawlik, M.; Benussi, L.; Ghidoni, R.; Levy, E. Neuroprotection mediated by cystatin C-loaded extracellular vesicles. Sci. Rep. 2019, 9, 11104. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jahić Mujkić, A.; Tušek Žnidarič, M.; Berbić, S.; Žerovnik, E. Synergy of the Inhibitory Action of Polyphenols Plus Vitamin C on Amyloid Fibril Formation: Case Study of Human Stefin B. Antioxidants 2021, 10, 1471. https://doi.org/10.3390/antiox10091471
Jahić Mujkić A, Tušek Žnidarič M, Berbić S, Žerovnik E. Synergy of the Inhibitory Action of Polyphenols Plus Vitamin C on Amyloid Fibril Formation: Case Study of Human Stefin B. Antioxidants. 2021; 10(9):1471. https://doi.org/10.3390/antiox10091471
Chicago/Turabian StyleJahić Mujkić, Alma, Magda Tušek Žnidarič, Selma Berbić, and Eva Žerovnik. 2021. "Synergy of the Inhibitory Action of Polyphenols Plus Vitamin C on Amyloid Fibril Formation: Case Study of Human Stefin B" Antioxidants 10, no. 9: 1471. https://doi.org/10.3390/antiox10091471
APA StyleJahić Mujkić, A., Tušek Žnidarič, M., Berbić, S., & Žerovnik, E. (2021). Synergy of the Inhibitory Action of Polyphenols Plus Vitamin C on Amyloid Fibril Formation: Case Study of Human Stefin B. Antioxidants, 10(9), 1471. https://doi.org/10.3390/antiox10091471