On the Antioxidant Properties of L-Kynurenine: An Efficient ROS Scavenger and Enhancer of Rat Brain Antioxidant Defense
Abstract
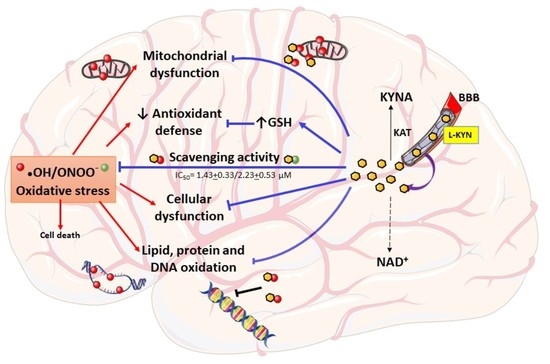
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Animals
2.3. Chemical Combinatory Assays
2.3.1. Superoxide Radicals Scavenging Assessment
2.3.2. Hydroxyl Radical Scavenging Assay
2.3.3. ONOO− Scavenging Activity Assessment
2.3.4. Hydrogen Peroxide Assay
2.3.5. •OH-Mediated Protein Degradation
2.3.6. •OH-Mediated DNA Degradation
2.4. In Vitro Assays
2.4.1. In Vitro Incubation
2.4.2. Determination of ROS Levels in Homogenates
2.4.3. Determination of Lipid Peroxidation (LP) in Homogenates
2.4.4. Determination of Cellular Function in Homogenates
2.5. Ex Vivo Assays
2.5.1. L-KYN Administration and Ex Vivo Incubation with Pro-Oxidants
2.5.2. GSH and GSSG Content
2.5.3. Glutathione Reductase (GR) Activity
2.5.4. Glutathione Peroxidase (GPx) Activity
2.5.5. Kynurenine Levels
2.5.6. Protein Assay
2.5.7. Statistical Analysis
3. Results
3.1. Scavenging Activity of L-KYN in Combinatorial Chemistry Assays
3.2. Effect of L-KYN on DNA and Protein Oxidative Degradation
3.3. Effect of L-KYN on ROS Production Induced by FeSO4 in Rat Brain Homogenates
3.4. Effect of L-KYN on Lipid Peroxidation and Cellular Dysfunction Induced by the Pro-Oxidant FeSO4 in Rat Brain Homogenates
3.5. Effect of Sub-Chronic Administration of L-KYN In Vivo on Redox Modulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beadle, G.W.; Mitchell, H.K.; Nyc, J.F. Kynurenine as an Intermediate in the Formation of Nicotinic Acid from Tryptophane by Neurospora. Proc. Natl. Acad. Sci. USA 1947, 33, 155–158. [Google Scholar] [CrossRef] [Green Version]
- Ciapala, K.; Mika, J.; Rojewska, E. The Kynurenine Pathway as a Potential Target for Neuropathic Pain Therapy Design: From Basic Research to Clinical Perspectives. Int. J. Mol. Sci. 2021, 22, 1055. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Iwahashi, H.; Sugata, R.; Kido, R. Oxidation of 3-hydroxykynurenine catalyzed by methemoglobin with hydrogen peroxide. Free Radic. Biol. Med. 1992, 13, 17–20. [Google Scholar] [CrossRef]
- Iwahashi, H.; Kawamori, H.; Fukushima, K. Quinolinic acid, alpha-picolinic acid, fusaric acid, and 2,6-pyridinedicarboxylic acid enhance the Fenton reaction in phosphate buffer. Chem. Biol. Interact. 1999, 118, 201–215. [Google Scholar] [CrossRef]
- Platenik, J.; Stopka, P.; Vejrazka, M.; Stipek, S. Quinolinic acid-iron(ii) complexes: Slow autoxidation, but enhanced hydroxyl radical production in the Fenton reaction. Free Radic. Res. 2001, 34, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Lugo-Huitron, R.; Blanco-Ayala, T.; Ugalde-Muniz, P.; Carrillo-Mora, P.; Pedraza-Chaverri, J.; Silva-Adaya, D.; Maldonado, P.D.; Torres, I.; Pinzon, E.; Ortiz-Islas, E.; et al. On the antioxidant properties of kynurenic acid: Free radical scavenging activity and inhibition of oxidative stress. Neurotoxicol. Teratol. 2011, 33, 538–547. [Google Scholar] [CrossRef]
- Pocivavsek, A.; Wu, H.Q.; Potter, M.C.; Elmer, G.I.; Pellicciari, R.; Schwarcz, R. Fluctuations in endogenous kynurenic acid control hippocampal glutamate and memory. Neuropsychopharmacology 2011, 36, 2357–2367. [Google Scholar] [CrossRef] [PubMed]
- Pocivavsek, A.; Thomas, M.A.; Elmer, G.I.; Bruno, J.P.; Schwarcz, R. Continuous kynurenine administration during the prenatal period, but not during adolescence, causes learning and memory deficits in adult rats. Psychopharmacology 2014, 231, 2799–2809. [Google Scholar] [CrossRef] [Green Version]
- Reyes-Ocampo, J.; Ramirez-Ortega, D.; Cervantes, G.I.; Pineda, B.; Balderas, P.M.; Gonzalez-Esquivel, D.; Sanchez-Chapul, L.; Lugo-Huitron, R.; Silva-Adaya, D.; Rios, C.; et al. Mitochondrial dysfunction related to cell damage induced by 3-hydroxykynurenine and 3-hydroxyanthranilic acid: Non-dependent-effect of early reactive oxygen species production. Neurotoxicology 2015, 50, 81–91. [Google Scholar] [CrossRef]
- Schwarcz, R.; Stone, T.W. The kynurenine pathway and the brain: Challenges, controversies and promises. Neuropharmacology 2017, 112, 237–247. [Google Scholar] [CrossRef] [Green Version]
- Goshima, N.; Wadano, A.; Miura, K. 3-Hydroxykynurenine as O2-. scavenger in the blowfly, Aldrichina grahami. Biochem. Biophys. Res. Commun. 1986, 139, 666–672. [Google Scholar] [CrossRef]
- Tanaka, M.; Toth, F.; Polyak, H.; Szabo, A.; Mandi, Y.; Vecsei, L. Immune Influencers in Action: Metabolites and Enzymes of the Tryptophan-Kynurenine Metabolic Pathway. Biomedicines 2021, 9, 734. [Google Scholar] [CrossRef]
- Knox, W.E.; Mehler, A.H. The conversion of tryptophan to kynurenine in liver. I. The coupled tryptophan peroxidase-oxidase system forming formylkynurenine. J. Biol. Chem. 1950, 187, 419–430. [Google Scholar] [CrossRef]
- Ruddick, J.P.; Evans, A.K.; Nutt, D.J.; Lightman, S.L.; Rook, G.A.; Lowry, C.A. Tryptophan metabolism in the central nervous system: Medical implications. Expert Rev. Mol. Med. 2006, 8, 1–27. [Google Scholar] [CrossRef] [PubMed]
- Gal, E.M.; Sherman, A.D. L-kynurenine: Its synthesis and possible regulatory function in brain. Neurochem. Res. 1980, 5, 223–239. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, K.; Beal, M.F. Neuroprotective effects of L-kynurenine on hypoxia-ischemia and NMDA lesions in neonatal rats. J. Cereb. Blood Flow Metab. 1992, 12, 400–407. [Google Scholar] [CrossRef]
- Robotka, H.; Sas, K.; Agoston, M.; Rozsa, E.; Szenasi, G.; Gigler, G.; Vecsei, L.; Toldi, J. Neuroprotection achieved in the ischaemic rat cortex with L-kynurenine sulphate. Life Sci. 2008, 82, 915–919. [Google Scholar] [CrossRef]
- Gigler, G.; Szenasi, G.; Simo, A.; Levay, G.; Harsing, L.G., Jr.; Sas, K.; Vecsei, L.; Toldi, J. Neuroprotective effect of L-kynurenine sulfate administered before focal cerebral ischemia in mice and global cerebral ischemia in gerbils. Eur. J. Pharmacol. 2007, 564, 116–122. [Google Scholar] [CrossRef]
- Sas, K.; Robotka, H.; Rozsa, E.; Agoston, M.; Szenasi, G.; Gigler, G.; Marosi, M.; Kis, Z.; Farkas, T.; Vecsei, L.; et al. Kynurenine diminishes the ischemia-induced histological and electrophysiological deficits in the rat hippocampus. Neurobiol. Dis. 2008, 32, 302–308. [Google Scholar] [CrossRef]
- Carrillo-Mora, P.; Mendez-Cuesta, L.A.; Perez-De La Cruz, V.; Fortoul-van Der Goes, T.I.; Santamaria, A. Protective effect of systemic L-kynurenine and probenecid administration on behavioural and morphological alterations induced by toxic soluble amyloid beta (25–35) in rat hippocampus. Behav. Brain Res. 2010, 210, 240–250. [Google Scholar] [CrossRef]
- Vecsei, L.; Beal, M.F. Intracerebroventricular injection of kynurenic acid, but not kynurenine, induces ataxia and stereotyped behavior in rats. Brain Res. Bull. 1990, 25, 623–627. [Google Scholar] [CrossRef]
- Miranda, A.F.; Sutton, M.A.; Beninger, R.J.; Jhamandas, K.; Boegman, R.J. Quinolinic acid lesion of the nigrostriatal pathway: Effect on turning behaviour and protection by elevation of endogenous kynurenic acid in Rattus norvegicus. Neurosci. Lett. 1999, 262, 81–84. [Google Scholar] [CrossRef]
- Santamaria, A.; Rios, C.; Solis-Hernandez, F.; Ordaz-Moreno, J.; Gonzalez-Reynoso, L.; Altagracia, M.; Kravzov, J. Systemic DL-kynurenine and probenecid pretreatment attenuates quinolinic acid-induced neurotoxicity in rats. Neuropharmacology 1996, 35, 23–28. [Google Scholar] [CrossRef]
- Silva-Adaya, D.; Perez-De La Cruz, V.; Villeda-Hernandez, J.; Carrillo-Mora, P.; Gonzalez-Herrera, I.G.; Garcia, E.; Colin-Barenque, L.; Pedraza-Chaverri, J.; Santamaria, A. Protective effect of L-kynurenine and probenecid on 6-hydroxydopamine-induced striatal toxicity in rats: Implications of modulating kynurenate as a protective strategy. Neurotoxicol. Teratol. 2011, 33, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Giles, G.I.; Collins, C.A.; Stone, T.W.; Jacob, C. Electrochemical and in vitro evaluation of the redox-properties of kynurenine species. Biochem. Biophys. Res. Commun. 2003, 300, 719–724. [Google Scholar] [CrossRef]
- Weiss, G.; Díez-Ruiz, A.; Murr, C.; Theur, I.; Fuchs, D. Tryptophan Metabolites as Scavengers of Reactive Oxygen and Chlorine Species. Pteridines 2002, 13, 140–144. [Google Scholar] [CrossRef]
- Fontana, M.; Mosca, L.; Rosei, M.A. Interaction of enkephalins with oxyradicals. Biochem. Pharmacol. 2001, 61, 1253–1257. [Google Scholar] [CrossRef]
- Floriano-Sanchez, E.; Villanueva, C.; Medina-Campos, O.N.; Rocha, D.; Sanchez-Gonzalez, D.J.; Cardenas-Rodriguez, N.; Pedraza-Chaverri, J. Nordihydroguaiaretic acid is a potent in vitro scavenger of peroxynitrite, singlet oxygen, hydroxyl radical, superoxide anion and hypochlorous acid and prevents in vivo ozone-induced tyrosine nitration in lungs. Free Radic. Res. 2006, 40, 523–533. [Google Scholar] [CrossRef]
- Blanco Ayala, T.; Lugo Huitron, R.; Carmona Aparicio, L.; Ramirez Ortega, D.; Gonzalez Esquivel, D.; Pedraza Chaverri, J.; Perez de la Cruz, G.; Rios, C.; Schwarcz, R.; Perez de la Cruz, V. Alternative kynurenic acid synthesis routes studied in the rat cerebellum. Front. Cell. Neurosci. 2015, 9, 178. [Google Scholar] [CrossRef] [Green Version]
- Beckman, J.S.; Chen, J.; Ischiropoulos, H.; Crow, J.P. Oxidative chemistry of peroxynitrite. Methods Enzymol. 1994, 233, 229–240. [Google Scholar] [CrossRef]
- Crow, J.P.; Beckman, J.S. The importance of superoxide in nitric oxide-dependent toxicity: Evidence for peroxynitrite-mediated injury. Adv. Exp. Med. Biol. 1996, 387, 147–161. [Google Scholar] [CrossRef]
- Long, L.H.; Evans, P.J.; Halliwell, B. Hydrogen peroxide in human urine: Implications for antioxidant defense and redox regulation. Biochem. Biophys. Res. Commun. 1999, 262, 605–609. [Google Scholar] [CrossRef]
- Galano, A.; Macias-Ruvalcaba, N.A.; Medina Campos, O.N.; Pedraza-Chaverri, J. Mechanism of the OH radical scavenging activity of nordihydroguaiaretic acid: A combined theoretical and experimental study. J. Phys. Chem. B 2010, 114, 6625–6635. [Google Scholar] [CrossRef]
- Kocha, T.; Yamaguchi, M.; Ohtaki, H.; Fukuda, T.; Aoyagi, T. Hydrogen peroxide-mediated degradation of protein: Different oxidation modes of copper- and iron-dependent hydroxyl radicals on the degradation of albumin. Biochim. Biophys. Acta 1997, 1337, 319–326. [Google Scholar] [CrossRef]
- Blanco Ayala, T.B.; Ramirez Ortega, D.R.; Ovalle Rodriguez, P.O.; Pineda, B.; Perez de la Cruz, G.P.; Gonzalez Esquivel, D.G.; Schwarcz, R.; Sathyasaikumar, K.V.; Jimenez Anguiano, A.J.; Perez de la Cruz, V.P. Subchronic N-acetylcysteine Treatment Decreases Brain Kynurenic Acid Levels and Improves Cognitive Performance in Mice. Antioxidants 2021, 10, 147. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Berridge, M.V.; Tan, A.S. Characterization of the cellular reduction of 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT): Subcellular localization, substrate dependence, and involvement of mitochondrial electron transport in MTT reduction. Arch. Biochem. Biophys. 1993, 303, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Carvalho-Silva, M.; Gomes, L.M.; de Pra, S.D.; Wessler, L.B.; Schuck, P.F.; Scaini, G.; de Bem, A.F.; Blum-Silva, C.H.; Reginatto, F.H.; de Oliveira, J.; et al. Evidence of hippocampal astrogliosis and antioxidant imbalance after L-tyrosine chronic administration in rats. Metab. Brain Dis. 2020, 35, 193–200. [Google Scholar] [CrossRef]
- Santana-Martinez, R.A.; Silva-Islas, C.A.; Fernandez-Orihuela, Y.Y.; Barrera-Oviedo, D.; Pedraza-Chaverri, J.; Hernandez-Pando, R.; Maldonado, P.D. The Therapeutic Effect of Curcumin in Quinolinic Acid-Induced Neurotoxicity in Rats is Associated with BDNF, ERK1/2, Nrf2, and Antioxidant Enzymes. Antioxidants 2019, 8, 388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, H.R.; Nielsen, J.B.; Nielsen, F.; Grandjean, P. Antioxidative enzyme activities in human erythrocytes. Clin. Chem. 1997, 43, 562–568. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Sundaram, G.; Brew, B.J.; Jones, S.P.; Adams, S.; Lim, C.K.; Guillemin, G.J. Quinolinic acid toxicity on oligodendroglial cells: Relevance for multiple sclerosis and therapeutic strategies. J. Neuroinflamm. 2014, 11, 204. [Google Scholar] [CrossRef] [Green Version]
- Torok, N.; Tanaka, M.; Vecsei, L. Searching for Peripheral Biomarkers in Neurodegenerative Diseases: The Tryptophan-Kynurenine Metabolic Pathway. Int. J. Mol. Sci. 2020, 21, 9338. [Google Scholar] [CrossRef]
- Detrait, M.; Pesse, M.; Calissi, C.; Bouyon, S.; Brocard, J.; Vial, G.; Pepin, J.L.; Belaidi, E.; Arnaud, C. Short-term intermittent hypoxia induces simultaneous systemic insulin resistance and higher cardiac contractility in lean mice. Physiol. Rep. 2021, 9, e14738. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Chavez, L.A.; Lugo Huitron, R.; Gonzalez Esquivel, D.; Pineda, B.; Rios, C.; Silva-Adaya, D.; Sanchez-Chapul, L.; Roldan-Roldan, G.; Perez de la Cruz, V. Relevance of Alternative Routes of Kynurenic Acid Production in the Brain. Oxid. Med. Cell. Longev. 2018, 2018, 5272741. [Google Scholar] [CrossRef] [Green Version]
- Genestet, C.; Le Gouellec, A.; Chaker, H.; Polack, B.; Guery, B.; Toussaint, B.; Stasia, M.J. Scavenging of reactive oxygen species by tryptophan metabolites helps Pseudomonas aeruginosa escape neutrophil killing. Free Radic. Biol. Med. 2014, 73, 400–410. [Google Scholar] [CrossRef]
- Zhuravlev, A.V.; Zakharov, G.A.; Shchegolev, B.F.; Savvateeva-Popova, E.V. Antioxidant Properties of Kynurenines: Density Functional Theory Calculations. PLoS Comput. Biol. 2016, 12, e1005213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matuszak, Z.; Reszka, K.; Chignell, C.F. Reaction of melatonin and related indoles with hydroxyl radicals: EPR and spin trapping investigations. Free Radic. Biol. Med. 1997, 23, 367–372. [Google Scholar] [CrossRef]
- Atherton, S.J.; Dillon, J.; Gaillard, E.R. A pulse radiolysis study of the reactions of 3-hydroxykynurenine and kynurenine with oxidizing and reducing radicals. Biochim. Biophys. Acta 1993, 1158, 75–82. [Google Scholar] [CrossRef]
- Rios, C.; Santamaria, A. Quinolinic acid is a potent lipid peroxidant in rat brain homogenates. Neurochem. Res. 1991, 16, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, H.; Robotka, H.; Kis, Z.; Rozsa, E.; Janaky, T.; Somlai, C.; Marosi, M.; Farkas, T.; Toldi, J.; Vecsei, L. Kynurenine administered together with probenecid markedly inhibits pentylenetetrazol-induced seizures. An electrophysiological and behavioural study. Neuropharmacology 2004, 47, 916–925. [Google Scholar] [CrossRef]
- Pocivavsek, A.; Notarangelo, F.M.; Wu, H.-Q.; Bruno, J.P.; Schwarcz, R. Chapter 25—Astrocytes as Pharmacological Targets in the Treatment of Schizophrenia: Focus on Kynurenic Acid. In Handbook of Behavioral Neuroscience; Pletnikov, M.V., Waddington, J.L., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 23, pp. 423–443. [Google Scholar]
- Zalachoras, I.; Hollis, F.; Ramos-Fernandez, E.; Trovo, L.; Sonnay, S.; Geiser, E.; Preitner, N.; Steiner, P.; Sandi, C.; Morato, L. Therapeutic potential of glutathione-enhancers in stress-related psychopathologies. Neurosci. Biobehav. Rev. 2020, 114, 134–155. [Google Scholar] [CrossRef] [PubMed]
- Sandhir, R.; Mehrotra, A. Quercetin supplementation is effective in improving mitochondrial dysfunctions induced by 3-nitropropionic acid: Implications in Huntington’s disease. Biochim. Biophys. Acta 2013, 1832, 421–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radi, R. Peroxynitrite, a stealthy biological oxidant. J. Biol. Chem. 2013, 288, 26464–26472. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Bohar, Z.; Vecsei, L. Are Kynurenines Accomplices or Principal Villains in Dementia? Maintenance of Kynurenine Metabolism. Molecules 2020, 25, 564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reszka, K.J.; Bilski, P.; Chignell, C.F.; Dillon, J. Free radical reactions photosensitized by the human lens component, kynurenine: An EPR and spin trapping investigation. Free Radic. Biol. Med. 1996, 20, 23–34. [Google Scholar] [CrossRef]
- Opitz, C.A.; Litzenburger, U.M.; Sahm, F.; Ott, M.; Tritschler, I.; Trump, S.; Schumacher, T.; Jestaedt, L.; Schrenk, D.; Weller, M.; et al. An endogenous tumour-promoting ligand of the human aryl hydrocarbon receptor. Nature 2011, 478, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Al-Karagholi, M.A.; Hansen, J.M.; Abou-Kassem, D.; Hansted, A.K.; Ubhayasekera, K.; Bergquist, J.; Vecsei, L.; Jansen-Olesen, I.; Ashina, M. Phase 1 study to access safety, tolerability, pharmacokinetics, and pharmacodynamics of kynurenine in healthy volunteers. Pharmacol. Res. Perspect. 2021, 9, e00741. [Google Scholar] [CrossRef]








| ONOO− | ONOO− + α-KYNA | |||||||
|---|---|---|---|---|---|---|---|---|
| Penicillamine | KYNA | L-KYN | Penicillamine | KYNA | L-KYN | |||
| Median | 98.17 | 47.22 | 52.38 | 59.78 | 103.4 | 46.68 | 91.76 | 50.08 |
| p-value | 0.0002 | 0.0237 | 0.0181 | >0.999 | 0.0101 | >0.999 | 0.0184 | |
| L-KYN + ●OH | ||||||||
|---|---|---|---|---|---|---|---|---|
| ●OH | 0.1 | 1 | 5 | 10 | 25 | 50 | ||
| DNA | Median | 65.1 | 86.85 | 99.11 | 102.6 | 100.4 | 101.7 | 105.4 |
| p-value | >0.999 | 0.5319 | 0.0266 | 0.0248 | 0.0231 | 0.0266 | ||
| Protein | Median | 54.45 | 60.8 | 77.11 | 80.03 | 85.29 | 83.38 | 81.59 |
| p-value | >0.999 | 0.3936 | 0.2118 | 0.0301 | 0.0117 | 0.0179 | ||
| FeSO4 | L-KYN (µM) + FeSO4 | KYNA (µM) + FeSO4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.1 | 1 | 10 | 100 | 0.1 | 1 | 10 | 100 | |||
| ROS production | Median | 153 | 108.4 | 100.8 | 101.5 | 85.83 | 141.6 | 139.9 | 120.4 | 99.65 |
| p-value | 0.3352 | 0.0114 | 0.0065 | 0.0003 | >0.9999 | 0.5333 | 0.0537 | 0.0032 | ||
| LP | Median | 156.2 | 126 | 109.7 | 109.9 | 107.6 | 151.3 | 150.3 | 137.1 | 113.8 |
| p-value | 0.3684 | 0.0142 | 0.0065 | 0.0016 | 0.9272 | 0.7538 | 0.0518 | 0.0011 | ||
| MTT reduction | Median | 47.09 | 67.67 | 99.55 | 92.84 | 90.43 | 46.51 | 66.63 | 64.19 | 100.4 |
| p-value | >0.9999 | 0.0004 | 0.0057 | 0.0072 | >0.9999 | >0.9999 | 0.4408 | 0.0007 | ||
| Saline | L-KYN | p-Value | |
|---|---|---|---|
| L-KYN (pmoles/mg protein) | 31.9 ± 8 | 32.1 ± 2 | 0.7000 |
| KYNA (fmoles/mg protein) | 60.4 ± 5 | 82.4 ± 7 | 0.1143 |
| 3-HK (pmoles/mg protein) | 0.86 ± 0.2 | 1.39 ± 0.2 | 0.0649 |
| Saline | L-KYN | ||
|---|---|---|---|
| GSH (nmoles/g of tissue) | Median | 1319 | 1429 |
| p-value | 0.0041 | ||
| GSSG (nmoles/g of tissue) | Median | 414.7 | 375.4 |
| p-value | 0.3829 | ||
| GR (U/mg protein) | Median | 0.07662 | 0.0965 |
| p-value | 0.0320 | ||
| GPx (U/mg protein) | Median | 0.02981 | 0.0371 |
| p-value | 0.0393 | ||
| FeSO4 | 3-NP | ONOO− | |||||
|---|---|---|---|---|---|---|---|
| Saline | L-KYN | Saline | L-KYN | Saline | L-KYN | ||
| ROS production | Median | 152.0 | 118.9 | 134.4 | 106.8 | 132.8 | 113.8 |
| p-value | 0.0004 | 0.0005 | 0.0317 | ||||
| LP | Median | 200.3 | 161.1 | 158.8 | 108.6 | 142.4 | 99.62 |
| p-value | 0.1143 | 0.0013 | 0.0238 | ||||
| MTT reduction | Median | 76.60 | 119.1 | 62.57 | 91.02 | 84.17 | 127.1 |
| p-value | <0.0001 | 0.0317 | 0.0002 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez Ortega, D.; Ugalde Muñiz, P.E.; Blanco Ayala, T.; Vázquez Cervantes, G.I.; Lugo Huitrón, R.; Pineda, B.; González Esquivel, D.F.; Pérez de la Cruz, G.; Pedraza Chaverrí, J.; Sánchez Chapul, L.; et al. On the Antioxidant Properties of L-Kynurenine: An Efficient ROS Scavenger and Enhancer of Rat Brain Antioxidant Defense. Antioxidants 2022, 11, 31. https://doi.org/10.3390/antiox11010031
Ramírez Ortega D, Ugalde Muñiz PE, Blanco Ayala T, Vázquez Cervantes GI, Lugo Huitrón R, Pineda B, González Esquivel DF, Pérez de la Cruz G, Pedraza Chaverrí J, Sánchez Chapul L, et al. On the Antioxidant Properties of L-Kynurenine: An Efficient ROS Scavenger and Enhancer of Rat Brain Antioxidant Defense. Antioxidants. 2022; 11(1):31. https://doi.org/10.3390/antiox11010031
Chicago/Turabian StyleRamírez Ortega, Daniela, Perla Eugenia Ugalde Muñiz, Tonali Blanco Ayala, Gustavo Ignacio Vázquez Cervantes, Rafael Lugo Huitrón, Benjamín Pineda, Dinora Fabiola González Esquivel, Gonzalo Pérez de la Cruz, José Pedraza Chaverrí, Laura Sánchez Chapul, and et al. 2022. "On the Antioxidant Properties of L-Kynurenine: An Efficient ROS Scavenger and Enhancer of Rat Brain Antioxidant Defense" Antioxidants 11, no. 1: 31. https://doi.org/10.3390/antiox11010031
APA StyleRamírez Ortega, D., Ugalde Muñiz, P. E., Blanco Ayala, T., Vázquez Cervantes, G. I., Lugo Huitrón, R., Pineda, B., González Esquivel, D. F., Pérez de la Cruz, G., Pedraza Chaverrí, J., Sánchez Chapul, L., Gómez-Manzo, S., & Pérez de la Cruz, V. (2022). On the Antioxidant Properties of L-Kynurenine: An Efficient ROS Scavenger and Enhancer of Rat Brain Antioxidant Defense. Antioxidants, 11(1), 31. https://doi.org/10.3390/antiox11010031