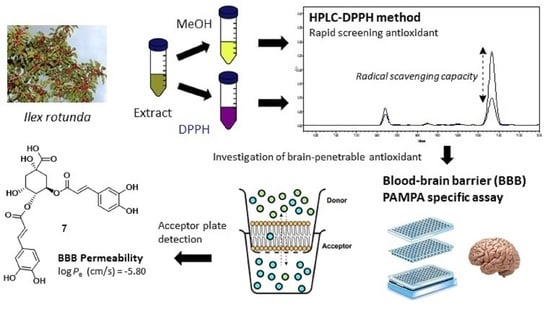
Analysis of Antioxidant Constituents from Ilex rotunda and Evaluation of Their Blood–Brain Barrier Permeability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Preparation of Extracts
2.3. DPPH Radical Scavenging Assay
2.4. ABTS Radical Scavenging Assay
2.5. Fractionation and Separation of Marker Compounds 1–8
2.6. Method Validation
2.6.1. Detection Wavelength
2.6.2. Preparation of Calibration Standard Solution
2.6.3. Chromatographic and Separation Conditions
2.6.4. Mass Conditions
2.7. HPLC-DPPH Method and ELISA Assay
2.8. Parallel Artificial Membrane Permeability Assay for the Blood–Brain Barrier (PAMPA-BBB)
2.9. Statistical Analysis
3. Results
3.1. Screened DPPH and ABTS Activities Guided Extraction and Solvent Selection
3.2. Antioxidant Activities of Fractions
3.3. Isolation and Identification of Marker Compounds 1–8
3.4. Method Validation of Marker Compounds (1–8) from I. rotunda
3.4.1. Optimization of HPLC Condition
3.4.2. Method Validation of Quantitative Analysis
Linearity, LODs, and LOQs
Precision, Accuracy, and Recovery
Quantification of Marker Compounds in I. rotunda
3.5. Screening of Antioxidants by HPLC-DPPH Method and ELISA Assay
3.6. Screening of Brain-Penetrable Antioxidants by PAMPA-BBB Method
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 12, 694. [Google Scholar] [CrossRef] [PubMed]
- Pham-Huy, L.A.; He, H.; Pham-Huy, C. Free radicals, antioxidants in disease and health. Int. J. Biomed. Sci. 2008, 4, 89–96. [Google Scholar] [PubMed]
- Martemucci, G.; Costagliola, C.; Mariano, M.; D’andrea, L.; Napolitano, P.; D’Alessandro, A.G. Free Radical Properties, Source and Targets, Antioxidant Consumption and Health. Oxygen 2022, 2, 48–78. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asadi, N.; Bahmani, M.; Kheradmand, A.; Rafieian-Kopaei, M. The Impact of Oxidative Stress on Testicular Function and the Role of Antioxidants in Improving it: A Review. J. Clin. Diagn Res. 2017, 11, IE01–IE05. [Google Scholar] [CrossRef] [PubMed]
- Rahman, K. Studies on free radicals, antioxidants, and co-factors. Clin. Interv. Aging. 2007, 2, 219–236. [Google Scholar]
- Hatami, S.; Hefler, J.; Freed, D.H. Inflammation and Oxidative Stress in the Context of Extracorporeal Cardiac and Pulmonary Support. Front. Immunol. 2022, 13, 831930. [Google Scholar] [CrossRef]
- Izzo, C.; Vitillo, P.; Di Pietro, P.; Visco, V.; Strianese, A.; Virtuoso, N.; Ciccarelli, M.; Galasso, G.; Carrizzo, A.; Vecchione, C. The Role of Oxidative Stress in Cardiovascular Aging and Cardiovascular Diseases. Life 2021, 11, 60. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N. Oxidative Stress-Induced Hypertension of Developmental Origins: Preventive Aspects of Antioxidant Therapy. Antioxidants 2022, 11, 511. [Google Scholar] [CrossRef]
- Pimentel, C.; Batista-Nascimento, L.; Rodrigues-Pousada, C.; Menezes, R.A. Oxidative stress in Alzheimer’s and Parkinson’s diseases: Insights from the yeast Saccharomyces cerevisiae. Oxid. Med. Cell Longev. 2012, 2012, 132146. [Google Scholar] [CrossRef] [PubMed]
- Salim, S. Oxidative Stress and the Central Nervous System. J. Pharmacol. Exp. Ther. 2017, 360, 201–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheignon, C.; Tomas, M.; Bonnefont-Rousselot, D.; Faller, P.; Hureau, C.; Collin, F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018, 14, 450–464. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, J.; Yuan, R.; Zhuo, Y.; Chen, Y.; Zhang, C.; Chen, M.; Gao, H.-W.; Liu, Z.; Feng, Y.; et al. Rapid identification of the chemical components of Ilex rotunda Thunb. using UPLC-Q-TOF-MS/MS. J. Chem. 2021, 16, 2021. [Google Scholar] [CrossRef]
- Han, Y.; Zhang, L.; Li, W.; Liu, X.; Xiao, J.; Chen, G.; Li, N. Natural CAC chemopreventive agents from Ilex rotunda Thunb. J. Nat. Med. 2019, 73, 456–467. [Google Scholar] [CrossRef]
- Li, C.; Li, Y.; Yao, C.; Wang, W. Free radical scavenging activity of extract from Ilex rotunda determined by electron spin resonance spectroscopy. J. Med. Plants Res. 2012, 6, 2224–2229. [Google Scholar]
- Le, D.; Han, S.; Ahn, J.; Yu, J.; Kim, C.-K.; Lee, M. Analysis of Antioxidant Phytochemicals and Anti-Inflammatory Effect from Vitex rotundifolia L. f Antioxidants 2022, 11, 454. [Google Scholar] [CrossRef]
- Di, L.; Kerns, E.H.; Fan, K.; McConnell, O.J.; Carter, G.T. High throughput artificial membrane permeability assay for blood-brain barrier. Eur. J. Med. Chem. 2003, 38, 223–232. [Google Scholar] [CrossRef]
- Shim, S.-Y.; Lee, Y.E.; Song, H.Y.; Lee, M. p-Hydroxybenzoic Acid β-d-Glucosyl Ester and Cimidahurinine with Antimelanogenesis and Antioxidant Effects from Pyracantha angustifolia via Bioactivity-Guided Fractionation. Antioxidants 2020, 9, 258. [Google Scholar] [CrossRef] [Green Version]
- Shim, S.-Y.; Lee, Y.E.; Lee, M. Antioxidant Compounds, Kirenol and Methyl ent-16α,17-dihydroxy-kauran-19-oate Bioactivity-Guided Isolated from Siegesbeckia glabrescens Attenuates MITF-Mediated Melanogenesis via Inhibition of Intracellular ROS Production. Molecules 2021, 26, 1940. [Google Scholar] [CrossRef]
- Könczöl, A.; Müller, J.; Földes, E.; Béni, Z.; Végh, K.; Kéry, A.; Balogh, G.T. Applicability of a blood-brain barrier specific artificial membrane permeability assay at the early stage of natural product-based CNS drug discovery. J. Nat. Prod. 2013, 76, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Könczöl, Á.; Rendes, K.; Dékány, M.; Müller, J.; Riethmüller, E.; Balogh, G.T. Blood-brain barrier specific permeability assay reveals N-methylated tyramine derivatives in standardised leaf extracts and herbal products of Ginkgo biloba. J. Pharm. Biomed. Anal. 2016, 131, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Martínez, J.D.; Bueno, M.; Alvarez-Rivera, G.; Tudela, J.; Ibañez, E.; Cifuentes, A. In vitro neuroprotective potential of terpenes from industrial orange juice by-products. Food Funct. 2021, 12, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.J.; Kim, S.I.; Ku, H.Y.; Lee, D.S.; Lee, J.W.; Kim, Y.S.; Seong, Y.H.; Song, K.S. Syringin from stem bark of Fraxinus rhynchophylla protects Abeta (25-35)-induced toxicity in neuronal cells. Arch. Pharm. Res. 2010, 33, 531–538. [Google Scholar] [CrossRef]
- Nakatani, N.; Kayano, S.; Kikuzaki, H.; Sumino, K.; Katagiri, K.; Mitani, T. Identification, quantitative determination, and antioxidative activities of chlorogenic acid isomers in prune (Prunus domestica L.). J Agric. Food Chem. 2000, 48, 5512–5516. [Google Scholar] [CrossRef]
- de Oliveira, D.M.; Siqueira, E.P.; Nunes, Y.R.F.; Cota, B.B. Flavonoids from leaves of Mauritia flexuosa. Rev. Bras. Farmacogn. 2013, 23, 614–620. [Google Scholar] [CrossRef]
- Kim, M.H.; Park, K.H.; Oh, M.H.; Kim, H.H.; Choe, K.I.; Park, S.H.; Lee, M.W. Two new hemiterpene glycosides from the leaves of Ilex rotunda. Thunb. Arch. Pharm. Res. 2012, 35, 1779–1784. [Google Scholar] [CrossRef]
- Yoon, M.H.; Cho, C.W.; Lee, J.W.; Kim, Y.S.; An, G.H.; Lim, C.H. Antithrombotic Compounds form the Leaves of Ligularia stenocephala M. Nat. Prod. Sci. 2008, 14, 62–67. [Google Scholar]
- Cheminat, A.; Zawatzky, R.; Becker, H.; Brouillard, R. Caffeoyl conjugates from Echinacea species: Structures and biological activity. Phytochemistry 1988, 27, 2787–2794. [Google Scholar] [CrossRef]
- Scarpati, M.L.; Guiso. M. Structure of the three dicaffoylquinic acid of coffee (Isochlorogenic acid). Tetrahedron Lett. 1964, 39, 2851–2853. [Google Scholar] [CrossRef]
- Ge, L.; Wan, H.; Tang, S.; Chen, H.; Li, J.; Zhang, K.; Zhou, B.; Fei, J.; Wu, S.; Zeng, X. Novel caffeoylquinic acid derivatives from Lonicera japonica Thunb. flower buds exert pronounced anti-HBV activities. RSC Adv. 2018, 8, 35374–35385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gosselet, F.; Loiola, R.A.; Roig, A.; Rosell, A.; Culot, M. Central nervous system delivery of molecules across the blood-brain barrier. Neurochem. Int. 2021, 144, 104952. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Cui, H.; Yang, W.; Zhao, Z. A systematic review: Botany, phytochemistry, traditional uses, pharmacology, toxicology, quality control and pharmacokinetics of Ilex rotunda Thunb. J. Ethnopharmacol. 2022, 298, 115419. [Google Scholar] [CrossRef] [PubMed]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, Q.; Xing, H.; Lu, X.; Zhao, L.; Qu, K.; Bi, K. Evaluation of antioxidant activity of ten compounds in different tea samples by means of an on-line HPLC-DPPH assay. Food Res. Int. 2013, 53, 847–856. [Google Scholar] [CrossRef]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Rojas-González, A.; Figueroa-Hernández, C.Y.; González-Rios, O.; Suárez-Quiroz, M.L.; González-Amaro, R.M.; Hernández-Estrada, Z.J.; Rayas-Duarte, P. Coffee Chlorogenic Acids Incorporation for Bioactivity Enhancement of Foods: A Review. Molecules 2022, 27, 3400. [Google Scholar] [CrossRef]
- Liang, N.; Kitts, D.D. Role of Chlorogenic Acids in Controlling Oxidative and Inflammatory Stress Conditions. Nutrients 2015, 8, 16. [Google Scholar] [CrossRef] [Green Version]
- Loganayaki, N.; Siddhuraju, P.; Manian, S. Antioxidant activity and free radical scavenging capacity of phenolic extracts from Helicteres isora L. and Ceiba pentandra L. J. Food Sci. Technol. 2013, 50, 687–695. [Google Scholar] [CrossRef] [Green Version]
- Su, L.J.; Zhang, J.H.; Gomez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [Green Version]
- Carpenter, T.S.; Kirshner, D.A.; Lau, E.Y.; Wong, S.E.; Nilmeier, J.P.; Lightstone, F.C. A method to predict blood-brain barrier permeability of drug-like compounds using molecular dynamics simulations. Biophys. J. 2014, 107, 630–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pajouhesh, H.; Lenz, G.R. Medicinal chemical properties of successful central nervous system drugs. NeuroRx. 2005, 2, 541–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geldenhuys, W.J.; Mohammad, A.S.; Adkins, C.E.; Lockman, P.R. Molecular determinants of blood-brain barrier permeation. Ther. Deliv. 2015, 6, 961–971. [Google Scholar] [CrossRef] [PubMed]






| Marker Compound | Concentration Range (µg/mL) | a Regression Equation | b Correlation Coefficient (R2) | c LOD (µg/mL) | d LOQ (µg/mL) |
|---|---|---|---|---|---|
| syringin (1) | 6.25 ~ 200 | y = 11,125x + 16,180 | 0.9999 | 0.18 | 0.55 |
| chlorogenic acid (2) | 12.5 ~ 400 | y = 2977.6x + 10,941 | 0.9993 | 0.45 | 1.38 |
| rutin (3) | 6.25 ~ 200 | y = 10,785x + 18,732 | 0.9998 | 0.13 | 0.42 |
| rotundarpenoside B (4) | 12.5 ~ 400 | y = 4358.5x + 4988.8 | 0.9999 | 0.26 | 0.81 |
| 3,4-dicaffeoylquinic acid (5) | 12.5 ~ 400 | y = 3462.4x − 8499.8 | 0.9995 | 0.65 | 1.98 |
| 3,5-dicaffeoylquinic acid (6) | 25 ~ 800 | y = 4918.7x − 13,540 | 0.9998 | 0.38 | 1.18 |
| 4,5-dicaffeoylquinic acid (7) | 25 ~ 800 | y = 5893x − 25,506 | 0.9993 | 0.27 | 0.78 |
| 3,4,5-tricaffeoylquinic acid (8) | 12.5 ~ 400 | y = 2617.1x + 916.7 | 0.9999 | 0.58 | 1.76 |
| Marker Compound | Concentration Range (µg/mL) | a Recovery (%) | b Precision (RSD %) | |
|---|---|---|---|---|
| Intraday | Interday | |||
| syringin (1) | 40 | 96.60 | 0.40 | 2.48 |
| 100 | 97.60 | |||
| 200 | 95.30 | |||
| chlorogenic acid (2) | 64 | 104.62 | 1.15 | 3.65 |
| 160 | 102.73 | |||
| 400 | 104.53 | |||
| rutin (3) | 40 | 104.03 | 0.46 | 2.53 |
| 100 | 102.10 | |||
| 200 | 100.47 | |||
| rotundarpenoside B (4) | 64 | 103.92 | 0.85 | 3.27 |
| 160 | 99.78 | |||
| 400 | 102.58 | |||
| 3,4-dicaffeoylquinic acid (5) | 64 | 104.7 | 0.51 | 2.81 |
| 160 | 97.26 | |||
| 400 | 100.21 | |||
| 3,5-dicaffeoylquinic acid (6) | 128 | 99.93 | 0.47 | 2.70 |
| 320 | 98.18 | |||
| 800 | 99.09 | |||
| 4,5-dicaffeoylquinic acid (7) | 128 | 99.44 | 0.68 | 3.04 |
| 320 | 97.88 | |||
| 800 | 98.79 | |||
| 3,4,5-tricaffeoylquinic acid (8) | 64 | 100.89 | 0.47 | 2.74 |
| 160 | 100.32 | |||
| 400 | 103.6 | |||
| Marker Compounds | a Reduction of the Peak Area (%) | EC50 Values (μM) |
|---|---|---|
| syringin (1) | 6.98 ± 0.44 | – |
| chlorogenic acid (2) | 60.51 ± 0.31 | 35.50 ± 0.38 |
| rutin (3) | 21.45 ± 0.90 | – |
| rotundarpenoside B (4) | 18.99 ± 0.90 | – |
| 3,4-dicaffeoylquinic acid (5) | 45.24 ± 0.67 | – |
| 3,5-dicaffeoylquinic acid (6) | 58.88 ± 0.44 | 10.88 ± 0.04 |
| 4,5-dicaffeoylquinic acid (7) | 83.67 ± 0.19 | 13.84 ± 0.24 |
| 3,4,5-tricaffeoylquinic acid (8) | 67.25 ± 1.00 | 10.89 ± 0.14 |
| Ascorbic acid * | – | 22.56 ± 0.77 |
| Marker Compounds | BBB Permeability log Pe (cm/s) | Cross BBB Potential a |
|---|---|---|
| syringin (1) | b n.d. | - |
| chlorogenic acid (2) | n.d. | - |
| rutin (3) | n.d. | - |
| rotundarpenoside B (4) | n.d. | - |
| 3,4-dicaffeoylquinic acid (5) | n.d. | - |
| 3,5-dicaffeoylquinic acid (6) | n.d. | - |
| 4,5-dicaffeoylquinic acid (7) | −5.85 ± 0.01 | + |
| 3,4,5-tricaffeoylquinic acid (8) | n.d. | - |
| c coumarin | −4.54 ± 0.01 | ++ |
| d caffeic acid | −9.08 ± 0.01 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.-K.; Ahn, J.; Yu, J.; Le, D.; Han, S.; Lee, M. Analysis of Antioxidant Constituents from Ilex rotunda and Evaluation of Their Blood–Brain Barrier Permeability. Antioxidants 2022, 11, 1989. https://doi.org/10.3390/antiox11101989
Kim C-K, Ahn J, Yu J, Le D, Han S, Lee M. Analysis of Antioxidant Constituents from Ilex rotunda and Evaluation of Their Blood–Brain Barrier Permeability. Antioxidants. 2022; 11(10):1989. https://doi.org/10.3390/antiox11101989
Chicago/Turabian StyleKim, Chang-Kwon, Jeongjun Ahn, Jayeon Yu, DucDat Le, Sanghee Han, and Mina Lee. 2022. "Analysis of Antioxidant Constituents from Ilex rotunda and Evaluation of Their Blood–Brain Barrier Permeability" Antioxidants 11, no. 10: 1989. https://doi.org/10.3390/antiox11101989
APA StyleKim, C. -K., Ahn, J., Yu, J., Le, D., Han, S., & Lee, M. (2022). Analysis of Antioxidant Constituents from Ilex rotunda and Evaluation of Their Blood–Brain Barrier Permeability. Antioxidants, 11(10), 1989. https://doi.org/10.3390/antiox11101989