Comparing Metabolomic and Essential Oil Fingerprints of Citrus australasica F. Muell (Finger Lime) Varieties and Their In Vitro Antioxidant Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Plant Materials and Non-Volatile Extract Preparation
2.3. Determination of the Total Polyphenol Content
2.4. UHPLC-DAD-HR-Orbitrap/ESI-MS Analyses
2.4.1. Quali-Quantitative Analyses of Phenols
2.4.2. Quali-Quantitative Analyses of Anthocyanins
2.5. Essential Oils (EOs) Hydrodistillation and Analysis
2.6. Cell Viability
2.7. Cell Treatment and Oxidative Stress
2.8. Statistical Analyses
3. Results and Discussion
3.1. Polyphenol Content of Finger Lime Fruits
3.2. Metabolomic Fingerprint and Quantitative Analysis of Non-Volatile Components
3.2.1. Phenol Composition
3.2.2. Anthocyanins Characterisation
3.2.3. Multivariate Statistical Analyses of the Non-Volatile Components
3.3. Essential Oil (EO) Composition of All of the Samples
Multivariate Statistical Analyses of the EO Compositions
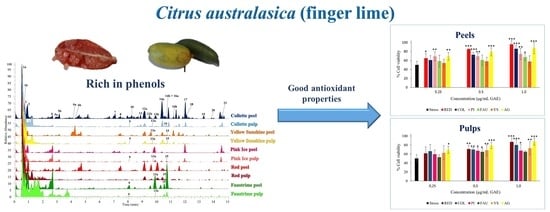
3.4. Cell Viability Assay and In Vitro Cellular Assessment of Antioxidant Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Favela-Hernández, J.M.J.; González-Santiago, O.; Ramírez-Cabrera, M.A.; Esquivel-Ferriño, P.C.; Camacho-Corona, M. Chemistry and pharmacology of Citrus sinensis. Molecules 2016, 21, 247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Ji, S.; Zang, W.; Wang, N.; Cao, J.; Li, X.; Sun, C. Identification of phenolic compounds from a unique Citrus species, finger lime (Citrus australasica) and their inhibition of LPS-induced NO-releasing in BV-2 cell line. Food Chem. Toxicol. 2019, 129, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, B.; Dutt, M.; Vashisth, T. Comparative phytochemical analysis of the fruits offour Florida-grown finger lime (Citrus australasica) selections. LWT-Food Sci.Technol. 2020, 135, 110003. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Heying, E.; Tanumihardjo, S.A. History, global distribution, and nutritional importance of Citrus fruits. Compr. Rev. Food Sci. Food Saf. 2012, 11, 530–545. [Google Scholar] [CrossRef]
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of Citrus flavonoids, naringin and naringenin, on metabolic syndrome and their mechanisms ofaction. Adv. Nutr. 2014, 5, 404417. [Google Scholar] [CrossRef] [Green Version]
- Benavente-García, O.; Castillo, J. Update on uses and properties of Citrus flavonoids: New findings in anticancer, cardiovascular, and anti-inflammatory activity. J. Agr. Food Chem. 2008, 56, 6185–6205. [Google Scholar] [CrossRef]
- Lv, X.; Zhao, S.; Ning, Z.; Zeng, H.; Shu, Y.; Tao, O.; Xiao, C.; Lu, C.; Liu, Y. Citrus fruits as a treasure trove of active natural metabolites that potentially provide benefits for human health. Chem. Cent. J. 2015, 9, 68. [Google Scholar] [CrossRef] [Green Version]
- Zou, Z.; Xi, W.; Hu, Y.; Nie, C.; Zhou, Z. Antioxidant activity of Citrus fruits. Food Chem. 2016, 196, 885–896. [Google Scholar] [CrossRef]
- Benavente-García, O.; Castillo, J.; Marin, F.R.; Ortuño, A.; Del Río, J.A. Uses and properties of Citrus flavonoids. J. Agric. Food Chem. 1997, 45, 4505–4515. [Google Scholar] [CrossRef]
- Delort, E.; Jaquier, A.; Decorzant, E.; Chapuis, C.; Casilli, A.; Frérot, E. Comparative analysis of three australian finger lime (Citrus australasica) cultivars: Identification of unique citrus chemotypes and new volatile molecules. Phytochemistry 2015, 109, 111–124. [Google Scholar] [CrossRef]
- Dugo, P.; Mondello, L.; Zappia, G.; Bonaccorsi, I.; Cotroneo, A.; Russo, M.T. The composition of the volatile fraction and the enantiomeric distribution of five volatile components of faustrime oil (Monocitrus australatica × Fortunella sp. × Citrus aurantifolia). J. Essential Oil Res. 2004, 16, 4. [Google Scholar] [CrossRef]
- Aznar, R.; Rodríguez-Pérez, C.; Rai, D.K. Comprehensive characterization and quantification of antioxidant compounds in finger lime (Citrus australasica L.) by HPLC-QTof-MS and UPLC-MS/MS. Appl. Sci. 2022, 12, 1712. [Google Scholar] [CrossRef]
- Da Pozzo, E.; De Leo, M.; Faraone, I.; Milella, L.; Cavallini, C.; Piragine, E.; Testai, L.; Calderone, V.; Pistelli, L.; Braca, A.; et al. Antioxidant and antisenescence effects of bergamot juice. Ox. Med. Cell. Longev. 2018, 2018, 9395804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Leo, M.; Piragine, E.; Pirone, A.; Braca, A.; Pistelli, L.; Calderone, V.; Miragliotta, V.; Testai, L. Protective effects of bergamot (Citrus bergamia Risso & Poiteau) juice in rats fed with high-fat diet. Planta Med. 2020, 86, 180–189. [Google Scholar] [CrossRef]
- Flamini, G.; Pistelli, L.; Nardoni, S.; Ebani, V.; Zinnai, A.; Mancianti, F.; Ascrizzi, R.; Pistelli, L. Essential oil composition and biological activity of “Pompia”, a sardinian Citrus ecotype. Molecules 2019, 24, 908. [Google Scholar] [CrossRef] [Green Version]
- Giovanelli, S.; Ciccarelli, D.; Giusti, G.; Mancianti, F.; Nardoni, S.; Pistelli, L. Comparative assessment of volatiles in juices and essential oils from minor Citrus fruits (Rutaceae). Flavour Fragr. J. 2020, 35, 639–652. [Google Scholar] [CrossRef]
- Testai, L.; De Leo, M.; Flori, L.; Polini, B.; Braca, A.; Nieri, P.; Pistelli, L.; Calderone, V. Contribution of irisin pathway in protective effects of mandarin juice (Citrus reticulata Blanco) on metabolic syndrome in rats fed with high fat diet. Pharmacol. Res. 2021, 35, 4324–4333. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Felice, F.; Fabiano, A.; De Leo, M.; Piras, A.M.; Beconcini, D.; Cesare, M.M.; Braca, A.; Zambito, Y.; Di Stefano, R. Antioxidant effect of cocoa by-product and cherry polyphenol extracts: A comparative study. Antioxidants 2020, 9, 132. [Google Scholar] [CrossRef] [Green Version]
- National Institute of Standards and Technology. NIST/EPA/NIH Mass Spectral Library. In NIST Standard Reference Database Number 69; (No. 2014); The NIST Mass Spectrometry Data Center: Gaithersburg, MD, USA, 2014. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Pub. Corp.: Carol Stream, IL, USA, 2007. [Google Scholar]
- Beconcini, D.; Fabiano, A.; Zambito, Y.; Berni, R.; Santoni, T.; Piras, A.M.; Di Stefano, R. Chitosan-based nanoparticles containing cherry extract from Prunus avium L. to improve the resistance of endothelial eells to oxidative stress. Nutrients 2018, 10, 1598. [Google Scholar] [CrossRef]
- Ascrizzi, R.; Flamini, G.; Giusiani, M.; Stefanelli, F.; Deriu, V.; Chericoni, S. VOCs as fingerprints for the chemical profiling of hashish samples analyzed by HS-SPME/GC–MS and multivariate statistical tools. Forensic Toxicol. 2018, 36, 243–260. [Google Scholar] [CrossRef]
- Parveen, I.; Winters, A.; Threadgill, M.D.; Hauck, B.; Morris, P. Extraction, structural characterisation and evaluation of hydroxycinnamate esters of orchard grass (Dactylis glomerata) as substrates for polyphenol oxidase. Phytochemistry 2008, 69, 2799–2806. [Google Scholar] [CrossRef] [PubMed]
- Strack, D.; Leicht, P.; Bokern, M.; Wray, V.; Grotjahn, L. Hydroxycinnamic acid esters of isocitric acid: Accumulation and enzymatic synthesis in Amaranthus cruentus. Phytochemistry 1987, 26, 2919–2922. [Google Scholar] [CrossRef]
- Russo, M.; Arigò, A.; Calabrò, M.L.; Farnetti, S.; Mondello, L.; Dugo, P. Bergamot (Citrus bergamia Risso) as a source of nutraceuticals: Limonoids and flavonoids. J. Funct. Foods 2016, 20, 10–19. [Google Scholar] [CrossRef]
- Biavatti, M.W.; Vieira, P.C.; Da Silva, M.F.; Fernandes, J.B.; Albuquerque, S. Limonoids from the endemic brazilian species Raulinoa echinata. Z. Naturforsch. C 2001, 56c, 570–574. [Google Scholar] [CrossRef]
- Fabroni, S.; Ballistreri, G.; Amenta, M.; Rapisarda, P. Anthocyanins in different Citrus species: An UHPLC-PDA-ESI/MSn-assisted qualitative and quantitative investigation. J. Sci. Food Agric. 2016, 96, 4797–4808. [Google Scholar] [CrossRef]
- Netzel, M.; Netzel, G.; Tian, Q.; Schwartz, S.; Konczak, I. Native Australian fruits—A novel source of antioxidants for food. Innov. Food Sci. Emerg. Technol. 2007, 8, 339–346. [Google Scholar] [CrossRef]
- Johnson, J.B.; Batley, R.; Manson, D.; White, S.; Naiker, M. Volatile compounds, phenolic acid profiles and phytochemical content of five Australian finger lime (Citrus australasica) cultivars. LWT 2022, 154, 112640. [Google Scholar] [CrossRef]
- Lota, M.-L.; de Rocca Serra, D.; Tomi, F.; Jacquemond, C.; Casanova, J. Volatile components of peel and leaf oils of lemon and lime species. J. Agric. Food Chem. 2002, 50, 796–805. [Google Scholar] [CrossRef]
- Trozzi, A.; Verzera, A.; d’Alcontres, I.S. Constituents of the cold-pressed oil of Faustrime, a trigeneric hybrid of Monocitrus australasica × Fortunella sp. × Citrus aurantifolia. J. Essent. Oil Res. 1995, 5, 97–100. [Google Scholar] [CrossRef]



| Finger Lime Varieties | Peel | Pulp |
|---|---|---|
| mg GAE/g DW ± SD | mg GAE/g DW ± SD | |
| Red | 9.1 ± 0.2 | 5.0 ± 0.2 |
| Collette | 7.4 ± 0.2 | 5.6 ± 0.2 |
| Pink Ice | 8.2 ± 0.2 | 6.4 ± 0.2 |
| Yellow Sunshine | 6.8 ± 0.2 | 2.6 ± 0.1 |
| Faustrime | 4.9 ± 0.1 | 3.1 ± 0.2 |
| N. a | Compound | tR(min) | [M-H]− | Formula | Error (ppm) | -ESI-MS/MS (m/z) b | Extract |
|---|---|---|---|---|---|---|---|
| Hydroxycinnamic acids | |||||||
| 1a | Caffeoylisocitric acid (isomer I) | 0.50 | 353.0723 | C15H14O10 | −0.8497 | 293.05; 191.02; 173.01; 111.01 | R; F; PI; C; YS |
| 1b | Caffeoylisocitric acid (isomer II) | 0.66 | 353.0723 | C15H14O10 | −0.8497 | 173.01; 120.20; 111.01; 87.01 | R; F; PI; C; YS |
| 2 | Caffeoylmethylisocitric acid | 0.7–2 | 367.0879 | C16H16O10 | −0.8172 | 205.03, 191.02; 179.06; 169.01; 161.05; 143.03; 111.00; 101.02; 89.02 | R; F; PI; C; YS |
| 3 | Methylisocitric acid derivative | 0.88 | 433.0596 | - | - | 401.04; 227.02; 205.03; 173.01; 143.03; 111.01; 87.01 | R; F; PI; C; YS |
| 4 | 3-Hydroxy-3-methylglutaric acid derivative | 2.62 | 365.1451 | - | - | 303.14; 263.11; 221.10; 161.04; 125.02; 99.04; 59.87; 57.03 | R; F; PI; C |
| 5a | p-Coumaroylglucoside acid (isomer I) | 2.87 | 325.0927 | C15H18O8 | −0.9228 | 163.04; 159.05; 145.03 | R; F (pe); PI; C; YS |
| 5b | p-Coumaroylglucoside acid (isomer II) | 3.11 | 325.0927 | C15H18O8 | −0.9228 | 163.04; 159.05; 145.03 | R; F(pe); PI; C; YS |
| 6a | Feruloylglucoside acid (isomer I) | 3.99 | 355.1034 | C16H20O9 | −0.2816 | 193.05; 175.04; 160.02 | R; F(pe); PI; C; YS |
| 6b | Feruloylglucoside acid (isomer II) | 4.19 | 355.1034 | C16H20O9 | −0.2816 | 193.05; 175.04; 160.02 | R; F(pe); PI; C; YS |
| Flavonoids | |||||||
| 7 | Rutin | 7.02 | 609.1458 | C27H30O16 | −0.4925 | 301.04; 300.03; 271.03 | R; F; PI; C; YS |
| 8 | Quercetin glucoside | 7.33 | 463.0880 | C21H20O12 | −0.4319 | 301.04; 300.03 | R; F; PI; C; YS |
| 9 | Neoeriocitrin/eriocitrin | 7.99 | 595.1666 | C27H32O15 | −0.3360 | 459.11; 287.06; 193.01; 161.02; 151.00; 135.04 | F; PI(pu); C(pu); YS(pu) |
| 10 | Luteolin 7-O-neohesperidoside/rutinoside | 8.19 | 593.1514 | C27H30O15 | +0.3372 | 529.27; 474.31; 285.04; 182.91 | R; F (pu); PI; C; YS |
| 11 | Kaempferol glucoside | 8.47 | 447.0933 | C21H20O11 | 0 | 327.05; 304.33; 285.04; 284.03; 256.04; 255.03; 227.03 | R; F(pe); PI; C; YS |
| 12a | Isorhamnetin glucoside (isomer I) | 9.07 | 477.1035 | C22H22O12 | −0.8384 | 357.06; 327.06; 315.05; 314.04; 286.05; 285.04; 271.02; 257.05; 243.03 | R; F; PI; C; YS |
| 12b | Isorhamnetin glucoside (isomer II) | 9.52 | 477.1035 | C22H22O12 | −0.8384 | 449.11; 357.06; 333.73; 315.04; 299.02; 285.04; 271.02; 243.03 | R; F; PI; C; YS |
| 13a | Naringin/Naringenin rutinoside | 9.50 | 579.1713 | C27H32O14 | −1.0360 | 471.43; 397.56; 313.07; 295.06; 285.08; 271.06; 151.00 | F; PI(pu); C(pu); YS |
| 13b | Naringin/Naringenin rutinoside | 9.94 | 579.1713 | C27H32O14 | −1.0360 | 313.07; 271.06; 151.00 | R; F; PI; C; YS |
| 14a | 3-Hydroxy-3-methylglutaryl isorhamnetin glucoside (isomer I) | 10.30 | 621.1457 | C28H30O16 | −0.6440 | 596.51; 559.15; 519.11; 477.10; 315.05; 299.02; 285.04; 271.02; 243.03 | R; F; PI; C; YS |
| 14b | 3-Hydroxy-3-methylglutaryl isorhamnetin glucoside (isomer II) | 10.59 | 621.1458 | C28H30O16 | −0.6440 | 559.15; 519.11; 477.10; 315.05; 300.03; 271.03 | R; F; C; PI; YS |
| 15 | Neodiosmin/diosmin | 10.46 | 607.1666 | C28H32O15 | −0.3294 | 341.07; 299.06; 284.03; 266.07; 255.03; 151.00 | R(pu); F; PI(pu); C(pu); YS |
| 16a | Neohesperidin/hesperidin | 10.65 | 609.1820 | C28H34O15 | −0.8208 | 418.95; 343.08; 301.07; 286.05; 151.00 | R; F; PI; C; YS |
| 16b | Neohesperidin/hesperidin | 11.01 | 609.1820 | C28H34O15 | −0.8208 | 301.07; 286.05; 151.00 | R; F; PI; C(pu); YS |
| 17 | Isosakuranetin rhamnosildiglucoside | 11.93 | 755.2415 | C34H44O19 | +1.4565 | 771.95; 755.24; 657.34; 490.63; 285.08 | R; F; PI; C; YS |
| 18 | Di-(3-hydroxy-3-methylglutaryl) isorhamnetin glucoside | 12.03 | 765.1881 | C34H38O20 | −0.3921 | 678.23; 642.82; 621.15; 519.12; 477.11; 315.05; 299.02; 271.03; 187.04; 151.00 | R; F; PI; C; YS |
| 19 | Kaempferol triglucoside | 13.29 | 771.2354 | C34H44O20 | +0.1297 | 527.23; 499.11; 408.51; 285.08; 251.90 | PI; C |
| 21 | Poncirin | 14.47 | 593.1878 | C28H34O14 | +0.1564 | 593.19; 427.38; 327.09; 285.08 | R; F; PI; C; YS |
| Limonoids | |||||||
| 20 | Limonexic acid | 13.76 | 501.1763 | C26H30O10 | −0.5986 | 457.18; 413.20; 271.89; 145.08 | F; C; YS |
| Anthocyanins | |||||||
| N.a | Compound | tR(min) | [M]+ | Formula | Error (ppm) | +ESI-MS/MS (m/z) | Extract |
| 22 | Cyanidin 3-O-glucoside | 2.78 | 449.1068 | C21H21O11+ | −2.2266 | 287.05; 241.05; 213.05; 185.06; 157.06 | R(pe, pu); C; PI |
| 23 | Petunidin rhamnosyldiglucoside | 3.68 | 787.2272 | C34H43O21+ | −2.4135 | 625.17; 479.12; 427.10; 317.06; 302.04 | R(pe, pu) |
| 24 | Cyanidin 3-(6′′-malonylglucoside) | 3.87 | 535.1071 | C24H23O14+ | −2.0557 | 287.05; 241.05; 213.05; 171.04 | R(pe, pu); C |
| 25 | Delfinidin rhamnosylglucoside | 4.76 | 611.1593 | C27H31O16+ | −2.1271 | 366.30; 303.05; 203.84; 173.85 | R(pe); C; PI |
| 26 | Peonidin 3-(6′′-malonylglucoside) | 4.85 | 549.1227 | C25H25O14+ | −2.0032 | 517.09; 449.11; 301.07; 287.05; 241.05; 213.05 | R(pe, pu); C; PI |
| Variety | |||||||
|---|---|---|---|---|---|---|---|
| Peak a | Compound | Collette | Yellow Sunshine | Pink Ice | Red | Faustrime | |
| 1a | Caffeoylisocitric acid (isomer I) | Peel | 1.18 ± 0.19 C | 1.45 ± 0.05 B | 2.01 ± 0.0 A | 1.23 ± 0.0 BC | 0.837 ± 0.026 D |
| Pulp | 0.911 ± 0.039 D | 1.47 ± 0.06 B | 2.23 ± 0.03 A | 1.03 ± 0.03 C | 0.919 ± 0.011 D | ||
| 1b | Caffeoylisocitric acid (isomer II) | Peel | 0.226 ± 0.031 C | 0.283 ± 0.013 B | 0.354 ± 0.012 A | 0.212 ± 0.004 C | 0.156 ± 0.014 D |
| Pulp | 0.215 ± 0.022 C | 0.379 ± 0.029 B | 0.451 ± 0.026 A | 0.179 ± 0.008 CD | 0.159 ± 0.004 D | ||
| 2 | Caffeoylmethylisocitric acid | Peel | 0.179 ± 0.023 A | 0.143 ± 0.002 B | 0.167 ± 0.002 A | 0.153 ± 0.006 B | 0.172 ± 0.003 A |
| Pulp | 0.114 ± 0.006 D | 0.143 ± 0.002 C | 0.329 ± 0.000 A | 0.071 ± 0.02 C | 0.069 ± 0.001 B | ||
| 5a | p-Coumaroylglucoside acid (isomer I) | Peel | 0.105 ± 0.008 A | 0.043 ± 0.007 C | 0.092 ± 0.009 AB | 0.080 ± 0.05 B | 0.0066 ± 0.0003 D |
| Pulp | 0.0072 ± 0.0005 BC | 0.0092 ± 0.001 B | 0.059 ± 0.006 A | 0.0083 ± 0.0004 B | Not detected C | ||
| 5b | p-Coumaroylglucoside acid (isomer II) | Peel | 0.187 ± 0.008 A | 0.089 ± 0.005 B | 0.189 ± 0.022 A | 0.171 ± 0.004 A | 0.012 ± 0.001 D |
| Pulp | 0.013 ± 0.001 B | 0.016 ± 0.001 B | 0.118 ± 0.006 A | 0.018 ± 0.000 B | Not detected C | ||
| 6a | Feruloylglucoside acid (isomer I) | Peel | 0.011 ± 0.000 C | 0.198 ± 0.018 A | 0.013 ± 0.001 C | 0.036 ± 0.002 B | 0.0033 ± 0.0001 C |
| Pulp | 0.0015 ±0.0001 D | 0.0031 ± 0.003 C | 0.010 ± 0.000 A | 0.0061 ± 0.0002 B | Not detected E | ||
| 6b | Feruloylglucoside acid (isomer II) | Peel | 0.064 ± 0.008 B | 0.388 ± 0.032 A | 0.021 ± 0.003 B | 0.060 ± 0.003 B | 0.017 ± 0.002 B |
| Pulp | 0.0030 ±0.0002 CD | 0.0045 ± 0.0005 BC | 0.025 ± 0.003 A | 0.0068 ± 0.0001 B | Not detected D | ||
| 7 | Rutin | Peel | 198 ± 13 A | 18.7 ± 2.0 C | 128 ± 12 B | 5.69 ± 0.49 C | 19.6 ± 1.3 C |
| Pulp | 7.50 ± 0.29 B | 2.64 ± 0.21 D | 21.1 ± 1.7 A | 0.534 ± 0.136 D | 4.72 ± 0.65 C | ||
| 8 | Quercetin glucoside | Peel | 105 ± 5 C | 145 ± 12 B | 106 ± 10 C | 245 ± 9 A | 2.32 ± 0.03 D |
| Pulp | 28.6 ± 1.4 A | 11.7 ± 0.8 B | 32.0 ± 7.5 A | 12.3 ± 0.2 B | 0.775 ± 0.050 C | ||
| 9 | Neoeriocitrin/eriocitrin | Peel | Not detected B | Not detected B | Not detected B | Not detected B | 316 ± 27 A |
| Pulp | 0.488 ± 0.109 B | 1.38 ± 0.06 B | 1.06 ± 0.05 B | Trace B | 56.8 ± 2.8 A | ||
| 10 | Luteolin 7-O-neohesperidoside/rutinoside | Peel | 660 ± 50 A | 14.7 ± 1.2 C | 224 ± 19 B | 7.72 ± 0.35 C | Not detected C |
| Pulp | 19.1 ± 0.9 B | 4.87 ± 0.25 C | 35.7 ± 2.7 A | 1.55 ± 0.06 C | 38.0 ± 2.4 A | ||
| 11 | Kaempferol glucoside | Peel | 11.8 ± 0.8 C | 14.3 ± 1.4 C | 76.0 ± 7.2 A | 43.8 ± 2.2 B | 2.55 ± 0.13 D |
| Pulp | 1.99 ± 0.12 BC | 1.06 ± 0.06 C | 10.7 ± 0.8 A | 2.89 ± 0.05 B | Not detected D | ||
| 12a | Isorhamnetin glucoside (isomer I) | Peel | 288 ± 14 B | 171 ± 11 C | 47.8 ± 3.9 D | 376 ± 21 A | 5.15 ± 0.31 E |
| Pulp | 69.3 ± 2.3 A | 32.2 ± 1.0 C | 44.1 ± 2.6 B | 65.0 ± 1.2 A | 3.31 ± 0.09 D | ||
| 12b | Isorhamnetin glucoside (isomer II) | Peel | 37.6 ± 1.2 C | 161 ± 10 B | 15.5 ± 1.0 D | 234 ± 4 A | 1.04 ± 0.06 E |
| Pulp | 15.4 ± 0.9 B | 20.9 ± 0.8 A | 7.86 ± 0.57 C | 20.5 ± 0.4 A | 0.426 ± 0.031 D | ||
| 13a | Naringin/naringenin rutinoside | Peel | Not detected B | 9.65 ± 0.72 B | Not detected B | Trace B | 145 ± 12 A |
| Pulp | 0.176 ± 0.049 C | 4.60 ± 0.20 B | 0.379 ± 0.050 C | Not detected C | 13.6 ± 0.5 A | ||
| 13b | Naringin/naringenin rutinoside | Peel | 21.9 ± 1.9 C | 146 ± 12 B | 22.0 ± 2.4 C | 1061 ± 63 A | 2.26 ± 0.25 C |
| Pulp | 15.1 ± 0.5 BC | 17.4 ± 1.1 B | 13.5 ± 0.8 C | 131 ± 1 A | Trace D | ||
| 14a | 3-Hydroxy-3-methylglutaryl isorhamnetin glucoside (I) | Peel | 573 ± 24 A | 92.3 ± 5.8 B | 36.1 ± 2.5 C | 102 ± 2 B | 7.53 ± 0.37 C |
| Pulp | 108 ± 6 A | 31.4 ± 1.6 C | 48.7 ± 3.2 B | 22.9 ± 0.3 C | 5.79 ± 0.25 D | ||
| 14b | 3-Hydroxy-3-methylglutaryl isorhamnetin glucoside (II) | Peel | 67.0 ± 4.2 A | 30.2 ± 1.8 C | 4.02 ± 0.34 D | 42.9 ± 2.2 B | 1.39 ± 0.10 D |
| Pulp | 25.8 ± 1.5 A | 9.53 ± 0.55 B | 5.21 ± 0.46 C | 10.3 ± 0.3 B | 0.553 ± 0.008 D | ||
| 15 | Neodiosmin/diosmin | Peel | Not detected C | 79.7 ± 7.5 B | Not detected C | Trace C | 606 ± 41 A |
| Pulp | 1.70 ± 0.18 C | 49.6 ± 3.1 B | 4.41 ± 1.10 C | 1.39 ± 0.07 C | 198 ± 8 A | ||
| 16a | Neohesperidin/hesperidin | Peel | 2.11 ± 0.20 B | 74.6 ± 7.4 B | 2.61 ± 0.13 B | 4.21 ± 1.03 B | 1495 ± 107 A |
| Pulp | 4.72 ± 1.20 C | 48.5 ± 1.7 B | 6.52 ± 0.49 C | 3.31 ± 0.14 C | 174 ± 5 A | ||
| 16b | Neohesperidin/hesperidin | Peel | Trace B | 502 ± 43 A | 2.92 ± 0.10 B | 18.0 ± 1.9 B | 10.5 ± 2.6 B |
| Pulp | 1.53 ± 0.43 B | 107 ± 7 A | 1.67 ± 0.11 B | 4.43 ± 0.18 B | 0.471 ± 0.149 B | ||
| 17 | Isosakuranetin rhamnosyldiglucoside | Peel | 1119 ± 102 A | 13.4 ± 1.2 C | 911 ± 92 B | 2.21 ± 0.00 C | 4.09 ± 0.45 C |
| Pulp | 157 ± 4 B | 2.17 ± 0.12 C | 272 ± 13 A | 1.49 ± 0.09 C | 0.348 ± 0.029 C | ||
| 18 | Di-(3-hydroxy-3-methylglutaryl) isorhamnetin glucoside | Peel | 114 ± 10 A | 28.2 ± 2.2 C | 4.11 ± 0.12 D | 41.2 ± 1.1 B | 11.6 ± 0.4 D |
| Pulp | 10.1 ± 0.6 A | 9.61 ± 0.58 A | 6.57 ± 0.53 B | 6.20 ± 0.01 B | 3.87 ± 0.22 C | ||
| 19 | Kaempferol triglucoside | Peel | 11.8 ± 0.8 A | Trace C | 9.85 ± 0.82 B | Not detected C | Trace C |
| Pulp | 2.09 ± 0.05 B | Not detected C | 3.24 ± 0.12 A | Not detected C | Not detected C | ||
| 21 | Poncirin | Peel | 221 ± 12 B | 15.6 ± 1.3 D | 767 ± 68 A | 120 ± 9.0 C | 3.68 ± 0.34 D |
| Pulp | 142 ± 5 B | 4.82 ± 0.20 D | 371 ± 6.6 A | 15.8 ± 0.2 C | 0.295 ± 0.006 D | ||
| Total flavonoids and phenolic acids | Peel | 3432 ± 239 | 1519 ± 121 | 2360 ± 220 | 2306 ± 117 | 2635 ± 193 | |
| Pulp | 612 ± 26 | 361 ± 19 | 896 ± 43 | 301 ± 4.0 | 502 ± 20 | ||
| Anthocyanins | |||||||
| Peak | Compound | Collette | Yellow Sunshine | Pink Ice | Red | Faustrime | |
| 22 | Cyanidin 3-O-glucoside | Peel | 20.0 ± 0.5 A | nd | nd | 12.3 ± 0.2 B | nd |
| Pulp | nd | nd | 0.925 ± 0.07 B | 1.44 ± 0.09 A | nd | ||
| 23 | Petunidin rhamnosyldiglucoside | Peel | Trace B | nd | Nd | 20.4 ± 0.3 A | nd |
| Pulp | nd | nd | Trace B | 3.41 ± 0.10 A | nd | ||
| 24 | Cyanidin 3-(6′′-malonylglucoside) | Peel | 20.3 ± 1.0 A | nd | nd | 17.1 ± 0.3 B | nd |
| Pulp | nd | nd | Trace B | 0.62 ± 0.14 A | nd | ||
| 25 | Delfinidin rhamnosylglucoside | Peel | 71.1 ± 1.0 A | nd | nd | 3.80 ± 0.15 B | nd |
| Pulp | nd | nd | 1.98 ± 0.01 A | Not detected B | nd | ||
| 26 | Peonidin 3-(6″-malonylglucoside) | Peel | 31.8 ± 0.7 B | nd | nd | 42.6 ± 1.3 A | nd |
| Pulp | nd | nd | 1.47 ± 0.03 B | 2.79 ± 0.23 A | nd | ||
| Total anthocyanins | Peel | 143.2 ± 3.2 | nd | nd | 96.2 ± 2.3 | nd | |
| Pulp | nd | nd | 4.38 ± 0.11 | 8.26 ± 0.56 | nd | ||
| Total phenols | Peel | 3575 ± 242 | 1519 ± 121 | 2360 ± 220 | 2402 ± 119 | 2635 ± 193 | |
| Pulp | 612 ± 26 | 361 ± 19 | 900 ± 43 | 309 ± 4.6 | 502 ± 20 | ||
| Compounds | l.r.i. a | Relative Abundance (%) ± SD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Collette | Pink Ice | Red | Yellow Sunshine | Faustrime | |||||||
| Peel | Pulp | Peel | Pulp | Peel | Pulp | Peel | Pulp | Peel | Pulp | ||
| α-Thujene | 931 | - | - | - | - | - | - | - | - | 0.1 ± 0.01 | - |
| α-Pinene | 941 | 0.7 ± 0.02 | - | 0.4 ± 0.05 | - | 0.1 ± 0.00 | 0.2 ± 0.24 | - | - | 1.1 ± 0.08 | 1.0 ± 0.08 |
| Sabinene | 976 | 1.7 ± 0.04 | - | 1.7 ± 0.13 | - | - | - | - | - | 0.3 ± 0.02 | 0.2 ± 0.02 |
| β-Pinene | 982 | 0.4 ± 0.02 | - | 0.1 ± 0.01 | - | - | - | - | - | 0.5 ± 0.03 | 0.2 ± 0.02 |
| Myrcene | 993 | 1.0 ± 0.03 | - | 0.7 ± 0.04 | - | 1.0 ± 0.03 | - | 0.3 ± 0.08 | - | 1.0 ± 0.06 | 1.0 ± 0.03 |
| Octanal | 1001 | - | - | - | - | - | - | - | - | 0.6 ± 0.03 | - |
| α-Phellandrene | 1005 | 4.1 ± 0.57 | - | 3.1 ± 0.18 | - | 0.1 ± 0.01 | - | - | - | 5.2 ± 0.30 | 7.2 ± 0.41 |
| δ-3-Carene | 1011 | 0.2 ± 0.04 | - | 0.1 ± 0.01 | - | 0.5 ± 0.01 | - | - | - | 0.3 ± 0.02 | 0.2 ± 0.01 |
| α-Terpinene | 1018 | 1.3 ± 0.16 | - | 3.7 ± 0.21 | - | - | - | - | - | 0.8 ± 0.04 | 2.1 ± 0.09 |
| p-Cymene | 1027 | 0.3 ± 0.02 | - | 0.2 ± 0.01 | - | - | - | - | - | 1.0 ± 0.05 | 0.4 ± 0.04 |
| Limonene | 1032 | 42.4 ± 5.64 | 1.4 ± 0.48 | 26.5 ± 1.75 | 1.0 ± 0.54 | 73.6 ± 4.41 | - | 40.0 ± 2.47 | 0.4 ± 0.11 | 31.5 ± 1.86 | 48.3 ± 3.71 |
| 1,8-Cineole | 1034 | 0.1 ± 0.01 | - | - | - | - | - | - | - | - | - |
| (Z)-β-Ocimene | 1042 | 0.3 ± 0.01 | - | 0.3 ± 0.01 | - | 1.2 ± 0.01 | - | 0.5 ± 0.05 | - | 0.7 ± 0.04 | 0.3 ± 0.01 |
| (E)-β-Ocimene | 1052 | 0.1 ± 0.01 | - | 0.2 ± 0.01 | - | 0.5 ± 0.00 | - | 0.4 ± 0.11 | - | 0.2 ± 0.01 | 0.1 ± 0.00 |
| γ-Terpinene | 1062 | 14.2 ± 1.96 | - | 7.3 ± 0.25 | - | 0.3 ± 0.01 | - | 0.4 ± 0.11 | - | 11.6 ± 0.5 | 10.8 ± 0.47 |
| Terpinolene | 1088 | 1.6 ± 0.24 | - | 2.0 ± 0.01 | - | 0.4 ± 0.01 | - | - | - | 1.2 ± 0.02 | 0.9 ± 0.01 |
| Linalool | 1101 | 0.9 ± 0.17 | 1.1 ± 0.11 | 0.3 ± 0.00 | - | - | - | - | 0.2 ± 0.04 | 2.6 ± 0.04 | 0.7 ± 0.01 |
| Nonanal | 1104 | - | - | - | - | - | - | - | - | 0.2 ± 0.01 | - |
| cis-p-Menth-2-en-1-ol | 1124 | 0.6 ± 0.03 | 1.2 ± 0.11 | 1.7 ± 0.05 | - | - | - | - | - | 1.1 ± 0.00 | 0.3 ± 0.00 |
| trans-p-Menth-2-en-1-ol | 1140 | 0.4 ± 0.03 | 1.2 ± 0.45 | 1.3 ± 0.04 | - | - | - | - | - | 0.8 ± 0.01 | 0.2 ± 0.01 |
| β-Terpineol | 1153 | - | - | - | - | - | - | - | - | 1.6 ± 0.02 | 0.1 ± 0.01 |
| Menthone | 1154 | 5.1 ± 0.14 | 1.0 ± 0.06 | - | - | - | - | - | - | - | 0.3 ± 0.01 |
| Citronellal | 1155 | 0.7 ± 0.04 | - | - | - | 1.7 ± 0.06 | - | 1.1 ± 0.12 | - | 9.4 ± 0.05 | - |
| isoBorneol | 1156 | - | - | - | - | - | - | - | - | 0.9 ± 0.03 | - |
| isoMenthone | 1164 | - | - | 2.6 ± 0.09 | - | - | - | - | - | 1.4 ± 0.03 | - |
| 4-Terpineol | 1178 | 8.4 ± 0.23 | 12.0 ± 1.07 | 38.3 ± 0.81 | 19.3 ± 4.14 | - | - | - | - | 2.1 ± 0.06 | 0.4 ± 0.02 |
| isoMenthol | 1179 | - | - | 0.2 ± 0.01 | - | - | - | - | - | - | - |
| Cryptone | 1187 | - | - | - | - | - | - | - | - | 0.2 ± 0.01 | - |
| α-Terpineol | 1189 | 1.2 ± 0.06 | 2.5 ± 0.31 | 1.9 ± 0.11 | - | - | - | - | 0.3 ± 0.04 | 3.1 ± 0.11 | 0.7 ± 0.06 |
| cis-Piperitol | 1195 | 0.2 ± 0.02 | - | 0.6 ± 0.04 | - | - | - | - | - | 0.3 ± 0.02 | - |
| Decanal | 1204 | - | - | 0.3 ± 0.04 | - | - | - | - | - | 0.5 ± 0.02 | 0.7 ± 0.06 |
| trans-Piperitol | 1207 | 0.4 ± 0.05 | 0.5 ± 0.20 | 1.0 ± 0.05 | - | - | - | - | - | 0.6 ± 0.04 | 0.2 ± 0.01 |
| Citronellol | 1230 | 1.1 ± 0.12 | - | 0.1 ± 0.08 | - | 0.4 ± 0.13 | - | - | - | 1.7 ± 0.11 | - |
| Neral | 1240 | - | - | - | - | - | - | - | - | 1.0 ± 0.07 | - |
| Piperitone | 1252 | 2.5 ± 0.15 | 2.7 ± 0.25 | 0.1 ± 0.07 | - | - | - | - | - | 6.9 ± 0.48 | 1.7 ± 0.16 |
| (E)-2-Decenal | 1260 | - | - | - | - | - | - | - | - | 0.1 ± 0.01 | - |
| Phellandral | 1272 | - | - | - | - | - | - | - | - | 0.1 ± 0.01 | - |
| trans-Citral | 1273 | - | - | - | - | - | - | - | - | 1.6 ± 0.13 | - |
| n-Tridecane | 1300 | - | - | - | - | - | - | - | - | - | 0.3 ± 0.04 |
| Undecanal | 1306 | - | - | - | - | - | - | - | - | - | 0.1 ± 0.01 |
| δ-Elemene | 1340 | 0.1 ± 0.01 | 0.4 ± 0.50 | - | - | 0.3 ± 0.07 | 1.8 ± 0.84 | 2.0 ± 0.00 | 0.6 ± 0.02 | - | - |
| Citronellyl acetate | 1354 | - | - | - | - | - | - | - | - | 0.2 ± 0.04 | 0.1 ± 0.02 |
| Eugenol | 1358 | - | - | - | 1.1 ± 0.04 | - | - | - | 0.4 ± 0.14 | - | - |
| β-Elemene | 1392 | - | - | - | - | - | - | 0.4 ± 0.00 | - | - | - |
| 1-Tetradecene | 1392 | - | - | - | - | - | - | - | - | - | 0.1 ± 0.08 |
| n-Tetradecane | 1400 | - | - | - | - | - | - | - | - | - | 0.1 ± 0.09 |
| Dodecanal | 1408 | - | - | 0.1 ± 0.07 | - | - | - | - | - | - | 0.4 ± 0.11 |
| cis-α-Bergamotene | 1416 | - | - | - | - | - | - | - | - | - | 0.1 ± 0.02 |
| β-Caryophyllene | 1420 | 0.7 ± 0.11 | 21.3 ± 1.05 | 0.4 ± 0.09 | 14.1 ± 1.00 | 0.2 ± 0.04 | 6.9 ± 1.20 | - | 0.6 ± 0.22 | 0.8 ± 0.16 | 2.3 ± 0.39 |
| trans-α-Bergamotene | 1438 | - | 1.2 ± 0.37 | - | 2.3 ± 0.01 | 0.2 ± 0.04 | 1.9 ± 0.56 | - | 0.9 ± 0.25 | 1.5 ± 0.31 | 2.9 ± 0.48 |
| α-Humulene | 1456 | - | 8.4 ± 0.13 | 0.6 ± 0.14 | 12.7 ± 0.52 | 0.2 ± 0.06 | 4.0 ± 0.37 | - | 0.3 ± 0.06 | 1.0 ± 0.21 | 4.6 ± 0.69 |
| (E)-β-Farnesene | 1460 | - | - | - | - | - | 0.2 ± 0.23 | - | - | - | 0.2 ± 0.04 |
| β-Santalene | 1463 | - | - | - | - | - | - | - | - | - | 0.1 ± 0.02 |
| γ-Gurjunene | 1474 | 0.5 ± 0.72 | 0.9 ± 0.25 | - | - | - | - | - | - | - | - |
| γ-Muurolene | 1477 | - | 0.7 ± 0.25 | - | - | - | - | - | - | - | - |
| Germacrene D | 1478 | 0.3 ± 0.06 | - | - | - | 0.3 ± 0.07 | 2.7 ± 0.04 | 2.6 ± 0.05 | 1.0 ± 0.08 | - | 0.2 ± 0.01 |
| β-Chamigrene | 1485 | - | - | - | - | - | - | - | - | - | 0.1 ± 0.03 |
| δ-Selinene | 1490 | - | - | - | - | 0.1 ± 0.07 | 1.0 ± 0.11 | - | 1.2 ± 0.11 | - | - |
| Bicyclogermacrene | 1496 | 4.9 ± 0.62 | 10.9 ± 0.33 | 1.9 ± 0.40 | 9.8 ± 0.74 | 6.9 ± 1.10 | 28 ± 1.61 | 39.8 ± 2.24 | 20.3 ± 0.07 | 0.8 ± 0.20 | 1.0 ± 0.20 |
| n-Pentadecane | 1500 | - | - | - | - | - | - | - | - | - | 1.1 ± 0.25 |
| (Z)-α-Bisabolene | 1504 | - | - | - | - | - | - | - | - | - | 0.4 ± 0.09 |
| α-Bulnesene | 1505 | - | - | - | - | - | - | 0.9 ± 0.07 | 0.3 ± 0.03 | - | - |
| Germacrene A | 1506 | - | - | - | - | 0.2 ± 0.03 | - | - | - | - | - |
| α-Chamigrene | 1508 | - | - | - | - | - | 2.4 ± 0.36 | 2.5 ± 0.14 | - | - | - |
| β-Bisabolene | 1509 | 0.8 ± 0.12 | 4.8 ± 0.4 | 0.7 ± 0.18 | 9.2 ± 0.35 | 2.0 ± 0.42 | 24.6 ± 0.14 | - | - | 2.6 ± 0.57 | 5.6 ± 1.05 |
| trans-γ-Cadinene | 1513 | - | - | - | - | - | - | - | 0.3 ± 0.06 | - | - |
| (Z)-γ-Bisabolene | 1515 | - | - | - | - | - | - | - | - | - | 0.1 ± 0.03 |
| Selina-3,7(11)-diene | 1542 | - | - | - | - | - | - | - | - | - | 0.1 ± 0.07 |
| Germacrene B | 1554 | - | - | - | - | 0.6 ± 0.15 | 5.2 ± 0.01 | 0.9 ± 0.02 | 0.2 ± 0.25 | - | 0.5 ± 0.11 |
| Palustrol | 1568 | - | 1.3 ± 0.06 | 0.2 ± 0.04 | - | 0.6 ± 0.12 | 1.7 ± 0.79 | - | 6.0 ± 0.00 | - | - |
| Spathulenol | 1576 | - | - | - | - | 0.1 ± 0.04 | - | 1.1 ± 0.15 | - | - | - |
| Caryophyllene oxide | 1581 | - | - | - | 15.6 ± 1.29 | - | - | - | - | - | - |
| Globulol | 1583 | 0.6 ± 0.12 | 9.5 ± 0.13 | 0.5 ± 0.13 | - | 1.8 ± 0.37 | 3.8 ± 0.99 | 2.9 ± 0.23 | 14.5± 0.15 | - | 0.1 ± 0.08 |
| Viridiflorol | 1590 | 0.7 ± 0.12 | 7.9 ± 0.30 | 0.4 ± 0.13 | 5.8 ± 1.58 | 1.7 ± 0.34 | 10.9 ± 0.21 | 1.2 ± 0.21 | 19.9 ± 0.01 | - | - |
| Guaiol | 1595 | 0.2 ± 0.03 | 1.1 ± 0.35 | 0.2 ± 0.03 | - | 0.5 ± 0.11 | 3.2 ± 0.05 | 0.3 ± 0.01 | 11.9 ± 0.71 | - | - |
| Cedrol | 1596 | 0.3 ± 0.12 | 1.4 ± 0.42 | 0.3 ± 0.08 | - | 0.8 ± 0.17 | 1.0 ± 1.45 | 0.8 ± 0.07 | 8.5 ± 0.08 | - | - |
| n-Hexadecane | 1600 | - | - | - | - | - | - | - | - | - | 0.6 ± 0.22 |
| 5-epi-7-epi-α-Eudesmol | 1603 | - | - | - | - | - | - | - | 2.2 ± 0.06 | - | - |
| Humulene epoxide II | 1608 | - | 2.0 ± 0.20 | - | 4.1 ± 0.83 | - | - | - | - | - | - |
| Selin-6-en-4-ol | 1618 | - | 1.2 ± 0.25 | - | - | 0.2 ± 0.06 | 0.5 ± 0.66 | - | 2.2 ± 0.08 | - | - |
| Caryophylla-4(14),8(15)-dien-5-ol | 1637 | - | - | - | 1.9 ± 0.36 | - | - | - | - | - | - |
| isoSpathulenol | 1639 | - | - | - | - | 0.1 ± 0.11 | - | - | - | - | - |
| epi-α-Cadinol | 1641 | - | - | - | 3.2 ± 1.03 | 0.2 ± 0.10 | - | 0.4 ± 0.05 | 2.6 ± 0.49 | - | - |
| Cubenol | 1643 | - | - | - | - | 0.2 ± 0.07 | - | 0.5 ± 0.04 | - | - | - |
| α-Cadinol | 1654 | - | 0.8 ± 0.35 | - | - | 0.3 ± 0.08 | - | 0.4 ± 0.10 | 1.9 ± 0.09 | - | - |
| β-Bisabolol | 1672 | - | - | - | - | - | - | - | - | 0.2 ± 0.25 | - |
| Tetradecanol | 1676 | - | - | - | - | - | - | - | - | - | 0.2 ± 0.08 |
| α-Bisabolol | 1683 | - | 1.4 ± 0.08 | - | - | 0.2 ± 0.04 | - | - | - | 0.5 ± 0.18 | 0.4 ± 0.18 |
| n-Heptadecane | 1700 | - | - | - | - | - | - | - | - | - | 0.2 ± 0.08 |
| Monoterpene hydrocarbons | 68.2 ± 4.11 | 1.4 ± 0.48 | 46.1 ± 2.68 | 1.0 ± 0.54 | 77.7 ± 4.37 | 0.2 ± 0.24 | 41.6 ± 2.83 | 0.4 ± 0.11 | 55.5 ± 3.05 | 72.8 ± 4.91 | |
| Oxygenated monoterpenes | 21.7 ± 1.05 | 22.2 ± 1.05 | 48.0 ± 1.34 | 19.3 ± 4.14 | 2.1 ± 0.18 | - | 1.1 ± 0.12 | 0.5 ± 0.08 | 35.5 ± 1.18 | 4.7 ± 0.27 | |
| Sesquiterpene hydrocarbons | 7.3 ± 1.65 | 48.5 ± 0.41 | 3.6 ± 0.81 | 48.1 ± 0.45 | 10.9 ± 2.04 | 78.8 ± 3.97 | 49.2 ± 2.38 | 25.7 ± 0.03 | 6.7 ± 1.45 | 18.2 ± 3.22 | |
| Oxygenated sesquiterpenes | 1.8 ± 0.39 | 26.6 ± 0.10 | 1.6 ± 0.41 | 30.5 ± 5.09 | 6.5 ± 1.60 | 21.1 ± 3.74 | 7.6 ± 0.60 | 69.8 ± 1.05 | 0.6 ± 0.43 | 0.5 ± 0.25 | |
| Phenylpropanoids | - | - | - | 1.1 ± 0.04 | - | - | - | 0.4 ± 0.14 | - | - | |
| Non-terpene derivatives | - | - | 0.3 ± 0.11 | - | - | - | - | - | 1.7 ± 0.00 | 3.7 ± 1.03 | |
| Total identified (%) | 99.0 ± 1.02 | 98.6 ± 1.02 | 99.6 ± 0.01 | 100 ± 0.01 | 97.2 ± 0.54 | 100 ± 0.01 | 99.5 ± 0.04 | 96.8 ± 0.74 | 100 ± 0.01 | 99.9 ± 0.13 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cioni, E.; Migone, C.; Ascrizzi, R.; Muscatello, B.; De Leo, M.; Piras, A.M.; Zambito, Y.; Flamini, G.; Pistelli, L. Comparing Metabolomic and Essential Oil Fingerprints of Citrus australasica F. Muell (Finger Lime) Varieties and Their In Vitro Antioxidant Activity. Antioxidants 2022, 11, 2047. https://doi.org/10.3390/antiox11102047
Cioni E, Migone C, Ascrizzi R, Muscatello B, De Leo M, Piras AM, Zambito Y, Flamini G, Pistelli L. Comparing Metabolomic and Essential Oil Fingerprints of Citrus australasica F. Muell (Finger Lime) Varieties and Their In Vitro Antioxidant Activity. Antioxidants. 2022; 11(10):2047. https://doi.org/10.3390/antiox11102047
Chicago/Turabian StyleCioni, Emily, Chiara Migone, Roberta Ascrizzi, Beatrice Muscatello, Marinella De Leo, Anna Maria Piras, Ylenia Zambito, Guido Flamini, and Luisa Pistelli. 2022. "Comparing Metabolomic and Essential Oil Fingerprints of Citrus australasica F. Muell (Finger Lime) Varieties and Their In Vitro Antioxidant Activity" Antioxidants 11, no. 10: 2047. https://doi.org/10.3390/antiox11102047
APA StyleCioni, E., Migone, C., Ascrizzi, R., Muscatello, B., De Leo, M., Piras, A. M., Zambito, Y., Flamini, G., & Pistelli, L. (2022). Comparing Metabolomic and Essential Oil Fingerprints of Citrus australasica F. Muell (Finger Lime) Varieties and Their In Vitro Antioxidant Activity. Antioxidants, 11(10), 2047. https://doi.org/10.3390/antiox11102047