Effects of Synthetic Short Cationic Antimicrobial Peptides on the Catalytic Activity of Myeloperoxidase, Reducing Its Oxidative Capacity
Abstract
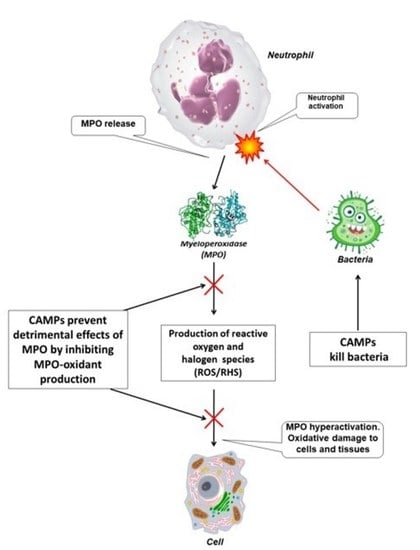
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Myeloperoxidase
2.3. Absorbance Spectra
2.4. Dityrosine Fluorescence Detection
2.5. Mass Spectrometry
2.6. Measurement of Sulfhydryl Groups
2.7. Myeloperoxidase Peroxidase Activity
2.8. Myeloperoxidase Chlorinating Activity
2.9. Calculation of Michaelis Constants, Maximal Rate, and Inhibition Constants
2.10. Data Analysis
3. Results
3.1. Characteristics of CAMPs Used in This Study
3.2. Detection of CAMP Effects on MPO Catalytic Activity Using Absorbance Spectra
3.3. Involvement of Peptide Tyrosine Residues in MPO Catalysis
3.3.1. Detection of MPO-Induced Oxidation of Peptide Tyrosine Residues Using Fluorometry
3.3.2. Detection of MPO-Induced Oxidation of Peptide Tyrosine Residues Using Mass Spectrometry
3.4. Involvement of Peptide Cysteine Residues in the Effects of Peptides on MPO Activity
3.5. Effects of CAMPs on MPO Peroxidase Activity
3.6. Effects of CAMPs on MPO Chlorinating Activity
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Moretta, A.; Scieuzo, C.; Petrone, A.M.; Salvia, R.; Manniello, M.D.; Franco, A.; Lucchetti, D.; Vassallo, A.; Vogel, H.; Sgambato, A.; et al. A new hope in biomedical and pharmaceutical fields. Front. Cell. Infect. Microbiol. 2021, 11, 668632. [Google Scholar] [CrossRef]
- Mwangi, J.; Hao, X.; Lai, R.; Zhang, Z.-Y. Antimicrobial peptides: New hope in the war against multidrug resistance. Zool. Res. 2019, 18, 488–505. [Google Scholar] [CrossRef]
- Mahlapuu, M.; Hakansson, J.; Ringstad, L.; Bjorn, C. Antimicrobial peptides: An emerging category of therapeutic agents. Front. Cell. Infect. Microbiol. 2016, 6, 194. [Google Scholar] [CrossRef] [Green Version]
- Vázquez, A.; Perdomo-Morales, R.; Montero-Alejo, V. Natural antimicrobial peptides. Biotecnol. Appl. 2018, 35, 4101–4107. [Google Scholar]
- Pasupuleti, M.; Schmidtchen, A.; Malmsten, M. Antimicrobial peptides: Key components of the innate immune system. Crit. Rev. Biotechnol. 2012, 32, 143–171. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.-J.; Gallo, R.L. Antimicrobial peptides. Curr. Biol. 2016, 26, R14–R19. [Google Scholar] [CrossRef] [PubMed]
- Ramazi, S.; Mohammadi, N.; Allahverdi, A.; Khalili, E.; Abdolmaleki, P. A review on antimicrobial peptides databases and the computational tools. Database 2022, 2022, baac011. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.M.; Edwards, M.A.; Li, J.; Yip, C.M.; Deber, C.M. Roles of hydrophobicity and charge distribution of cationic antimicrobial peptides in peptide-membrane interactions. J. Biol. Chem. 2012, 287, 7738–7745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, L.T.; Haney, E.F.; Vogel, H.J. The expanding scope of antimicrobial peptide structure and their modes of action. Trends Biotechnol. 2011, 29, 464–472. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Yan, Z.-B.; Meng, Y.-M.; Hong, X.-Y.; Shao, G.; Ma, J.-J.; Cheng, X.-R.; Liu, J.; Kang, J.; Fu, C.-Y. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Military Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef]
- Spohn, R.; Daruka, L.; Lazar, V.; Martins, A.; Vidovics, F.; Grezal, G.; Mehi, J.; Kintses, B.; Szamel, M.; Janger, P.; et al. Integrated evolutionary analysis reveals antimicrobial peptides with limited resistance. Nat. Commun. 2019, 10, 4538. [Google Scholar] [CrossRef]
- Guilhelmelli, F.; Vilela, N.; Albuquerque, P.; Derengowski, L.S.; Silva-Pereira, I.; Kyaw, C.M. Antibiotic development challenges: The various mechanisms of action of antimicrobial peptides and of bacterial resistance. Front. Microbiol. 2013, 4, 353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benfield, A.H.; Henriques, S.T. Mode-of-action of antimicrobial peptides: Membrane disruption vs. intracellular mechanisms. Front. Med. Technol. 2020, 2, 610997. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, K. Control of cell selectivity of antimicrobial peptides. Biochim. Biophys. Acta 2009, 1788, 1687–1692. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.Y.; Weibel, D.B. Organization and function of anionic phospholipids in bacteria. Appl. Microbiol. Biotechnol. 2016, 100, 4255–4267. [Google Scholar] [CrossRef]
- Vance, J.E. Phospholipid synthesis and transport in mammalian cells. Traffic 2015, 16, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, J.H. Synthetic peptide API manufacturing: A mini review of current perspectives for peptide manufacturing. Bioorg. Med. Chem. 2018, 26, 2914–2918. [Google Scholar] [CrossRef]
- Cardoso, P.; Glossop, H.; Meikle, T.G.; Aburto-Medina, A.; Conn, C.E.; Sarojini, V.; Valery, C. Molecular engineering of antimicrobial peptides: Microbial targets, peptide motifs and translation opportunities. Biophys. Rev. 2021, 13, 35–69. [Google Scholar] [CrossRef] [PubMed]
- Tucker, A.T.; Leonard, S.P.; DuBois, C.D.; Knauf, G.A.; Cunningham, A.L.; Wilke, C.O.; Trent, M.S.; Davies, B.W. Discovery of next-generation antimicrobials through bacterial self-screening of surface-displayed peptide libraries. Cell 2018, 172, 618–628. [Google Scholar] [CrossRef]
- Grafskaia, E.N.; Pavlova, E.R.; Latsis, I.A.; Malakhova, M.V.; Ivchenkov, D.V.; Bashkirov, P.V.; Kot, E.F.; Mineev, K.S.; Arseniev, A.S.; Klinov, D.V.; et al. Non-toxic antimicrobial peptide Hm-AMP2 from leech metagenome proteins identified by the gradient-boosting approach. Mater. Des. 2022, 224, 111364. [Google Scholar] [CrossRef]
- Kim, H.; Jang, J.H.; Kim, S.C.; Cho, J.H. De novo generation of short antimicrobial peptides with enhanced stability and cell specificity. J. Antimicrob. Chemother. 2014, 69, 121–132. [Google Scholar] [CrossRef]
- Lima, B.; Ricci, M.; Garro, A.; Juhász, T.; Szigyártó, I.C.; Papp, Z.I.; Feresin, G.; Torre, J.G.; Cascales, J.L.; Fülöp, L.; et al. New short cationic antibacterial peptides. Synthesis, biological activity and mechanism of action. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183665. [Google Scholar] [CrossRef]
- Vakhrusheva, T.V.; Moroz, G.D.; Basyreva, L.Y.; Shmeleva, E.V.; Gusev, S.A.; Mikhalchik, E.V.; Grafskaia, E.N.; Latsis, I.A.; Panasenko, O.M.; Lazarev, V.N. Effects of medicinal leech-related cationic antimicrobial peptides on human blood cells and plasma. Molecules 2022, 27, 5848. [Google Scholar] [CrossRef]
- Svenson, J.; Brandsdal, B.-O.; Stensen, W.; Svendsen, J.-S. Albumin binding of short cationic antimicrobial micropeptides and its influence on the in vitro bactericidal effect. J. Med. Chem. 2007, 50, 3334–3339. [Google Scholar] [CrossRef] [PubMed]
- Sivertsen, A.; Isaksson, J.; Leiros, H.-K.S.; Svenson, J.; Svendsen, J.-S.; Brandsdal, B.-O. Synthetic cationic antimicrobial peptides bind with their hydrophobic parts to drug site II of human serum albumin. BMC Struct. Biol. 2014, 14, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilpert, K.; McLeod, B.; Yu, J.; Elliott, M.R.; Rautenbach, M.; Ruden, S.; Bürck, J.; Muhle-Goll, C.; Ulrich, A.S.; Keller, S.; et al. Short cationic antimicrobial peptides interact with ATP. Antimicrob. Agents Chemother. 2010, 54, 4480–4483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Othman, A.; Sekheri, M.; Filep, J.G. Roles of neutrophil granule proteins in orchestrating inflammation and immunity. FEBS J. 2022, 289, 3932–3953. [Google Scholar] [CrossRef]
- Davies, M.J.; Hawkins, C.L. The role of myeloperoxidase in biomolecule modification, chronic inflammation, and disease. Antioxid. Redox Signal. 2020, 32, 957–981. [Google Scholar] [CrossRef] [Green Version]
- Arnhold, J.; Furtmüller, P.G.; Obinger, C. Redox properties of myeloperoxidase. Redox Rep. 2003, 8, 179–186. [Google Scholar] [CrossRef]
- Davies, M. Myeloperoxidase-derived oxidation: Mechanisms of biological damage and its prevention. J. Clin. Biochem. Nutr. 2011, 48, 8–19. [Google Scholar] [CrossRef] [Green Version]
- Does, A.M.; Hensbergen, P.J.; Bogaards, S.J.; Cansoy, M.; Deelder, A.M.; Leeuwen, H.C.; Drijfhout, J.W.; Dissel, J.T.; Nibbering, P.H. The human lactoferrin-derived peptide hLF1-11 exerts immunomodulatory effects by specific inhibition of myeloperoxidase activity. J. Immunol. 2012, 188, 5012–5019. [Google Scholar] [CrossRef] [PubMed]
- Grafskaia, E.; Pavlova, E.; Babenko, V.; Latsis, I.; Malakhova, M.; Lavrenova, V.; Bashkirov, P.; Belousov, D.; Klinov, D.; Lazarev, V. The Hirudo medicinalis microbiome is a source of new antimicrobial peptides. Int. J. Mol. Sci. 2020, 21, 7141. [Google Scholar] [CrossRef] [PubMed]
- Beers, R.F.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Sokolov, A.V.; Kostevich, V.A.; Zakharova, E.T.; Samygina, V.R.; Panasenko, O.M.; Vasilyev, V.B. Interaction of ceruloplasmin with eosinophil peroxidase as compared to its interplay with myeloperoxidase: Reciprocal effect on enzymatic properties. Free Radic. Res. 2015, 46, 800–811. [Google Scholar] [CrossRef]
- Furtmüller, P.G.; Burner, U.; Obinger, C. Reaction of myeloperoxidase compound I with chloride, bromide, iodide, and thiocyanate. Biochemistry 1998, 37, 17923–17930. [Google Scholar] [CrossRef]
- Bekard, I.B.; Dunstan, D.E. Tyrosine autofluorescence as a measure of bovine insulin fibrillation. Biophys. J. 2009, 97, 2521–2531. [Google Scholar] [CrossRef] [Green Version]
- Verzini, S.; Shah, M.; Theillet, F.-X.; Belsom, A.; Bieschke, J.; Wanker, E.E.; Rappsilber, J.; Binolfi, A.; Selenko, P. Megadalton-sized dityrosine aggregates of a-synuclein retain high degrees of structural disorder and internal dynamics. J. Mol. Biol. 2020, 432, 166689. [Google Scholar] [CrossRef]
- Kostevich, V.A.; Sokolov, A.V. Oxidation of cysteine by ceruloplasmin leads to formation of hydrogen peroxide, which can be utilized by myeloperoxidase. Biochem. Biophys. Res. Commun. 2018, 503, 2146–2151. [Google Scholar] [CrossRef]
- Reut, V.E.; Kozlov, S.O.; Kudryavtsev, I.V.; Grudinina, N.A.; Kostevich, V.A.; Gorbunov, N.P.; Grigorieva, D.V.; Kalvinkovskaya, J.A.; Bushuk, S.B.; Varfolomeeva, E.Y.; et al. New application of the commercially available dye celestine blue B as a sensitive and selective fluorescent “turn-on” probe for endogenous detection of HOCl and reactive halogenated species. Antioxidants 2022, 11, 1719. [Google Scholar] [CrossRef]
- Osorio, D.; Rondón-Villarreal, P.; Torres, R. Peptides: A package for data mining of antimicrobial peptides. Small 2015, 7, 4–14. [Google Scholar] [CrossRef]
- Marquez, L.A.; Huang, J.T.; Dunford, H.B. Spectral and kinetic studies on the formation of myeloperoxidase compounds I and II: Roles of hydrogen peroxide and superoxide. Biochemistry 1994, 33, 1447–1454. [Google Scholar] [CrossRef]
- Marquez, L.A.; Dunford, H.B. Kinetics of oxidation of tyrosine and dityrosine by myeloperoxidase compounds I and II. J. Biol. Chem. 1995, 270, 30434–30440. [Google Scholar] [CrossRef] [Green Version]
- Hoogland, H.; Dekker, H.L.; van Riel, C.; van Kuilenburg, A.; Muijsers, A.O.; Wever, R. A steady state study on the formation of Compounds II and III of myeloperoxidase. Biochim. Biophys. Acta 1988, 955, 337–345. [Google Scholar] [CrossRef]
- Marquez, L.A.; Dunford, H.B.; Wart, H.V. Kinetic studies on the reaction of Compound II of myeloperoxidase with ascorbic acid. J. Biol. Chem. 1990, 265, 5666–5670. [Google Scholar] [CrossRef]
- Kettle, A.J.; Winterbourn, C.C. Myeloperoxidase: A key regulator of neutrophil oxidant production. Redox Rep. 1997, 3, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Vlasova, I.I.; Sokolov, A.V.; Arnhold, J. The free amino acid tyrosine enhances the chlorinating activity of human myeloperoxidase. J. Inorg. Biochem. 2012, 106, 76–83. [Google Scholar] [CrossRef]
- Tien, M. Myeloperoxidase-catalyzed oxidation of tyrosine. Arch. Biochem. Biophys. 1999, 367, 61–66. [Google Scholar] [CrossRef]
- Michon, T.; Chenu, M.; Kellershon, N.; Desmadril, M.; Guéguen, J. Horseradish peroxidase oxidation of tyrosine-containing peptides and their subsequent polymerization: A kinetic study. Biochemistry 1997, 36, 8504–8513. [Google Scholar] [CrossRef]
- Steffensen, C.L.; Mattinen, M.-L.; Andersen, H.J.; Kruus, K.; Buchert, J.; Nielsen, J.H. Cross-linking of tyrosine-containing peptides by hydrogen peroxide-activated Coprinus cinereus peroxidase. Eur. Food Res. Technol. 2008, 227, 57–67. [Google Scholar] [CrossRef]
- Burner, U.; Jantschko, W.; Obinger, C. Kinetics of oxidation of aliphatic and aromatic thiols by myeloperoxidase compounds I and II. FEBS Lett. 1999, 443, 290–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeng, J.; Fenna, R.E. X-ray crystal structure of canine myeloperoxidase at 3 A resolution. J. Mol. Biol. 1992, 226, 185–207. [Google Scholar] [CrossRef] [PubMed]
- Bolscher, B.G.; Wever, R. A kinetic study of the reaction between human myeloperoxidase, hydroperoxides and cyanide. Inhibition by chloride and thiocyanate. Biochim. Biophys. Acta 1984, 788, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Hori, H.; Fenna, R.E.; Kimura, S.; Ikeda-Saito, M. Aromatic substrate molecules bind at the distal heme pocket of myeloperoxidase. J. Biol. Chem. 1994, 269, 8388–8392. [Google Scholar] [CrossRef] [PubMed]
- Ramos, D.R.; García, M.V.; Canle, L.M.; Santaballa, J.A.; Furtmüller, P.G.; Obinger, C. Myeloperoxidase-catalyzed chlorination: The quest for the active species. J. Inorg. Biochem. 2008, 102, 1300–1311. [Google Scholar] [CrossRef] [PubMed]
- Heinecke, J.W.; Li, W.; Daehnke, H.L., III; Goldstein, J.A. Dityrosine, a specific marker of oxidation, is synthesized by the myeloperoxidase-hydrogen peroxide system of human neutrophils and macrophages. J. Biol. Chem. 1993, 268, 4069–4077. [Google Scholar] [CrossRef]
- Jacob, J.S.; Cistola, D.P.; Hsu, F.F.; Muzaffar, S.M.; Mueller, D.M.; Hazen, S.L.; Heinecke, J.W. Human phagocytes employ the myeloperoxidase-hydrogen peroxide system to synthesize dityrosine, trityrosine, pulcherosine, and isodityrosine by a tyrosyl radical-dependent pathway. J. Biol. Chem. 1996, 271, 19950–19956. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Jing, X.; Shi, Y.; Xu, H.; Du, J.; Guan, T.; Weihrauch, D.; Jones, D.W.; Wang, W.; Gourlay, D.; et al. N-acetyl lysyltyrosylcysteine amide inhibits myeloperoxidase, a novel tripeptide inhibitor. J. Lipid Res. 2013, 54, 3016–3029. [Google Scholar] [CrossRef]













| Peptide Name | Amino Acid Sequence | Mol. Mass, Da | Length | Net Charge (pH 7) | * Hydro- Phobicity | * Aliphatic Index | pI |
|---|---|---|---|---|---|---|---|
| Hm-AMP1 | RLKRFKRVALRREKTARNFRSIVS | 2988.61 | 24 | +9 | −0.95 | 81.25 | 12.9 |
| pept_1545 | FLIGKAIKRKFCLRSVWNA | 2250.81 | 19 | +5 | 0.33 | 107.8 | 11.6 |
| Hm-AMP8 | RAVIYKIPYNAIASRWIIAPKKC | 2675.31 | 23 | +5 | 0.21 | 114.78 | 10.6 |
| Hm-AMP2 | EKRWRRLIFNYF | 1728.05 | 12 | +3 | −1.00 | 65 | 11.4 |
| Decline in Peptide’s Sulfhydryl Groups as a Result of Peptide Incubation with MPO/H2O2 | ||
|---|---|---|
| 5 min Incubation | 90 min Incubation | |
| Pept_1545 | * 80 ± 4% | * 76 ± 5% |
| Hm-AMP8 | 93 ± 5% | * 79 ± 6% |
| Kinetic Parameters of MPO Peroxidase Activity towards Amplex Red | |||
|---|---|---|---|
| MPO | MPO/20 μM pept_1545 | MPO/2 μM Hm-AMP8 | |
| Michaelis constant (KM), μM | 2.4 | 3.4 | 6.5 |
| Maximum reaction rate (Vmax), a. u. | 235,917 | 214,729 | 175,664 |
| Type of inhibition | mixed | mixed | |
| Inhibition constant (Ki), μM | 48.3 | 1.1 | |
| Kinetic parameters of MPO peroxidase activity towards H2O2 | |||
| MPO | MPO/20μM pept_1545 | MPO/2μM Hm-AMP8 | |
| Michaelis constant (KM), μM | 2.85 | 2.64 | 1.71 |
| Maximum reaction rate (Vmax), a. u. | 288,269 | 210,265 | 152,160 |
| Type of inhibition | uncompetitive | uncompetitive | |
| Inhibition constant (Ki), μM | 52.8 | 1.79 | |
| Kinetic Parameters of MPO Chlorinating Activity towards Cl− | |||
|---|---|---|---|
| MPO | MPO/8 μM pept_1545 | MPO/0.8 μM Hm-AMP8 | |
| Michaelis constant (KM), μM | 90 | 127 | 41.5 |
| Maximum reaction rate (Vmax), s−1 | 45.4 | 45.2 | 18.2 |
| Type of inhibition | competitive | uncompetitive | |
| Inhibition constant (Ki), μM | 19.5 | 0.42 | |
| Kinetic parameters of MPO chlorinating activity towards H2O2 | |||
| MPO | MPO/8μM pept_1545 | MPO/0.8μM Hm-AMP8 | |
| Michaelis constant (KM), μM | 27.0 | 16.0 | 8.9 |
| Maximum reaction rate (Vmax), s−1 | 50.3 | 29.0 | 17.1 |
| Type of inhibition | uncompetitive | uncompetitive | |
| Inhibition constant (Ki), μM | 10.8 | 0.28 | |
| Interaction of CAMPs with MPO | |||
|---|---|---|---|
| H2O2-Induced Formation and Decay of MPO Compound II (CII). Oxidation of Peptide C and Y Residues | Inhibition of MPO Peroxidase Activity (Amplex Red Assay) | Inhibition of MPO Chlorinating Activity (CB Assay) | |
| Hm-AMP8 RAVIYKIPYNAIASRWIIAPKKC | CII accumulation; –SH oxidation | IC50 = 2 μM Uncompetitive—H2O2 Mixed—Amplex Red | IC50 = 0.3 μM Uncompetitive—H2O2 Uncompetitive—Cl− |
| Pept_1545 FLIGKAIKRKFCLRSVWNA | Depending on concentration, acceleration of CII reduction to native MPO and CII accumulation; –SH oxidation | IC50 = 11 μM Uncompetitive—H2O2 Mixed—Amplex Red | IC50 = 7 μM Uncompetitive—H2O2 Competitive—Cl− |
| Hm-AMP2 EKRWRRLIFNYF | Acceleration of CII reduction to native MPO; diY formation | No effect | No effect |
| Hm-AMP1 RLKRFKRVALRREKTARNFRSIVS | No effect | No effect | No effect |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vakhrusheva, T.V.; Sokolov, A.V.; Moroz, G.D.; Kostevich, V.A.; Gorbunov, N.P.; Smirnov, I.P.; Grafskaia, E.N.; Latsis, I.A.; Panasenko, O.M.; Lazarev, V.N. Effects of Synthetic Short Cationic Antimicrobial Peptides on the Catalytic Activity of Myeloperoxidase, Reducing Its Oxidative Capacity. Antioxidants 2022, 11, 2419. https://doi.org/10.3390/antiox11122419
Vakhrusheva TV, Sokolov AV, Moroz GD, Kostevich VA, Gorbunov NP, Smirnov IP, Grafskaia EN, Latsis IA, Panasenko OM, Lazarev VN. Effects of Synthetic Short Cationic Antimicrobial Peptides on the Catalytic Activity of Myeloperoxidase, Reducing Its Oxidative Capacity. Antioxidants. 2022; 11(12):2419. https://doi.org/10.3390/antiox11122419
Chicago/Turabian StyleVakhrusheva, Tatyana V., Alexey V. Sokolov, Grigoriy D. Moroz, Valeria A. Kostevich, Nikolay P. Gorbunov, Igor P. Smirnov, Ekaterina N. Grafskaia, Ivan A. Latsis, Oleg M. Panasenko, and Vassili N. Lazarev. 2022. "Effects of Synthetic Short Cationic Antimicrobial Peptides on the Catalytic Activity of Myeloperoxidase, Reducing Its Oxidative Capacity" Antioxidants 11, no. 12: 2419. https://doi.org/10.3390/antiox11122419
APA StyleVakhrusheva, T. V., Sokolov, A. V., Moroz, G. D., Kostevich, V. A., Gorbunov, N. P., Smirnov, I. P., Grafskaia, E. N., Latsis, I. A., Panasenko, O. M., & Lazarev, V. N. (2022). Effects of Synthetic Short Cationic Antimicrobial Peptides on the Catalytic Activity of Myeloperoxidase, Reducing Its Oxidative Capacity. Antioxidants, 11(12), 2419. https://doi.org/10.3390/antiox11122419