Differences in Oxidative Stress Markers and Antioxidant Enzyme Activities in Black Bean Aphid Morphs (Aphis fabae Scop.) Fed on the Primary Host Viburnum opulus L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plants and Aphids
2.2. Assay
2.3. H2O2 Assay
2.4. MDA Assay
2.5. SOD Assay
2.6. CAT Assay
2.7. APX Assay
2.8. Protein Assay
2.9. Statistical Analyses
3. Results
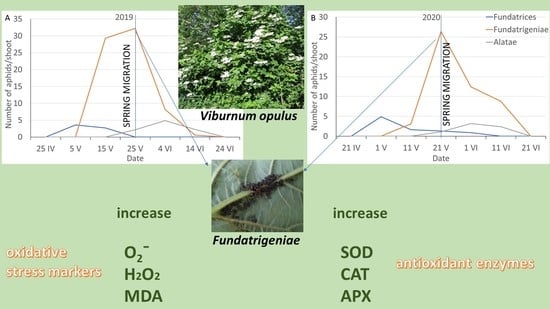
3.1. Population Dynamics of A. fabae on V. opulus
3.2. , H2O2 and MDA Content in A. fabae Morphs
3.3. SOD, CAT and APX Activity in A. fabae Morphs
3.4. Changes in , H2O2 and MDA Content in the Fundatrigeniae of A. fabae during Their Occurrence on the Primary Host
3.5. Changes in SOD, CAT and APX Activities in the Fundatrigeniae of A. fabae during Their Occurrence on the Primary Host
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Esmaeili-Vardanjani, M.; Askarianzadeh, A.; Saeidi, Z.; Hasanshahi, G.H.; Karimi, J.; Nourbakhsh, S.H. A study on common bean cultivars to identify sources of resistance against the black bean aphid, Aphis fabae Scopoli (Hemiptera: Aphididae). Arch. Phytopathol. Plant Prot. 2013, 46, 1598–1608. [Google Scholar] [CrossRef]
- Powell, G.; Hardie, J. A potent, morph-specific parturition stimulant in the overwintering host plant of the black bean aphid, Aphis fabae. Physiol. Entomol. 2001, 26, 194–201. [Google Scholar] [CrossRef]
- Fernandez-Quintanilla, C.; Fereres, C.A.; Godfrey, L.; Norris, R.F. Development and reproduction of Myzus persicae and Aphis fabae (Hom., Aphididae) on selected weed species surrounding sugar beet fields. J. Appl. Entomol. 2002, 126, 198–202. [Google Scholar] [CrossRef]
- Kafel, A.; Nadgórska-Socha, A.; Gospodarek, J.; Babczyńska, A.; Skowronek, M.; Kandziora, M.; Rozpendek, K. The effects of Aphis fabae infestation on the antioxidant response and heavy metal content in field grown Philadelphus coronarius plants. Sci. Total Environ. 2010, 408, 1111–1119. [Google Scholar] [CrossRef]
- Wilkinson, T.L.; Adams, D.; Minto, L.B.; Douglas, A.E. The impact of host plant on the abundance and function of symbiotic bacteria in an aphid. J. Exp. Biol. 2001, 204, 3027–3038. [Google Scholar] [CrossRef]
- Webster, B.; Bruce, T.; Pickett, J.; Hardie, J. Volatiles functioning as host cues in a blend become nonhost cues when presented alone to the black bean aphid. Anim. Behav. 2010, 79, 451–457. [Google Scholar] [CrossRef]
- Fajinmi, A.A.; Odebode, C.A.; Fajinmi, O.B. The effect of agro-ecological zones on the incidence and distribution of aphid vectors of pepper veinal mottle virus, on cultivated pepper (Capsicum annuum L.) in Nigeria. J. Central Eur. Agric. 2011, 12, 528–542. [Google Scholar] [CrossRef]
- Hulle, M.; d’Acier, A.C.; Bankhead-Dronnet, S.; Harrington, R. Aphids in the face of global change. Comptes Rendus Biol. 2010, 333, 497–503. [Google Scholar] [CrossRef]
- Fericean, L.M.; Horablaga, N.M.; Bănăţean-Dunea, I.; Rada, O.; Ostan, M. The behaviour, life cycle and biometrical measurements of Aphis fabae. Res. J. Agric. Sci. 2012, 44, 31. [Google Scholar]
- Sytykiewicz, H.; Goławska, S.; Chrzanowski, G. Effect of the bird cherry-oat aphid Rhopalosiphum padi L. feeding on phytochemical responses within the bird cherry Prunus padus L. Pol. J. Ecol. 2011, 59, 329–338. [Google Scholar]
- Goławska, S.; Łukasik, I. Antifeedant activity of luteolin and genistein against the pea aphid, Acyrthosiphon pisum. J. Pest. Sci. 2012, 85, 443–450. [Google Scholar] [CrossRef] [Green Version]
- Gildow, F.; Damsteegt, V.; Stone, A.; Schneider, W.; Luster, D.; Levy, L. Plum pox in North America: Identification of aphid vectors and a potential role for fruit in virus spread. Phytopathology 2004, 94, 868–874. [Google Scholar] [CrossRef]
- Nebreda, M.; Moreno, A.; Pérez, N.; Palacios, I.; Seco-Fernández, V.; Fereres, A. Activity of aphids associated with lettuce and broccoli in Spain and their efficiency as vectors of Lettuce mosaic virus. Virus Res. 2004, 100, 83–88. [Google Scholar] [CrossRef]
- Jovičić, I.; Radonjić, A.; Petrović-Obradović, O. Flight activity of aphids as potential vectors of viral infection of alfalfa in Serbia. Pestic. Phytomed. (Belgrade) 2017, 32, 173–179. [Google Scholar] [CrossRef] [Green Version]
- Lobstein, A.; Weniger, B.; Malécot, V.; Um, B.H.; Alzate, F.; Anton, R. Polyphenolic content of two Colombian Viburnum species (Caprifoliaceae). Biochem. Syst. Ecol. 2003, 31, 95–97. [Google Scholar] [CrossRef]
- Jordheim, M.; Giske, N.H.; Andersen, Ø.M. Anthocyanins in Caprifoliaceae. Biochem. Syst. Ecol. 2006, 35, 153–159. [Google Scholar] [CrossRef]
- Heinonen, M. Antioxidant activity and antimicrobial effect of berry phenolics—Finnish perspective. Mol. Nutr. Food Res. 2007, 51, 684–691. [Google Scholar] [CrossRef]
- Li, W.; Hydamaka, A.W.; Lowry, L.; Beta, T. Comparison of antioxidant capacity and phenolic compounds of berries, chokecherry and sea buckthorn. Cent. Eur. J. Biol. 2009, 4, 499–506. [Google Scholar]
- Šavikin, K.; Zdunić, G.; Janković, T.; Tasić, S.; Menković, N.; Stević, T.; Dordević, B. Phenolic content and radical scavenging capacity of berries and related jams from certificated area in Serbia. Plant Foods Hum. Nutr. 2009, 64, 212–221. [Google Scholar] [CrossRef]
- Calis, I.; Yuruker, A.; Ruegger, H.; Wright, A.D.; Sticher, O. Lantanoside, a monocyclic C10 iridoid glucoside from Viburnum lantana. Phytochemistry 1995, 38, 163–165. [Google Scholar] [CrossRef]
- Tomassini, L.; Brkic, D.; Foddai, S.; Nicoletti, M. Iridoid glucosides from Viburnum rhytidophyllum. Phytochemistry 1997, 44, 751–753. [Google Scholar] [CrossRef]
- Deineka, V.I.; Sorokopudov, V.N.; Deineka, L.A.; Shaposhnik, E.I.; Kol′tsov, S.V. Anthocyanins from fruit of some plants of the Caprifoliaceae family. Chem. Nat. Compd. 2005, 41, 162–164. [Google Scholar] [CrossRef]
- Gatehouse, J.A. Plant resistance towards insect herbivores: Dynamic interaction. New Phytol. 2002, 156, 145–169. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Kuźniak, E.; Urbanek, H. The involvement of hydrogen peroxide in plant responses to stresses. Acta Physiol. Plant. 2000, 22, 195–203. [Google Scholar] [CrossRef]
- Groβ, F.; Durner, J.; Gaupels, F. Nitric oxide, antioxidants and prooxidants in plant defence responses. Front. Plant Sci. 2013, 4, 419. [Google Scholar]
- Shankar, P.; Yinghua, H. Elevated production of reactive oxygen species is related to host plant resistance to sugarcane aphid in sorghum. Plant Signal. Behav. 2021, 16, 1849523. [Google Scholar]
- Łukasik, I.; Goławska, S.; Wójcicka, A. Antioxidant defense mechanisms of cereal aphids based on ascorbate and ascorbate peroxidase. Biologia 2009, 64, 994–998. [Google Scholar] [CrossRef]
- Łukasik, I.; Goławska, S.; Wójcicka, A.; Goławski, A. Effect of host plants on antioxidant system of pea aphid Acyrthosiphon pisum. Bull. Insectol. 2011, 64, 153–158. [Google Scholar]
- Łukasik, I.; Leszczyński, B.; Dixon, A.F.G. Changes in bird cherry-oat metabolism while occuring on primary host. In Aphids in a New Millenium, 1st ed.; Simon, J.C., Dedryver, C.A., Rispe, C., Hulle, M., Eds.; INRA: Paris, France, 2004; pp. 463–469. [Google Scholar]
- Łukasik, I. Effect of host alternation on the activity of adaptive enzymes of the bird cherry-oat aphid Rhopalosiphum padi (L.). J. Pest Sci. 2009, 82, 203–209. [Google Scholar] [CrossRef]
- Green, M.J.; Hill, H.A. Chemistry of dioxygen. Meth. Enzymol. 1984, 105, 3–22. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M.C. Role of free radicals and catalytic metal ions in human disease: An overview. Meth. Enzymol. 1990, 186, 1–85. [Google Scholar]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Meth. Enzymol. 1978, 52, 302–310. [Google Scholar]
- Marklund, S.L. Pyrogallol autooxidation. In CRC Handbook of Methods for Oxygen Radical Research, 1st ed.; Greenwald, R.A., Ed.; CRC Press: Boca Raton, FL, USA, 1985; pp. 243–247. [Google Scholar]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar]
- Asada, K. Chloroplasts: Formation of active oxygen species and its scavenging. Methods Enzymol. 1984, 105, 422–429. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Legrand, M.A.; Colinet, H.; Vernon, P.; Hance, T. Autumn, winter and spring dynamics of aphid Sitobion avenae and parasitoid Aphidius rhopalosiphi interactions. Ann. Appl. Biol. 2004, 145, 139–144. [Google Scholar] [CrossRef]
- Jaśkiewicz, B. Aphids (Homoptera, Aphidodea) inhabiting the shrubs of Cotoneaster divaricatus Rehder et E. H. Wilson in the urban green area of Lublin. Part I. The population dynamics. EJPAU Ser. Hortic. 2004, 7, 2. [Google Scholar]
- Michaud, J.P.; Belliure, B. Consequences of foundress aggregation in the brown citrus aphid Toxoptera citricida. Ecol. Entomol. 2000, 25, 307–314. [Google Scholar] [CrossRef]
- Angeli, G.; Simoni, S. Apple cultivars acceptance by Dysaphis plantaginea Passerini (Homoptera: Aphididae). J. Pest Sci. 2006, 79, 175–179. [Google Scholar] [CrossRef]
- Hodgson, E.W.; Venette, R.C.; Abrahamson, M.; Ragsdale, D.W. Alate production of soybean aphid (Homoptera: Aphididae) in Minnesota. Environ. Entomol. 2005, 3, 1456–1463. [Google Scholar] [CrossRef] [Green Version]
- Voegtlin, D.J.; O’Neil, R.J.; Graves, W.R.; Lagos, D.; Yoo, H.J.S. Potential winter hosts of soybean aphid. Ann. Entomol. Soc. Am. 2005, 98, 690–693. [Google Scholar] [CrossRef]
- Braendle, C.; Davis, G.K.; Brisson, J.A.; Stern, D.L. Wing dimorphism in aphids. Heredity 2006, 97, 192–199. [Google Scholar] [CrossRef] [Green Version]
- Sandström, J.; Talang, A.; Moran, N.A. Nutritional enhacement of host plants by aphids–a comparison of three aphid species on grasses. J. Insect Physiol. 2000, 46, 33–40. [Google Scholar] [CrossRef]
- Douglas, A.E. Phloem-sap feeding by animals: Problems and solutions. J. Exp. Bot. 2006, 57, 747–754. [Google Scholar] [CrossRef] [Green Version]
- Cam, M.; Hisil, Y.; Kuscu, A. Organic acid, phenolic content, and antioxidant capacity of fruit flesh and seed of Viburnum opulus. Chem. Nat. Compd. 2007, 43, 460–461. [Google Scholar] [CrossRef]
- Perova, I.B.; Zhogova, A.A.; Cherkashin, A.V.; Éller, K.I.; Ramenskaya, G.V. Biologically active sunstances from European guelder berry fruits. Pharm. Chem. J. 2014, 48, 332–339. [Google Scholar] [CrossRef]
- Ersoy, N.; Ercisli, S.; Gundogdu, M. Evaluation of European Cranberrybush (Viburnum opulus L.) genotypes for agro-morphological, biochemical and bioactive characteristics in Turkey. Folia Hortic. 2017, 29, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Polka, D.; Podsędek, A.; Koziołkiewicz, M. Comparison of chemical composition and antioxidant capacity of fruit, flower and bark of Viburnum opulus. Plant Foods Hum. Nutr. 2019, 74, 436–442. [Google Scholar] [CrossRef] [Green Version]
- Kajszczak, D.; Zakłos-Szyda, M.; Podsędek, A. Viburnum opulus L.—A review of phytochemistry and biological effects. Nutrients 2020, 12, 3398. [Google Scholar] [CrossRef]
- Czerniewicz, P.; Leszczyński, B.; Chrzanowski, G.; Sempruch, C.; Sytykiewicz, H. Effects of host plant phenolics on spring migration of bird cherry-oat aphid (Rhopalosiphum padi L.). Allelopath. J. 2011, 27, 309–316. [Google Scholar]
- Eleftherianos, I.; Vamvatsikos, P.; Ward, D.; Gravanis, F. Changes in the levels of plant total phenols and free amino acids induced by two cereal aphids and effects on aphid fecundity. J. Appl. Entomol. 2006, 130, 15–19. [Google Scholar] [CrossRef]
- Lahtinen, M.; Salminen, J.-P.; Kapari, L.; Lempa, K.; Ossipov, V.; Sinkkonen, J.; Valkama, E.; Haukioja, E.; Pihlaja, K. Defensive effect surface flavonoid aglycones of Betula pubescens leaves against first instar Epirrita autumnata larvae. J. Chem. Ecol. 2004, 30, 2257–2268. [Google Scholar] [CrossRef]
- Urbańska, A. Occurrence and source of hydrogen peroxide in aphids. EJPAU 2009, 12, 27. [Google Scholar]
- Łukasik, I.; Goławska, S. Effect of plant o-dihydroxyphenols and quinone on generation of reactive oxygen species within the grain aphid tissues. Pestycydy/Pesticides 2008, 3–4, 117–124. [Google Scholar]
- Łukasik, I.; Goławska, S.; Leszczyński, B. Biochemical markers of oxidative stress within cereal aphid tissues. Acta Biol. Hung. 2009, 60, 263–272. [Google Scholar] [CrossRef] [Green Version]
- Łukasik, I.; Goławska, S. Effect of host plant on levels of reactive oxygen species and antioxidants in the cereal aphids Sitobion avenae and Rhopalosiphum padi. Biochem. Syst. Ecol. 2013, 51, 232–239. [Google Scholar] [CrossRef]
- Madhusudhan, V.V.; Miles, P.W. Mobility of salivary components as a possible reason for differences in response of alfalfa to the spotted alfalfa aphid and pea aphid. Entomol. Exp. Appl. 1998, 86, 25–39. [Google Scholar] [CrossRef]
- Łukasik, I.; Goławska, S.; Wójcicka, A. Effect of host plants on biochemical markers of oxidative stress within tissues of pea aphid. J. Plant Prot. Res. 2012, 52, 59–63. [Google Scholar] [CrossRef]
- Czerniewicz, P.; Chrzanowski, G. The effect of Santolina chamaecyparissus and Tagetes patula essential oils on biochemical markers of oxidative stress in aphids. Insects 2021, 12, 360. [Google Scholar] [CrossRef]
- Esterbauer, H. Estimation of peroxidative damage. A critical review. Pathol. Biol. 1996, 44, 25–28. [Google Scholar]
- Walling, L.L. Avoiding effective defenses: Strategies employed by phloem-feeding insects. Plant Physiol. 2008, 146, 859–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukasik, I. Changes in activity of superoxide dismutase and catalase within cereal aphids in response to plant o-dihydroxyphenols. J. Appl. Entomol. 2007, 131, 209–214. [Google Scholar] [CrossRef]
- Durak, R.; Molon, M.; Durak, T.; Chrzanowski, G. The enzymatic markers of the adaptation of Cinara tujafilina to changing the host plant. Ethol. Ecol. Evol. 2018, 30, 416–429. [Google Scholar] [CrossRef]
- Abdelsalam, S.A.; Awad, A.M.A.; Abdelrahman, M.A.A.; Nasser, M.A.K.; Abdelhamid, N.M.R. Antioxidant defense response of the green peach aphid, Myzus persicae against secondary metabolites of the host plants cumin, anise, and coriander. J. Agric. Sci. Tech. 2016, 18, 1583–1592. [Google Scholar]
- Lee, K.; Berenbaum, M.R. Food utilization and antioxidant enzyme activities of black swallowtail in response to plant phototoxins. Arch. Insect Biochem. Physiol. 1993, 23, 79–89. [Google Scholar] [CrossRef]
- Khurshid, A.; Inayat, R.; Tamkeen, A.; Haq, I.U.; Li, C.; Boamah, S.; Zhou, J.-J.; Liu, C. Antioxidant enzymes and heat-shock protein genes of green peach aphid (Myzus persicae) under short-time heat stresss. Front. Physiol. 2021, 12, 805509. [Google Scholar] [CrossRef] [PubMed]
- Leszczyński, B.; Łukasik, I.; Urbańska, A.; Jóźwiak, B. Biochemiczne oddziaływania podczas zmiany roślinnych żywicieli przez mszycę czeremchowo-zbożową. In Biochemiczne Oddziaływania Środowiskowe, 1st ed.; Oleszek, K., Głowniak, B., Leszczyński, B., Eds.; Akademia Medyczna: Lublin, Poland, 2001; pp. 128–209. [Google Scholar]
- Urbańska, A. Location and variability of catalase activity within aphids. EJPAU 2007, 10, 38. [Google Scholar]
- Rup, P.J.; Sohal, S.K.; Kaur, H. Studies on the role of six enzymes in the metabolism of kinetin in mustard aphid, Lipaphis erysimi (Kalt.). J. Environ. Biol. 2006, 27, 579–584. [Google Scholar]
- Zhang, M.; Fang, T.; Pu, G.; Sun, X.; Zhou, X.; Cai, Q. Xenobiotic metabolism of plant secondary compounds in the English grain aphid, Sitobion avenae (F.) (Hemiptera: Aphididae). Pestic. Biochem. Physiol. 2013, 107, 44–49. [Google Scholar] [CrossRef]
- Durak, R.; Dampc, J.; Kula-Maximenko, M.; Molon, M.; Durak, T. Changes in antioxidative, oxidoreductive and detoxification enzymes during development of aphids and temperature increase. Antioxidants 2021, 10, 1181. [Google Scholar] [CrossRef] [PubMed]
- Dampc, J.; Kula-Maximenko, M.; Molon, M.; Durak, R. Enzymatic defense response of apple aphid Aphis pomi to increased temperature. Insects 2020, 11, 436. [Google Scholar] [CrossRef] [PubMed]











Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Łukasik, I.; Goławska, S.; Sytykiewicz, H. Differences in Oxidative Stress Markers and Antioxidant Enzyme Activities in Black Bean Aphid Morphs (Aphis fabae Scop.) Fed on the Primary Host Viburnum opulus L. Antioxidants 2022, 11, 2476. https://doi.org/10.3390/antiox11122476
Łukasik I, Goławska S, Sytykiewicz H. Differences in Oxidative Stress Markers and Antioxidant Enzyme Activities in Black Bean Aphid Morphs (Aphis fabae Scop.) Fed on the Primary Host Viburnum opulus L. Antioxidants. 2022; 11(12):2476. https://doi.org/10.3390/antiox11122476
Chicago/Turabian StyleŁukasik, Iwona, Sylwia Goławska, and Hubert Sytykiewicz. 2022. "Differences in Oxidative Stress Markers and Antioxidant Enzyme Activities in Black Bean Aphid Morphs (Aphis fabae Scop.) Fed on the Primary Host Viburnum opulus L." Antioxidants 11, no. 12: 2476. https://doi.org/10.3390/antiox11122476
APA StyleŁukasik, I., Goławska, S., & Sytykiewicz, H. (2022). Differences in Oxidative Stress Markers and Antioxidant Enzyme Activities in Black Bean Aphid Morphs (Aphis fabae Scop.) Fed on the Primary Host Viburnum opulus L. Antioxidants, 11(12), 2476. https://doi.org/10.3390/antiox11122476