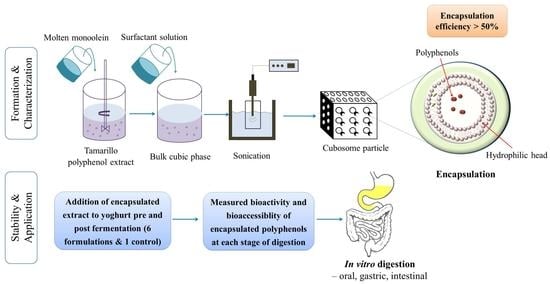
Tamarillo Polyphenols Encapsulated-Cubosome: Formation, Characterization, Stability during Digestion and Application in Yoghurt
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Tamarillo Extract (EXT) Preparation and Identtification of Polyphenol Components
2.3. Preparation of Cubosome and Cubosome Containing Tamarillo Extract
2.4. Polarized Light Microscopy (PLM) and Scanning Electron Microscopy (SEM)
2.5. Dynamic Light Scattering (DLS)
2.6. Determination of the Entrapment Efficiency (EE)
2.7. Yoghurt Fermentation and Fortification with Tamarillo Polyphenol Loaded-Cubosome
2.8. Determination of Physicochemical Properties of Fortified Yoghurts
2.9. In Vitro Digestion
2.10. Total Phenolic Content (TPC) and Antioxidant Activity
2.11. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Cubosomal Suspensions Containing Tamarillo Extract (CUBTAM)
3.2. TPC and Antioxidant Activities of Encapsulated and Non-Encapsulated Extracts during In Vitro Digestion
3.3. Release of Tamarillo Polypehnols from Cubosomes during Digestion
3.4. Physicochemical Properties of Yoghurt Fortified with CUBTAM
3.5. Total Phenol Content, Antioxidant Activity and Release of Polyphenol Compounds in Yoghurt Fortified with CUBTAM during Digestion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mezzenga, R.; Seddon, J.M.; Drummond, C.J.; Boyd, B.J.; Schröder-Turk, G.E.; Sagalowicz, L. Nature-Inspired design and application of lipidic lyotropic liquid crystals. Adv. Mater. 2019, 31, 1900818. [Google Scholar] [CrossRef] [PubMed]
- Barriga, H.M.; Holme, M.N.; Stevens, M.M. Cubosomes: The next generation of smart lipid nanoparticles? Angew. Chem. Int. Ed. 2019, 58, 2958–2978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meikle, T.G.; Dharmadana, D.; Hoffmann, S.V.; Jones, N.C.; Drummond, C.J.; Conn, C.E. Analysis of the structure, loading and activity of six antimicrobial peptides encapsulated in cubic phase lipid nanoparticles. J. Colloid Interface Sci. 2021, 587, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Boge, L.; Browning, K.L.; Nordström, R.; Campana, M.; Damgaard, L.S.; Seth Caous, J.; Hellsing, M.; Ringstad, L.; Andersson, M. Peptide-loaded cubosomes functioning as an antimicrobial unit against Escherichia coli. ACS Appl. Mater. Interfaces 2019, 11, 21314–21322. [Google Scholar] [CrossRef] [PubMed]
- Kwon, T.K.; Hong, S.K.; Kim, J.-C. In vitro skin permeation of cubosomes containing triclosan. J. Ind. Eng. Chem. 2012, 18, 563–567. [Google Scholar] [CrossRef]
- Luo, Q.; Lin, T.; Zhang, C.Y.; Zhu, T.; Wang, L.; Ji, Z.; Jia, B.; Ge, T.; Peng, D.; Chen, W. A novel glyceryl monoolein-bearing cubosomes for gambogenic acid: Preparation, cytotoxicity and intracellular uptake. Int. J. Pharm. 2015, 493, 30–39. [Google Scholar] [CrossRef]
- Saber, M.M.; Al-Mahallawi, A.M.; Nassar, N.N.; Stork, B.; Shouman, S.A. Targeting colorectal cancer cell metabolism through development of cisplatin and metformin nano-cubosomes. BMC Cancer 2018, 18, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zabara, M.; Senturk, B.; Gontsarik, M.; Ren, Q.; Rottmar, M.; Maniura-Weber, K.; Mezzenga, R.; Bolisetty, S.; Salentinig, S. Multifunctional nano-biointerfaces: Cytocompatible antimicrobial nanocarriers from stabilizer-free cubosomes. Adv. Funct. Mater. 2019, 29, 1904007. [Google Scholar] [CrossRef]
- Cortesi, R.; Cappellozza, E.; Drechsler, M.; Contado, C.; Baldisserotto, A.; Mariani, P.; Carducci, F.; Pecorelli, A.; Esposito, E.; Valacchi, G. Monoolein aqueous dispersions as a delivery system for quercetin. Biomed. Microdevices 2017, 19, 41. [Google Scholar] [CrossRef]
- Puglia, C.; Cardile, V.; Panico, A.M.; Crascì, L.; Offerta, A.; Caggia, S.; Drechsler, M.; Mariani, P.; Cortesi, R.; Esposito, E. Evaluation of monooleine aqueous dispersions as tools for topical administration of curcumin: Characterization, in vitro and ex-vivo studies. J. Pharm. Sci. 2013, 102, 2349–2361. [Google Scholar] [CrossRef]
- Archana, A.; Vijayasri, K.; Madhurim, M.; Kumar, C. Curcumin loaded nano cubosomal hydrogel: Preparation, in vitro characterization and antibacterial activity. Chem. Sci. Trans. 2015, 4, 75–80. [Google Scholar]
- Tu, Y.; Fu, J.; Sun, D.; Zhang, J.; Yao, N.; Huang, D.; Shi, Z. Preparation, characterisation and evaluation of curcumin with piperine-loaded cubosome nanoparticles. J. Microencaps. 2014, 31, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Diep, T.; Pook, C.; Yoo, M. Phenolic and Anthocyanin Compounds and Antioxidant Activity of Tamarillo (Solanum betaceum Cav.). Antioxidants 2020, 9, 169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The role of polyphenols in human health and food systems: A mini-review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diep, T.T.; Rush, E.C.; Yoo, M.J.Y. Tamarillo (Solanum betaceum Cav.): A Review of Physicochemical and Bioactive Properties and Potential Applications. Food Rev. Int. 2020, 1–25. [Google Scholar] [CrossRef]
- Martínez-Ballesta, M.; Gil-Izquierdo, Á.; García-Viguera, C.; Domínguez-Perles, R. Nanoparticles and controlled delivery for bioactive compounds: Outlining challenges for new “smart-foods” for health. Foods 2018, 7, 72. [Google Scholar] [CrossRef] [Green Version]
- Piovesana, A.; Noreña, C.P.Z. Microencapsulation of bioactive compounds from hibiscus calyces using different encapsulating materials. Int. J. Food Eng. 2018, 14. [Google Scholar] [CrossRef]
- Boge, L.; Hallstensson, K.; Ringstad, L.; Johansson, J.; Andersson, T.; Davoudi, M.; Larsson, P.T.; Mahlapuu, M.; Håkansson, J.; Andersson, M. Cubosomes for topical delivery of the antimicrobial peptide LL-37. Eur. J. Pharm. Biopharm. 2019, 134, 60–67. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, J.-C. In vitro anti-inflammatory efficacy of Bambusae Caulis in Taeniam extract loaded in monoolein cubosomes. J. Ind. Eng. Chem. 2019, 77, 189–197. [Google Scholar] [CrossRef]
- Seo, S.R.; Kang, G.; Ha, J.W.; Kim, J.-C. In vivo hair growth-promoting efficacies of herbal extracts and their cubosomal suspensions. J. Ind. Eng. Chem. 2013, 19, 1331–1339. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, J.; Zheng, Y.; Gong, Y.; Fu, M.; Liu, C.; Xu, L.; Sun, C.C.; Gao, Y.; Qian, S. Cubosomes with surface cross-linked chitosan exhibit sustained release and bioavailability enhancement for vinpocetine. RSC Adv. 2019, 9, 6287–6298. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Guo, C.; Chen, H.; Zhang, Y.; Peng, X.; Zhu, P. Determination of sinomenine in cubosome nanoparticles by HPLC technique. J. Anal. Methods Chem. 2015, 2015, 931687. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.P.; Pawara, D.D.; Gudewar, C.S.; Tekade, A.R. Nanostructured cubosomes in an in situ nasal gel system: An alternative approach for the controlled delivery of donepezil HCl to brain. J. Liposome Res. 2019, 29, 264–273. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Kristo, E.; LaPointe, G. Adding apple pomace as a functional ingredient in stirred-type yogurt and yogurt drinks. Food Hydrocoll. 2020, 100, 105453. [Google Scholar] [CrossRef]
- Kristo, E.; Miao, Z.; Corredig, M. The role of exopolysaccharide produced by Lactococcus lactis subsp. cremoris in structure formation and recovery of acid milk gels. Int. Dairy J. 2011, 21, 656–662. [Google Scholar] [CrossRef]
- Zhang, R.; Yoo, M.J.; Gathercole, J.; Reis, M.G.; Farouk, M.M. Effect of animal age on the nutritional and physicochemical qualities of ovine bresaola. Food Chem. 2018, 254, 317–325. [Google Scholar] [CrossRef]
- Caffrey, M.; Cherezov, V. Crystallizing membrane proteins using lipidic mesophases. Nat. Protoc. 2009, 4, 706–731. [Google Scholar] [CrossRef] [Green Version]
- Flak, D.K.; Adamski, V.; Nowaczyk, G.; Szutkowski, K.; Synowitz, M.; Jurga, S.; Held-Feindt, J. AT101-Loaded Cubosomes as an Alternative for Improved Glioblastoma Therapy. Int. J. Nanomed. 2020, 15, 7415. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M. Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Ortega, N.; Macià, A.; Romero, M.-P.; Reguant, J.; Motilva, M.-J. Matrix composition effect on the digestibility of carob flour phenols by an in-vitro digestion model. Food Chem. 2011, 124, 65–71. [Google Scholar] [CrossRef]
- Flores, F.P.; Singh, R.K.; Kerr, W.L.; Phillips, D.R.; Kong, F. In vitro release properties of encapsulated blueberry (Vaccinium ashei) extracts. Food Chem. 2015, 168, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Ydjedd, S.; Bouriche, S.; López-Nicolás, R.N.; Sánchez-Moya, T.; Frontela-Saseta, C.; Ros-Berruezo, G.; Rezgui, F.; Louaileche, H.; Kati, D.-E. Effect of in vitro gastrointestinal digestion on encapsulated and nonencapsulated phenolic compounds of carob (Ceratonia siliqua L.) pulp extracts and their antioxidant capacity. J. Agric. Food Chem. 2017, 65, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Saura-Calixto, F.; Serrano, J.; Goñi, I. Intake and bioaccessibility of total polyphenols in a whole diet. Food Chem. 2007, 101, 492–501. [Google Scholar] [CrossRef] [Green Version]
- Pavan, V.; Sancho, R.A.S.; Pastore, G.M. The effect of in vitro digestion on the antioxidant activity of fruit extracts (Carica papaya, Artocarpus heterophillus and Annona marcgravii). LWT-Food Sci. Technol. 2014, 59, 1247–1251. [Google Scholar] [CrossRef] [Green Version]
- da Silva Haas, I.C.; Toaldo, I.M.; Gomes, T.M.; Luna, A.S.; de Gois, J.S.; Bordignon-Luiz, M.T. Polyphenolic profile, macro-and microelements in bioaccessible fractions of grape juice sediment using in vitro gastrointestinal simulation. Food Biosci. 2019, 27, 66–74. [Google Scholar] [CrossRef]
- Ferreyra, S.; Torres-Palazzolo, C.; Bottini, R.; Camargo, A.; Fontana, A. Assessment of in-vitro bioaccessibility and antioxidant capacity of phenolic compounds extracts recovered from grapevine bunch stem and cane by-products. Food Chem. 2021, 348, 129063. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Verzelloni, E.; Bertolini, D.; Conte, A. In vitro bio-accessibility and antioxidant activity of grape polyphenols. Food Chem. 2010, 120, 599–606. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Gonçalves, S.; Hernanz, D.; Heredia, F.J.; Romano, A. Effects of in vitro gastrointestinal digestion on phenolic compounds and antioxidant activity of different white winemaking byproducts extracts. Food Res. Int. 2018, 109, 433–439. [Google Scholar] [CrossRef]
- Wojtunik-Kulesza, K.; Oniszczuk, A.; Oniszczuk, T.; Combrzyński, M.; Nowakowska, D.; Matwijczuk, A. Influence of in vitro digestion on composition, bioaccessibility and antioxidant activity of food polyphenols—A non-systematic review. Nutrients 2020, 12, 1401. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Helal, A.; Verzelloni, E.; Conte, A. The type and concentration of milk increase the in vitro bioaccessibility of coffee chlorogenic acids. J. Agric. Food Chem. 2012, 60, 11056–11064. [Google Scholar] [CrossRef]
- Celep, E.; Charehsaz, M.; Akyüz, S.; Acar, E.T.; Yesilada, E. Effect of in vitro gastrointestinal digestion on the bioavailability of phenolic components and the antioxidant potentials of some Turkish fruit wines. Food Res. Int. 2015, 78, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Kamiloglu, S.; Ozkan, G.; Isik, H.; Horoz, O.; Van Camp, J.; Capanoglu, E. Black carrot pomace as a source of polyphenols for enhancing the nutritional value of cake: An in vitro digestion study with a standardized static model. LWT 2017, 77, 475–481. [Google Scholar] [CrossRef]
- Sengul, H.; Surek, E.; Nilufer-Erdil, D. Investigating the effects of food matrix and food components on bioaccessibility of pomegranate (Punica granatum) phenolics and anthocyanins using an in-vitro gastrointestinal digestion model. Food Res. Int. 2014, 62, 1069–1079. [Google Scholar] [CrossRef] [Green Version]
- De Santiago, E.; Pereira-Caro, G.; Moreno-Rojas, J.M.; Cid, C.N.; De Pena, M.-P. Digestibility of (poly) phenols and antioxidant activity in raw and cooked cactus cladodes (Opuntia ficus-indica). J. Agric. Food Chem. 2018, 66, 5832–5844. [Google Scholar] [CrossRef] [PubMed]
- Bermúdez-Soto, M.-J.; Tomás-Barberán, F.-A.; García-Conesa, M.-T. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007, 102, 865–874. [Google Scholar] [CrossRef]
- McDougall, G.; Fyffe, S.; Dobson, P.; Stewart, D. Anthocyanins from red wine–their stability under simulated gastrointestinal digestion. Phytochemistry 2005, 66, 2540–2548. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, Y.; Huang, Z.; Chen, J.; Cui, Y.; Niu, B.; Huang, Y.; Pan, X.; Wu, C. Smart phase transformation system based on lyotropic liquid crystalline@ hard capsules for sustained release of hydrophilic and hydrophobic drugs. Drug Deliv. 2020, 27, 449–459. [Google Scholar] [CrossRef] [Green Version]
- Martiel, I.; Baumann, N.; Vallooran, J.J.; Bergfreund, J.; Sagalowicz, L.; Mezzenga, R. Oil and drug control the release rate from lyotropic liquid crystals. J. Control. Release 2015, 204, 78–84. [Google Scholar] [CrossRef]
- Cui, B.; Lu, Y.-m.; Tan, C.-p.; Wang, G.-q.; Li, G.-H. Effect of cross-linked acetylated starch content on the structure and stability of set yoghurt. Food Hydrocoll. 2014, 35, 576–582. [Google Scholar] [CrossRef]
- Tavakoli, H.; Hosseini, O.; Jafari, S.M.; Katouzian, I. Evaluation of physicochemical and antioxidant properties of yogurt enriched by olive leaf phenolics within nanoliposomes. J. Agric. Food Chem. 2018, 66, 9231–9240. [Google Scholar] [CrossRef]
- De Moura, S.C.; Schettini, G.N.; Garcia, A.O.; Gallina, D.A.; Alvim, I.D.; Hubinger, M.D. Stability of hibiscus extract encapsulated by ionic gelation incorporated in yogurt. Food Bioprocess Technol. 2019, 12, 1500–1515. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Zhou, J.; Wadhwa, S.S. Effects of adding apple polyphenols before and after fermentation on the properties of drinking yoghurt. Food Bioprocess Technol. 2012, 5, 2674–2686. [Google Scholar] [CrossRef]
- Sun-Waterhouse, D.; Zhou, J.; Wadhwa, S.S. Drinking yoghurts with berry polyphenols added before and after fermentation. Food Control 2013, 32, 450–460. [Google Scholar] [CrossRef]
- Ribeiro, T.B.; Bonifácio-Lopes, T.; Morais, P.; Miranda, A.; Nunes, J.; Vicente, A.A.; Pintado, M. Incorporation of olive pomace ingredients into yoghurts as a source of fibre and hydroxytyrosol: Antioxidant activity and stability throughout gastrointestinal digestion. J. Food Eng. 2021, 297, 110476. [Google Scholar] [CrossRef]
- Remanan, M.K.; Zhu, F. Encapsulation of rutin using quinoa and maize starch nanoparticles. Food Chem. 2021, 353, 128534. [Google Scholar] [CrossRef] [PubMed]
- Altin, G.; Gültekin-Özgüven, M.; Ozcelik, B. Liposomal dispersion and powder systems for delivery of cocoa hull waste phenolics via Ayran (drinking yoghurt): Comparative studies on in-vitro bioaccessibility and antioxidant capacity. Food Hydrocoll. 2018, 81, 364–370. [Google Scholar] [CrossRef]
- Martins, A.; Barros, L.; Carvalho, A.M.; Santos-Buelga, C.; Fernandes, I.P.; Barreiro, F.; Ferreira, I.C. Phenolic extracts of Rubus ulmifolius Schott flowers: Characterization, microencapsulation and incorporation into yogurts as nutraceutical sources. Food Funct. 2014, 5, 1091–1100. [Google Scholar] [CrossRef]
- Aravind, S.M.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of dietary polyphenols on gut microbiota, their metabolites and health benefits. Food Res. Int. 2021, 142, 110189.s. [Google Scholar] [CrossRef]




| Bioactive/Phases | Tamarillo Extract | Tamarillo Polyphenol Loaded-Cubosome | ||||
|---|---|---|---|---|---|---|
| Oral | Gastric | Intestinal | Oral | Gastric | Intestinal | |
| Phenolics | ||||||
| Gallic Acid | 3.86 ± 0.84 a | 46.5 ± 6.02 b | 20.4 ± 1.86 c | 4.51 ± 0.79 a | 8.57 ± 0.85 a | 52.9 ± 11.7 d |
| Catechin | 24.5 ± 5.01 a | 27.2 ± 7.93 a | 25.4 ± 6.09 a | 6.37 ± 0.52 b | 23.5 ± 4.09 a | 16.3 ± 0.39 |
| Caffeic Acid | 24.1 ± 4.55 a | 31.4 ± 3.90 b | 21.9 ± 7.06 a | 81.2 ± 15.2 c | 2.73 ± 0.91 d | 8.03 ± 0.35 e |
| Chlorogenic Acid | 9.40 ± 1.24 a | 67.7 ± 12.3 b | 4.82 ± 1.12 c | 4.99 ± 0.44 c | 9.33 ± 0.43 a | 28.8 ± 4.02 d |
| Epicatechin | 24.2 ± 6.22 a | 55.2 ± 10.3 b | 13.9 ± 1.10 c | 9.42 ± 0.49 d | 16.6 ± 2.62 c | 16.3 ± 3.66 ac |
| p-coumaric acid | 38.8 ± 8.36 a | 39.4 ± 6.29 a | 4.14 ± 0.36 b | 31.1 ± 1.16 c | 16.2 ± 0.97 d | 23.2 ± 4.20 e |
| Ferulic Acid | 5.55 ± 0.98 a | 13.4 ± 2.62 b | 17.8 ± 2.64 b | 54.4 ± 8.75 c | 3.42 ± 0.98 a | 32.3 ± 7.54 d |
| Rutin | 31.0 ± 6.09 a | 38.7 ± 7.40 a | 23.0 ± 5.96 b | 16.5 ± 1.39 bc | 14.7 ± 2.69 c | 24.0 ± 5.79 b |
| Kaempferol rutinoside | 32.1 ± 8.67 a | 37.6 ± 5.87 a | 15.5 ± 2.00 b | 21.0 ± 3.76 c | 21.5 ± 4.49 c | 29.1 ± 4.72 ac |
| Isorhamnetin rutinoside | 8.21 ± 1.04 a | 10.3 ± 1.08 b | 9.81 ± 1.08 ab | 3.76 ± 0.52 c | 10.2 ± 2.20 b | 9.43 ± 1.89 ab |
| Kaempferol | 43.8 ± 12.1 a | 20.4 ± 5.01 b | 5.83 ± 0.70 c | 29.6 ± 4.51 b | 20.2 ± 5.21 b | 33.1 ± 3.25 d |
| Anthocyanins | ||||||
| Delphinidin rutinoside | 10.1 ± 0.76 a | 24.6 ± 5.97 b | 7.87 ± 0.40 c | 5.30 ± 0.32 d | 10.3 ± 1.16 a | 19.4 ± 1.14 b |
| Cyanidin rutinoside | 14.3 ± 1.26 a | 35.9 ± 6.17 b | 4.31 ± 0.73 c | 8.68 ± 0.55 d | 17.1 ± 6.90 a | 25.2 ± 0.09 e |
| Pelargonidin rutinoside | 20.6 ± 1.49 a | 48.7 ± 5.26 b | 6.16 ± 0.31 c | 10.3 ± 1.46 d | 14.6 ± 2.80 d | 27.8 ± 1.36 e |
| Parameters/Samples | Control | POS5 | POS10 | POS15 | PRE5 | PRE10 | PRE15 |
|---|---|---|---|---|---|---|---|
| pH | 4.35 ± 0.03 a | 4.27 ± 0.02 b | 4.14 ± 0.01 c | 4.09 ± 0.02 d | 4.30 ± 0.00 a | 4.18 ± 0.01 c | 4.10 ± 0.02 cd |
| Syneresis (%) | 29.3 ± 0.91 a | 28.8 ± 1.48 a | 27.1 ± 1.53 ab | 26.6 ± 1.91 b | 28.7 ± 1.36 a | 27.9 ± 1.70 b | 26.9 ± 1.52 b |
| Textural parameters | |||||||
| Firmness (N) | 1.027 ± 0.005 a | 1.031 ± 0.004 a | 1.034 ± 0.002 ab | 1.042 ± 0.008 b | 1.033 ± 0.004 a | 1.035 ± 0.003 ab | 1.045 ± 0.006 b |
| Consistency (N.sec) | 16.08 ± 0.004 a | 16.12 ± 0.029 a | 16.21 ± 0.021 b | 17.19 ± 0.068 c | 16.09 ± 0.102 a | 16.23 ± 0.039 b | 17.22 ± 0.065 d |
| Cohesiveness (N) | −0.005 ± 0.003 a | −0.007 ± 0.002 a | −0.011 ± 0.004 ab | −0.014 ± 0.005 b | −0.008 ± 0.003 a | −0.010 ± 0.001 ab | −0.016 ± 0.007 b |
| Rheological parameters | |||||||
| Consistency coefficient (K, Pa.s) | <0.005 a | 0.009 ± 0.003 b | 0.010 ± 0.003 b | 0.014 ± 0.007 b | 0.008 ± 0.02 b | 0.012 ± 0.005 b | 0.015 ± 0.008 b |
| Flow behaviours index (n) | 0.781 ± 0.012 a | 0.729 ± 0.022 a | 0.658 ± 0.010 b | 0.604 ± 0.018 c | 0.735 ± 0.014 a | 0.678 ± 0.020 b | 0.642 ± 0.015 b |
| Viscosity at 350 s−1 (Pa.s) | 0.026 ± 0.000 a | 0.030 ± 0.000 b | 0.033 ± 0.001 c | 0.041 ± 0.001 d | 0.032 ± 0.000 b | 0.037 ± 0.002 cd | 0.045 ± 0.003 d |
| Elastic modulus (Pa) | N/A | 0.003 ± 0.000 a | 0.005 ± 0.000 b | 0.005 ± 0.001 b | 0.003 ± 0.000 a | 0.004 ± 0.001 b | 0.006 ± 0.002 b |
| Phases | Samples | TPC | CUPRAC | FRAP |
|---|---|---|---|---|
| Oral | CUBTAM | 5.07 | 10.55 | 6.97 |
| POS5 | 7.64 | 15.50 | 8.38 | |
| POS10 | 8.19 | 14.47 | 7.83 | |
| POS15 | 8.59 | 13.34 | 8.33 | |
| PRE5 | 8.14 | 15.25 | 8.67 | |
| PRE10 | 8.86 | 14.36 | 7.93 | |
| PRE15 | 8.87 | 12.89 | 9.45 | |
| Gastric | CUBTAM | 29.13 | 22.77 | 24.70 |
| POS5 | 28.34 | 31.04 | 31.84 | |
| POS10 | 26.79 | 27.05 | 25.88 | |
| POS15 | 29.20 | 26.86 | 28.24 | |
| PRE5 | 27.75 | 29.41 | 30.18 | |
| PRE10 | 28.18 | 27.29 | 25.39 | |
| PRE15 | 27.19 | 24.84 | 29.25 | |
| Intestinal | CUBTAM | 77.03 | 58.53 | 65.27 |
| POS5 | 79.20 | 50.83 | 87.22 | |
| POS10 | 81.72 | 50.27 | 77.47 | |
| POS15 | 86.30 | 52.49 | 81.92 | |
| PRE5 | 94.90 | 59.64 | 101.19 | |
| PRE10 | 90.41 | 54.74 | 79.94 | |
| PRE15 | 81.08 | 49.99 | 85.62 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diep, T.T.; Yoo, M.J.Y.; Rush, E. Tamarillo Polyphenols Encapsulated-Cubosome: Formation, Characterization, Stability during Digestion and Application in Yoghurt. Antioxidants 2022, 11, 520. https://doi.org/10.3390/antiox11030520
Diep TT, Yoo MJY, Rush E. Tamarillo Polyphenols Encapsulated-Cubosome: Formation, Characterization, Stability during Digestion and Application in Yoghurt. Antioxidants. 2022; 11(3):520. https://doi.org/10.3390/antiox11030520
Chicago/Turabian StyleDiep, Tung Thanh, Michelle Ji Yeon Yoo, and Elaine Rush. 2022. "Tamarillo Polyphenols Encapsulated-Cubosome: Formation, Characterization, Stability during Digestion and Application in Yoghurt" Antioxidants 11, no. 3: 520. https://doi.org/10.3390/antiox11030520
APA StyleDiep, T. T., Yoo, M. J. Y., & Rush, E. (2022). Tamarillo Polyphenols Encapsulated-Cubosome: Formation, Characterization, Stability during Digestion and Application in Yoghurt. Antioxidants, 11(3), 520. https://doi.org/10.3390/antiox11030520