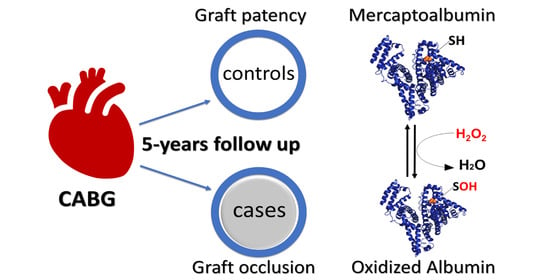
Mercaptoalbumin Is Associated with Graft Patency in Patients Undergoing Coronary Artery Bypass Grafting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Quantitation of S-Thiolated Albumin by Mass Spectrometry (MS)
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Study Participants and Study Workflow
3.2. Albumin Isoforms Differences between Occluded and Patent Grafts
3.3. HSA-SH as a Predictor of Graft Occlusion
3.4. Thio-HSA Increased Significantly after Surgery but Was Not a Predictor of Graft Occlusion
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fihn, S.D.; Blankenship, J.C.; Alexander, K.P.; Bittl, J.A.; Byrne, J.G.; Fletcher, B.J.; Fonarow, G.C.; Lange, R.A.; Levine, G.N.; Maddox, T.M.; et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2014, 64, 1929–1949. [Google Scholar] [PubMed] [Green Version]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. The Task Force on myocardial revascularization of the European Society of Cardiology (ESC) and European Association for Cardio-Thoracic Surgery (EACTS). G. Ital. Cardiol. (Rome) 2019, 20, 1S–61S. [Google Scholar]
- Gaudino, M.; Antoniades, C.; Benedetto, U.; Deb, S.; Di Franco, A.; Di Giammarco, G.; Fremes, S.; Glineur, D.; Grau, J.; He, G.W.; et al. Mechanisms, Consequences, and Prevention of Coronary Graft Failure. Circulation 2017, 136, 1749–1764. [Google Scholar] [CrossRef] [PubMed]
- Caliskan, E.; de Souza, D.R.; Boning, A.; Liakopoulos, O.J.; Choi, Y.H.; Pepper, J.; Gibson, C.M.; Perrault, L.P.; Wolf, R.K.; Kim, K.B.; et al. Saphenous vein grafts in contemporary coronary artery bypass graft surgery. Nat. Rev. Cardiol. 2020, 17, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Parolari, A.; Poggio, P.; Myasoedova, V.; Songia, P.; Pilozzi, A.; Alamanni, F.; Tremoli, E. Molecular pathways activation in coronary artery bypass surgery: Which role for pump avoidance? J. Cardiovasc. Med. (Hagerstown) 2016, 17, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Veglia, F.; Werba, J.P.; Tremoli, E.; Squellerio, I.; Sisillo, E.; Parolari, A.; Minardi, F.; Cavalca, V. Assessment of oxidative stress in coronary artery bypass surgery: Comparison between the global index OXY-SCORE and individual biomarkers. Biomarkers 2009, 14, 465–472. [Google Scholar] [CrossRef]
- Akila; D’souza, B.; Vishwanath, P.; D’souza, V. Oxidative injury and antioxidants in coronary artery bypass graft surgery: Off-pump CABG significantly reduces oxidative stress. Clin. Chim. Acta 2007, 375, 147–152. [Google Scholar] [CrossRef]
- Karu, I.; Taal, G.; Zilmer, K.; Pruunsild, C.; Starkopf, J.; Zilmer, M. Inflammatory/oxidative stress during the first week after different types of cardiac surgery. Scand. Cardiovasc. J. 2010, 44, 119–124. [Google Scholar] [CrossRef]
- Chien, S.C.; Chen, C.Y.; Lin, C.F.; Yeh, H.I. Critical appraisal of the role of serum albumin in cardiovascular disease. Biomark. Res. 2017, 5, 31. [Google Scholar] [CrossRef]
- Roche, M.; Rondeau, P.; Singh, N.R.; Tarnus, E.; Bourdon, E. The antioxidant properties of serum albumin. FEBS Lett. 2008, 582, 1783–1787. [Google Scholar] [CrossRef]
- Colombo, G.; Clerici, M.; Giustarini, D.; Rossi, R.; Milzani, A.; Dalle-Donne, I. Redox albuminomics: Oxidized albumin in human diseases. Antioxid. Redox Signal. 2012, 17, 1515–1527. [Google Scholar] [CrossRef] [PubMed]
- Laussac, J.P.; Sarkar, B. Characterization of the copper(II)- and nickel(II)-transport site of human serum albumin. Studies of copper(II) and nickel(II) binding to peptide 1-24 of human serum albumin by 13C and 1H NMR spectroscopy. Biochemistry 1984, 23, 2832–2838. [Google Scholar] [CrossRef] [PubMed]
- Regazzoni, L.; Del Vecchio, L.; Altomare, A.; Yeum, K.J.; Cusi, D.; Locatelli, F.; Carini, M.; Aldini, G. Human serum albumin cysteinylation is increased in end stage renal disease patients and reduced by hemodialysis: Mass spectrometry studies. Free Radic. Res. 2013, 47, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Zoanni, B.; Brioschi, M.; Mallia, A.; Gianazza, E.; Eligini, S.; Carini, M.; Aldini, G.; Banfi, C. Novel insights about albumin in cardiovascular diseases: Focus on heart failure. Mass Spectrom. Rev. 2021, e21743. [Google Scholar] [CrossRef] [PubMed]
- Bonanata, J.; Turell, L.; Antmann, L.; Ferrer-Sueta, G.; Botasini, S.; Mendez, E.; Alvarez, B.; Coitino, E.L. The thiol of human serum albumin: Acidity, microenvironment and mechanistic insights on its oxidation to sulfenic acid. Free Radic. Biol. Med. 2017, 108, 952–962. [Google Scholar] [CrossRef] [PubMed]
- Parolari, A.; Cavallotti, L.; Andreini, D.; Myasoedova, V.; Banfi, C.; Camera, M.; Poggio, P.; Barili, F.; Pontone, G.; Mussoni, L.; et al. D-dimer is associated with arterial and venous coronary artery bypass graft occlusion. J. Thorac. Cardiovasc. Surg. 2018, 155, 200–207e3. [Google Scholar] [CrossRef] [Green Version]
- Brioschi, M.; Gianazza, E.; Mallia, A.; Zoanni, B.; Altomare, A.; Martinez Fernandez, A.; Agostoni, P.; Aldini, G.; Banfi, C. S-Thiolation Targets Albumin in Heart Failure. Antioxidants 2020, 9, 763. [Google Scholar] [CrossRef]
- Liu, M.; Chan, C.P.; Yan, B.P.; Zhang, Q.; Lam, Y.Y.; Li, R.J.; Sanderson, J.E.; Coats, A.J.; Sun, J.P.; Yip, G.W.; et al. Albumin levels predict survival in patients with heart failure and preserved ejection fraction. Eur. J. Heart Fail. 2012, 14, 39–44. [Google Scholar] [CrossRef]
- Hartopo, A.B.; Gharini, P.P.; Setianto, B.Y. Low serum albumin levels and in-hospital adverse outcomes in acute coronary syndrome. Int. Heart J. 2010, 51, 221–226. [Google Scholar] [CrossRef] [Green Version]
- de la Cruz, K.I.; Bakaeen, F.G.; Wang, X.L.; Huh, J.; LeMaire, S.A.; Coselli, J.S.; Chu, D. Hypoalbuminemia and long-term survival after coronary artery bypass: A propensity score analysis. Ann. Thorac. Surg. 2011, 91, 671–675. [Google Scholar] [CrossRef]
- Malhotra, R.; Bakken, K.; D’Elia, E.; Lewis, G.D. Cardiopulmonary Exercise Testing in Heart Failure. JACC Heart Fail. 2016, 4, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Camera, M.; Brambilla, M.; Canzano, P.; Cavallotti, L.; Parolari, A.; Tedesco, C.C.; Zara, C.; Rossetti, L.; Tremoli, E. Association of Microvesicles With Graft Patency in Patients Undergoing CABG Surgery. J. Am. Coll. Cardiol. 2020, 75, 2819–2832. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulos, A.S.; Odutayo, A.; Oikonomou, E.K.; Trivella, M.; Petrou, M.; Collins, G.S.; Antoniades, C.; SAFINOUS-CABG (Saphenous Vein Graft Failure—An Outcomes Study in Coronary Artery Bypass Grafting) Group. Development of a risk score for early saphenous vein graft failure: An individual patient data meta-analysis. J. Thorac. Cardiovasc. Surg. 2020, 160, 116–127.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vukicevic, P.; Klisic, A.; Neskovic, V.; Babic, L.; Mikic, A.; Bogavac-Stanojevic, N.; Matkovic, M.; Milicevic, V.; Aleksic, N.; Kotur-Stevuljevic, J. Oxidative Stress in Patients before and after On-Pump and Off-Pump Coronary Artery Bypass Grafting: Relationship with Syntax Score. Oxid. Med. Cell Longev. 2021, 2021, 3315951. [Google Scholar] [CrossRef] [PubMed]
- Altomare, A.; Baron, G.; Brioschi, M.; Longoni, M.; Butti, R.; Valvassori, E.; Tremoli, E.; Carini, M.; Agostoni, P.; Vistoli, G.; et al. N-Acetyl-Cysteine Regenerates Albumin Cys34 by a Thiol-Disulfide Breaking Mechanism: An Explanation of Its Extracellular Antioxidant Activity. Antioxidants 2020, 9, 367. [Google Scholar] [CrossRef]




| Variable | Patent Grafts (n = 38) | Occluded Grafts (n = 36) | p-Value |
|---|---|---|---|
| Age | 63.97 ± 2.66 # | 63.16 ± 7.97 # | 0.56 |
| Male | 34 (89.5) | 31 (86.1) | 0.66 |
| BMI | 27.02 ± 3.35 # | 25.73 ± 3.03 # | 0.09 |
| Risk factors | |||
| Diabetes mellitus | 13 (34.2) | 14 (38.9) | 0.68 |
| Hypertension | 26 (68.4) | 27 (75) | 0.54 |
| Hyperlipidemia | 32 (84.2) | 31 (86.1) | 0.82 |
| Current smoker | 4 (10.5) | 8 (22.2) | 0.21 * |
| Medications | |||
| Antiplatelet | 28 (77.8) | 23 (69.7) | 0.45 |
| Hypoglycemic | 8 (22.2) | 11 (33.3) | 0.31 |
| Antihypertensive | 32 (88.9) | 30 (90.9) | 0.99 * |
| Antiarrhythmic | 1 (2.8) | 2 (6.1) | 0.60 * |
| Hypolipidemic | 25 (69.4) | 24 (72.7) | 0.77 |
| Preoperative characteristics | |||
| LVEF (%) | 61.95 ± 6.21 # | 54.89 ± 11.26 # | 0.001 |
| Additive EuroSCORE | 2 (1; 3) § | 3 (1; 4) § | 0.004 |
| Logistic EuroSCORE | 1.3 (1.1; 1.8) § | 1.7 (1.3; 3.1) § | 0.02 |
| D-dimer T0 (ng/mL) | 606.1 (366.11; 976.05) § | 796.66 (476.81; 1224.33) § | 0.039 |
| CRP T0 (mg/L) | 2.29 (1.08; 4.42) § | 2.89 (1.29; 7.81) § | 0.22 |
| Fibrinogen T0 (mg/dL) | 400.18 (356.19; 422.67) § | 417.7 (365.85; 461.01) § | 0.12 |
| Creatinine T0 (mg/dL) | 1.03 ± 0.2 # | 1.07 ± 0.23 # | 0.43 |
| Diseased coronary vessels (n) | 2.63 ± 0.61 # | 2.91 ± 0.29 # | 0.016 |
| Bypass grafts (n) | 2.68 ± 0.74 # | 3.28 ± 0.74 # | 0.002 ** |
| Anastomoses (n) | 2.97 ± 0.88 # | 3.6 ± 0.9 # | 0.01 ** |
| Surgery parameters | |||
| GSV use | 34 (89.5) | 36 (100) | 0.11 * |
| LITA use | 38 (100) | 36 (100) | - |
| RITA use | 5 (13.2) | 7 (19.4) | 0.47 |
| Radial artery use | 2 (5.3) | 2 (5.6) | 0.99 * |
| Surgery time (h) | 4 (3.5; 5) § | 4.48 (4; 5) § | 0.03 |
| ECC time (min) | 85.5 (70; 105) § | 107.5 (87.5; 127.5) § | 0.001 |
| Clamp time (min) | 63 (45; 76) § | 75.5 (61.5; 88.5) § | 0.004 |
| Variable | HSA-SH T0 | Thio-HSA T0 | ||
|---|---|---|---|---|
| R | p-Value | R | p-Value | |
| Age | −0.083 | 0.4682 | 0.067 | 0.5594 |
| Sex | −0.029 | 0.8013 | −0.002 | 0.9844 |
| BMI | 0.015 | 0.8934 | 0.013 | 0.9106 |
| Risk factors | ||||
| Diabetes | 0.113 | 0.3267 | −0.235 | 0.0383 |
| Hypertension | −0.038 | 0.7414 | 0.095 | 0.4085 |
| Hypercholesterolemia | 0.123 | 0.2829 | −0.084 | 0.4666 |
| Smoke | −0.109 | 0.341 | 0.06 | 0.5997 |
| Medications | ||||
| Antiplatelet | 0.261 | 0.0255 | −0.269 | 0.0216 |
| Hypoglycemic | 0.078 | 0.5143 | −0.204 | 0.0835 |
| Antihypertensive | −0.104 | 0.3823 | 0.254 | 0.0302 |
| Antiarrhythmic | −0.043 | 0.7206 | 0.043 | 0.7206 |
| Hypolipidemic | 0.027 | 0.8187 | −0.016 | 0.8945 |
| Preoperative characteristics | ||||
| LVEF | 0.041 | 0.7187 | 0.012 | 0.914 |
| Additive EuroSCORE | −0.214 | 0.0612 | 0.186 | 0.1059 |
| Logistic EuroSCORE | −0.236 | 0.039 | 0.199 | 0.0834 |
| D-dimer T0 | −0.324 | 0.0038 | 0.241 | 0.0332 |
| CRP T0 | −0.197 | 0.0852 | 0.184 | 0.1087 |
| Fibrinogen T0 | −0.25 | 0.0272 | 0.211 | 0.0641 |
| Creatinine T0 | −0.346 | 0.0019 | 0.302 | 0.0072 |
| Diseased coronary vessels | 0.017 | 0.8908 | −0.061 | 0.6164 |
| Intraoperative characteristics | ||||
| GSV use | 0.034 | 0.7695 | 0.01 | 0.9276 |
| RITA use | 0.147 | 0.1997 | −0.18 | 0.115 |
| RAD use | −0.271 | 0.0164 | 0.142 | 0.215 |
| ECC time | −0.233 | 0.0459 | 0.293 | 0.0113 |
| Variable | ODDS RATIO | 95% Wald | p-Value | |
|---|---|---|---|---|
| Confidence Limits | ||||
| Model A | ||||
| HSA-SH T0 | 0.847 | 0.733 | 0.978 | 0.0236 |
| * D-dimer T0 | 1.400 | 0.763 | 2.588 | 0.2747 |
| Logistic EuroSCORE | 1.726 | 1.027 | 2.902 | 0.0393 |
| Model B | ||||
| HSA-SH T0 | 0.81 | 0.665 | 0.988 | 0.0372 |
| * D-dimer T0 | 1.453 | 0.757 | 2.79 | 0.2613 |
| ECC time | 1.025 | 1.003 | 1.048 | 0.0278 |
| Logistic EuroSCORE | 1.72 | 0.898 | 3.298 | 0.1022 |
| Variable | HSA-SH T1 | Thio-HSA T1 | ||
|---|---|---|---|---|
| R | p-Value | R | p-Value | |
| D-dimer T1 | −0.21294 | 0.0666 | 0.20649 | 0.0755 |
| CRP T1 | −0.26765 | 0.0203 | 0.13935 | 0.2331 |
| Graft occlusion | −0.03696 | 0.7529 | 0.05297 | 0.6517 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brioschi, M.; Gianazza, E.; Andreini, D.; Mushtaq, S.; Cavallotti, L.; Veglia, F.; Tedesco, C.C.; Colombo, G.I.; Pepi, M.; Polvani, G.; et al. Mercaptoalbumin Is Associated with Graft Patency in Patients Undergoing Coronary Artery Bypass Grafting. Antioxidants 2022, 11, 702. https://doi.org/10.3390/antiox11040702
Brioschi M, Gianazza E, Andreini D, Mushtaq S, Cavallotti L, Veglia F, Tedesco CC, Colombo GI, Pepi M, Polvani G, et al. Mercaptoalbumin Is Associated with Graft Patency in Patients Undergoing Coronary Artery Bypass Grafting. Antioxidants. 2022; 11(4):702. https://doi.org/10.3390/antiox11040702
Chicago/Turabian StyleBrioschi, Maura, Erica Gianazza, Daniele Andreini, Saima Mushtaq, Laura Cavallotti, Fabrizio Veglia, Calogero C. Tedesco, Gualtiero I. Colombo, Mauro Pepi, Gianluca Polvani, and et al. 2022. "Mercaptoalbumin Is Associated with Graft Patency in Patients Undergoing Coronary Artery Bypass Grafting" Antioxidants 11, no. 4: 702. https://doi.org/10.3390/antiox11040702
APA StyleBrioschi, M., Gianazza, E., Andreini, D., Mushtaq, S., Cavallotti, L., Veglia, F., Tedesco, C. C., Colombo, G. I., Pepi, M., Polvani, G., Tremoli, E., Parolari, A., & Banfi, C. (2022). Mercaptoalbumin Is Associated with Graft Patency in Patients Undergoing Coronary Artery Bypass Grafting. Antioxidants, 11(4), 702. https://doi.org/10.3390/antiox11040702