Aging-Related Decline of Autophagy in Patients with Atrial Fibrillation—A Post Hoc Analysis of the ATHERO-AF Study
Abstract
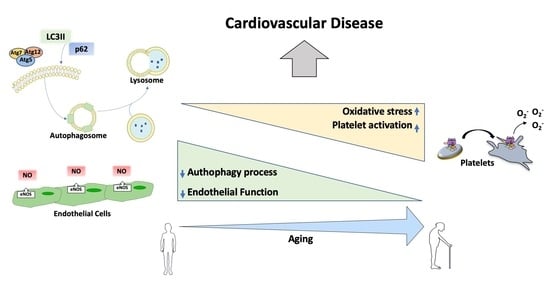
:1. Introduction
2. Materials and Methods
2.1. Population Study
2.2. Preparation of Serum, Plasma, and Platelets
2.3. Serum sNox2-dp Release
2.4. Serum H2O2 Determination
2.5. Serum Hydrogen Peroxide Scavenging Activity
2.6. Serum Nitric Oxide
2.7. Plasma sP-Selectin Assay
2.8. Plasma CD40L Assay
2.9. Plasmatic ATG5 Detection
2.10. Plasmatic P62 Detection
2.11. Statistical Analysis
3. Results
3.1. Characteristics of Population
3.2. Oxidative Stress Analysis
3.3. NO Production Analysis
3.4. Platelet Activation Analysis
3.5. Autophagy Process Analysis
3.6. Correlation Analysis in the AF Patients and CS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Christensen, K.; Doblhammer, G.; Rau, R.; Vaupel, J.W. Ageing populations: The challenges ahead. Lancet 2009, 374, 1196–1208. [Google Scholar] [CrossRef] [Green Version]
- Roth, G.A.; Johnson, C.; Abajobir, A.; Abd-Allah, F.; Abera, S.F.; Abyu, G.; Ahmed, M.; Aksut, B.; Alam, T.; Alam, K.; et al. Global, Regional, and National Burden of Cardiovascular Diseases for 10 Causes, 1990 to 2015. J. Am. Coll. Cardiol. 2017, 70, 1–25. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Trogdon, J.G.; Khavjou, O.A.; Butler, J.; Dracup, K.; Ezekowitz, M.D.; Finkelstein, E.A.; Hong, Y.; Johnston, S.C.; Khera, A.; et al. Forecasting the future of cardiovascular disease in the United States: A policy statement from the American Heart Association. Circulation 2011, 123, 933–944. [Google Scholar] [CrossRef] [Green Version]
- Guzik, T.J.; Touyz, R.M. Oxidative Stress, Inflammation, and Vascular Aging in Hypertension. Hypertension 2017, 70, 660–667. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Loffredo, L.; Carnevale, R.; Pignatelli, P.; Pastori, D. Atherothrombosis and Oxidative Stress: Mechanisms and Management in Elderly. Antioxid. Redox Signal. 2017, 27, 1083–1124. [Google Scholar] [CrossRef] [PubMed]
- Shirakabe, A.; Ikeda, Y.; Sciarretta, S.; Zablocki, D.K.; Sadoshima, J. Aging and Autophagy in the Heart. Circ. Res. 2016, 118, 1563–1576. [Google Scholar] [CrossRef] [Green Version]
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef] [Green Version]
- Sciarretta, S.; Maejima, Y.; Zablocki, D.; Sadoshima, J. The Role of Autophagy in the Heart. Annu. Rev. Physiol. 2018, 80, 1–26. [Google Scholar] [CrossRef]
- Sciarretta, S.; Zhai, P.; Volpe, M.; Sadoshima, J. Pharmacological modulation of autophagy during cardiac stress. J. Cardiovasc. Pharmacol. 2012, 60, 235–241. [Google Scholar] [CrossRef] [Green Version]
- Alfaras, I.; Di Germanio, C.; Bernier, M.; Csiszar, A.; Ungvari, Z.; Lakatta, E.G.; de Cabo, R. Pharmacological Strategies to Retard Cardiovascular Aging. Circ. Res. 2016, 118, 1626–1642. [Google Scholar] [CrossRef] [PubMed]
- Sciarretta, S.; Forte, M.; Castoldi, F.; Frati, G.; Versaci, F.; Sadoshima, J.; Kroemer, G.; Maiuri, M.C. Caloric restriction mimetics for the treatment of cardiovascular diseases. Cardiovasc. Res. 2020, 117, 1434–1449. [Google Scholar] [CrossRef]
- Li, H.; Qiu, S.; Li, X.; Li, M.; Peng, Y. Autophagy biomarkers in CSF correlates with infarct size, clinical severity and neurological outcome in AIS patients. J. Transl. Med. 2015, 13, 359. [Google Scholar] [CrossRef] [Green Version]
- Castellazzi, M.; Patergnani, S.; Donadio, M.; Giorgi, C.; Bonora, M.; Bosi, C.; Brombo, G.; Pugliatti, M.; Seripa, D.; Zuliani, G.; et al. Autophagy and mitophagy biomarkers are reduced in sera of patients with Alzheimer’s disease and mild cognitive impairment. Sci. Rep. 2019, 9, 20009. [Google Scholar] [CrossRef] [Green Version]
- Ball, J.; Carrington, M.J.; McMurray, J.J.; Stewart, S. Atrial fibrillation: Profile and burden of an evolving epidemic in the 21st century. Int. J. Cardiol. 2013, 167, 1807–1824. [Google Scholar] [CrossRef] [PubMed]
- Violi, F.; Pastori, D.; Pignatelli, P.; Loffredo, L. Antioxidants for prevention of atrial fibrillation: A potentially useful future therapeutic approach? A review of the literature and meta-analysis. Europace 2014, 16, 1107–1116. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; Pastori, D.; Carnevale, R.; Farcomeni, A.; Cangemi, R.; Nocella, C.; Bartimoccia, S.; Vicario, T.; Saliola, M.; Lip, G.Y.; et al. Serum NOX2 and urinary isoprostanes predict vascular events in patients with atrial fibrillation. Thromb. Haemost. 2015, 113, 617–624. [Google Scholar] [CrossRef] [Green Version]
- Pastori, D.; Pignatelli, P.; Angelico, F.; Farcomeni, A.; Del Ben, M.; Vicario, T.; Bucci, T.; Raparelli, V.; Cangemi, R.; Tanzilli, G.; et al. Incidence of myocardial infarction and vascular death in elderly patients with atrial fibrillation taking anticoagulants: Relation to atherosclerotic risk factors. Chest 2015, 147, 1644–1650. [Google Scholar] [CrossRef] [PubMed]
- Pastori, D.; Farcomeni, A.; Pignatelli, P.; Violi, F.; Lip, G.Y. ABC (Atrial fibrillation Better Care) Pathway and Healthcare Costs in Atrial Fibrillation: The ATHERO-AF Study. Am. J. Med. 2019, 132, 856–861. [Google Scholar] [CrossRef]
- Pastori, D.; Pignatelli, P.; Farcomeni, A.; Menichelli, D.; Nocella, C.; Carnevale, R.; Violi, F. Aging-Related Decline of Glutathione Peroxidase 3 and Risk of Cardiovascular Events in Patients With Atrial Fibrillation. J. Am. Heart Assoc. 2016, 5, e003682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastori, D.; Pignatelli, P.; Farcomeni, A.; Nocella, C.; Bartimoccia, S.; Carnevale, R.; Violi, F. Age-related increase of thromboxane B2 and risk of cardiovascular disease in atrial fibrillation. Oncotarget 2016, 7, 39143–39147. [Google Scholar] [CrossRef] [Green Version]
- Carnevale, R.; Silvestri, R.; Loffredo, L.; Novo, M.; Cammisotto, V.; Castellani, V.; Bartimoccia, S.; Nocella, C.; Violi, F. Oleuropein, a component of extra virgin olive oil, lowers postprandial glycaemia in healthy subjects. Br. J. Clin. Pharmacol. 2018, 84, 1566–1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferroni, P.; Martini, F.; Riondino, S.; La Farina, F.; Magnapera, A.; Ciatti, F.; Guadagni, F. Soluble P-selectin as a marker of in vivo platelet activation. Clin. Chim. Acta Int. J. Clin. Chem. 2009, 399, 88–91. [Google Scholar] [CrossRef] [PubMed]
- Dewitte, A.; Tanga, A.; Villeneuve, J.; Lepreux, S.; Ouattara, A.; Desmouliere, A.; Combe, C.; Ripoche, J. New frontiers for platelet CD154. Exp. Hematol. Oncol. 2015, 4, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, X.; Zhou, X.J.; Zhang, H. Exploring the Role of Autophagy-Related Gene 5 (ATG5) Yields Important Insights Into Autophagy in Autoimmune/Autoinflammatory Diseases. Front. Immunol. 2018, 9, 2334. [Google Scholar] [CrossRef]
- Bjorkoy, G.; Lamark, T.; Pankiv, S.; Overvatn, A.; Brech, A.; Johansen, T. Monitoring autophagic degradation of p62/SQSTM1. Methods Enzymol. 2009, 452, 181–197. [Google Scholar] [CrossRef]
- Perri, L.; Pastori, D.; Pignatelli, P.; Violi, F.; Loffredo, L. Flow-mediated dilation is associated with cardiovascular events in non-valvular atrial fibrillation patients. Int. J. Cardiol. 2015, 179, 139–143. [Google Scholar] [CrossRef]
- Blankenberg, S.; Rupprecht, H.J.; Bickel, C.; Torzewski, M.; Hafner, G.; Tiret, L.; Smieja, M.; Cambien, F.; Meyer, J.; Lackner, K.J.; et al. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N. Engl. J. Med. 2003, 349, 1605–1613. [Google Scholar] [CrossRef] [Green Version]
- Wagner, S.; Dantz, C.; Flebbe, H.; Azizian, A.; Sag, C.M.; Engels, S.; Möllencamp, J.; Dybkova, N.; Islam, T.; Shah, A.M.; et al. NADPH oxidase 2 mediates angiotensin II-dependent cellular arrhythmias via PKA and CaMKII. J. Mol. Cell. Cardiol. 2014, 75, 206–215. [Google Scholar] [CrossRef]
- Shingu, Y.; Takada, S.; Yokota, T.; Shirakawa, R.; Yamada, A.; Ooka, T.; Katoh, H.; Kubota, S.; Matsui, Y. Correlation between increased atrial expression of genes related to fatty acid metabolism and autophagy in patients with chronic atrial fibrillation. PLoS ONE 2020, 15, e0224713. [Google Scholar] [CrossRef] [Green Version]
- Wiersma, M.; Meijering, R.A.M.; Qi, X.Y.; Zhang, D.; Liu, T.; Hoogstra-Berends, F.; Sibon, O.C.M.; Henning, R.H.; Nattel, S.; Brundel, B. Endoplasmic Reticulum Stress Is Associated with Autophagy and Cardiomyocyte Remodeling in Experimental and Human Atrial Fibrillation. J. Am. Heart Assoc. 2017, 6, e006458. [Google Scholar] [CrossRef] [Green Version]
- Garcia, L.; Verdejo, H.E.; Kuzmicic, J.; Zalaquett, R.; Gonzalez, S.; Lavandero, S.; Corbalan, R. Impaired cardiac autophagy in patients developing postoperative atrial fibrillation. J. Thorac. Cardiovasc. Surg. 2012, 143, 451–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.C.; Chang, J.P.; Wang, Y.H.; Liu, W.H.; Ho, W.C.; Chang, H.W. Autophagy as a mechanism for myolysis of cardiomyocytes in mitral regurgitation. Eur. J. Clin. Investig. 2011, 41, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Dong, Y.; Cai, X.; Yang, H.; Guo, T. Identification and Validation of Autophagy-Related Genes as Potential Biomarkers and Therapeutic Targets in Atrial Fibrillation. Int. J. Gen. Med. 2021, 14, 7783–7796. [Google Scholar] [CrossRef] [PubMed]
- Chao, X.; Dai, W.; Li, S.; Jiang, C.; Jiang, Z.; Zhong, G. Identification of circRNA-miRNA-mRNA Regulatory Network and Autophagy Interaction Network in Atrial Fibrillation Based on Bioinformatics Analysis. Int. J. Gen. Med. 2021, 14, 8527–8540. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pastori, D.; Guo, Y.; Wang, Y.; Lip, G.Y.H. Risk factors for new-onset atrial fibrillation: A focus on Asian populations. Int. J. Cardiol. 2018, 261, 92–98. [Google Scholar] [CrossRef]
- Pastori, D.; Carnevale, R.; Pignatelli, P. Is there a clinical role for oxidative stress biomarkers in atherosclerotic diseases? Intern. Emerg. Med. 2014, 9, 123–131. [Google Scholar] [CrossRef]
- Rubinsztein, D.C.; Marino, G.; Kroemer, G. Autophagy and aging. Cell 2011, 146, 682–695. [Google Scholar] [CrossRef] [Green Version]
- Mejias-Pena, Y.; Estebanez, B.; Rodriguez-Miguelez, P.; Fernandez-Gonzalo, R.; Almar, M.; de Paz, J.A.; Gonzalez-Gallego, J.; Cuevas, M.J. Impact of resistance training on the autophagy-inflammation-apoptosis crosstalk in elderly subjects. Aging 2017, 9, 408–418. [Google Scholar] [CrossRef] [Green Version]
- Mejias-Pena, Y.; Rodriguez-Miguelez, P.; Fernandez-Gonzalo, R.; Martinez-Florez, S.; Almar, M.; de Paz, J.A.; Cuevas, M.J.; Gonzalez-Gallego, J. Effects of aerobic training on markers of autophagy in the elderly. Age 2016, 38, 33. [Google Scholar] [CrossRef] [Green Version]
- Frati, G.; Vecchione, C.; Sciarretta, S. Novel Beneficial Cardiovascular Effects of Natural Activators of Autophagy. Circ. Res. 2018, 123, 947–949. [Google Scholar] [CrossRef]
- Carnevale, R.; Nocella, C.; Schiavon, S.; Cammisotto, V.; Cotugno, M.; Forte, M.; Valenti, V.; Marchitti, S.; Vecchio, D.; Biondi Zoccai, G.; et al. Beneficial effects of a combination of natural product activators of autophagy on endothelial cells and platelets. Br. J. Pharmacol. 2021, 178, 2146–2159. [Google Scholar] [CrossRef] [PubMed]


| Risk Factor | Control Subjects | AF Patients | p-Value |
|---|---|---|---|
| Arterial hypertension | 14.0% | 85.3% | <0.001 |
| Diabetes | 4.6% | 27.3% | <0.001 |
| Smoking | 8.7% | 12.7% | n.s. |
| Heart failure | 0% | 18.0% | <0.001 |
| Previous cardiovascular disease | 2% | 28.0% | <0.001 |
| Previous thromboembolism | 0% | 12.7% | <0.001 |
| Time in therapeutic range (%) | - | 67.0 (54–82.0) * | - |
| Creatinine Clearance (sMDRD) ml/min | 88.9 (73.1–93.3) * | 78.4 (65.6–93.3) * | n.s. |
| CHA2DS2VASc score | - | 3.0 (2.0–4.0) * | - |
| Variables | Total (n = 300) | Control Subjects (n = 150) | AF Patients (n = 150) | p-Value |
|---|---|---|---|---|
| Age (years) | 66.6 ± 9.5 | 66.8 ± 9.1 | 0.840 | |
| Women (%) | 39.3 | 37.3 | 0.812 | |
| Serum sNox2-dp (pg/mL) | 12.25 ± 0.58 | 14.60 ± 1.0012 | 0.04 | |
| Serum H2O2 (µM) | 18.68 ± 0.71 | 30.36 ± 1.38 | <0.0001 | |
| HBA (%) | 44.89 ± 2,14 | 30.10 ± 1,79 | <0.0001 | |
| Plasma CD40L (ng/mL) | 2.84 ± 0.14 | 6.01 ± 0.28 | <0.0001 | |
| Plasma sP-selectin (ng/mL) | 7.958 ± 0.39 | 12.48 ± 0.40 | <0.0001 | |
| Serum NO (µM) | 38.23 ± 2,27 | 19.93 ± 1.08 | <0.0001 | |
| Plasma ATG5 (ng/mL) | 128.0 ± 4.45 | 93.21 ± 4.60 | <0.0001 | |
| Plasma P62 (ng/mL) | 50.26 ± 3.023 | 66.74 ± 3.81 | <0.001 |
| Control Subjects Group | ||||||
| H2O2 (µM) | P62 (ng/mL) | ATG5 (ng/mL) | NO (µM) | CD40L (ng/mL) | sP-Selectin (ng/mL) | |
| H2O2 (µM) | - | |||||
| P62 (ng/mL) | 0.174 * | - | ||||
| ATG5 (ng/mL) | −0.233 ** | −0.207 * | - | |||
| NO (µM) | −0.144 | −0.025 | 0.085 | - | ||
| CD40L (ng/mL) | 0.214 ** | 0.230 ** | −0.185 * | −0.125 | - | |
| sP-selectin (ng/mL) | 0.244 ** | 0.111 | −0.271 ** | −0.067 | 0.417 ** | - |
| Atrial Fibrillation Patients | ||||||
| H2O2 (µM) | P62 (ng/mL) | ATG5 (ng/mL) | NO (µM) | CD40L (ng/mL) | sP-Selectin (ng/mL) | |
| H2O2 (µM) | - | |||||
| P62 (ng/mL) | 0.126 | - | ||||
| ATG5 (ng/mL) | −0.209 * | −0.294 ** | - | |||
| NOµM | −0.205 * | −0.214 ** | 0.250 ** | - | ||
| CD40L (ng/mL) | 0.080 | 0.234 ** | −0.172 * | −0.274 ** | - | |
| sP-selectin (ng/mL) | 0.273 ** | 0.242 ** | −0.347 ** | −0.241 ** | 0.281 ** | - |
| CHA2DS2VASc score | 0.245 ** | 0.241 ** | −0.118 | −0.326 ** | 0.116 | 0.474 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Versaci, F.; Valenti, V.; Forte, M.; Cammisotto, V.; Nocella, C.; Bartimoccia, S.; Schirone, L.; Schiavon, S.; Vecchio, D.; D’Ambrosio, L.; et al. Aging-Related Decline of Autophagy in Patients with Atrial Fibrillation—A Post Hoc Analysis of the ATHERO-AF Study. Antioxidants 2022, 11, 698. https://doi.org/10.3390/antiox11040698
Versaci F, Valenti V, Forte M, Cammisotto V, Nocella C, Bartimoccia S, Schirone L, Schiavon S, Vecchio D, D’Ambrosio L, et al. Aging-Related Decline of Autophagy in Patients with Atrial Fibrillation—A Post Hoc Analysis of the ATHERO-AF Study. Antioxidants. 2022; 11(4):698. https://doi.org/10.3390/antiox11040698
Chicago/Turabian StyleVersaci, Francesco, Valentina Valenti, Maurizio Forte, Vittoria Cammisotto, Cristina Nocella, Simona Bartimoccia, Leonardo Schirone, Sonia Schiavon, Daniele Vecchio, Luca D’Ambrosio, and et al. 2022. "Aging-Related Decline of Autophagy in Patients with Atrial Fibrillation—A Post Hoc Analysis of the ATHERO-AF Study" Antioxidants 11, no. 4: 698. https://doi.org/10.3390/antiox11040698
APA StyleVersaci, F., Valenti, V., Forte, M., Cammisotto, V., Nocella, C., Bartimoccia, S., Schirone, L., Schiavon, S., Vecchio, D., D’Ambrosio, L., Spinosa, G., D’Amico, A., Chimenti, I., Violi, F., Frati, G., Pignatelli, P., Sciarretta, S., Pastori, D., & Carnevale, R. (2022). Aging-Related Decline of Autophagy in Patients with Atrial Fibrillation—A Post Hoc Analysis of the ATHERO-AF Study. Antioxidants, 11(4), 698. https://doi.org/10.3390/antiox11040698