Salvia officinalis L. Essential Oil: Characterization, Antioxidant Properties, and the Effects of Aromatherapy in Adult Patients
Abstract
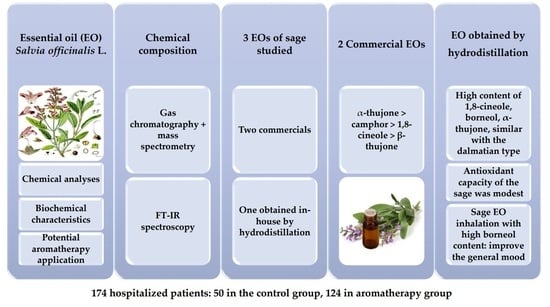
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. GC-MS Determination of the Chemical Composition of the Essential Oil from Salvia officinalis
2.3. ATR-FTIR Spectroscopy
2.4. Antioxidant Activity (DPPH and ABTS Assays)
2.5. Aromatherapy Effects: Clinical Application
2.6. Statistical Analysis
3. Results
3.1. Chemical Analyses
3.1.1. GC-MS Analyses
3.1.2. ATR-FTIR Spectroscopy
3.1.3. Antioxidant Capacity
3.2. Patients’ Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, R.; Zhang, D.; Yang, J.; Wu, Z.; Luo, C.; Han, L.; Yang, F.; Lin, J.; Yang, M. Review of Aromatherapy Essential Oils and Their Mechanism of Action Against Migraines. J. Ethnopharmacol. 2021, 265, 113326. [Google Scholar] [CrossRef] [PubMed]
- Winkelman, W.J. Aromatherapy, Botanicals, and Essential Oils in Acne. Clin. Dermatol. 2018, 36, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Lizarraga-Valderrama, L.R. Effects of Essential Oils on Central Nervous System: Focus on Mental Health. Phytother. Res. 2021, 35, 657–679. [Google Scholar] [CrossRef]
- Zeinalian, M.; Eshaghi, M.; Hadian, M.; Naji, H.; Marandi, S.M.M.; Asgary, S. Eight Essential Foods in Iranian Traditional Medicine and their Role in Health Promotion and Well-being. Int. J. Prev. Med. 2017, 8, 2. [Google Scholar] [CrossRef] [PubMed]
- Contrada, M.; Cerasa, A.; Tonin, P.; Bagetta, G.; Scuteri, D. Aromatherapy in Stroke Patients: Is It Time to Begin? Front. Behav. Neurosci. 2021, 15, 749353. [Google Scholar] [CrossRef] [PubMed]
- Fismer, K.L.; Pilkington, K. Lavender and Sleep: A systematic Review of the Evidence. Eur. J. Integr. Med. 2012, 4, E436–E447. [Google Scholar] [CrossRef]
- Lillehei, A.S.; Halcon, L.L. A Systematic Review of the Effect of Inhaled Essential Oils on Sleep. J. Altern. Complement. Med. 2014, 20, 441–451. [Google Scholar] [CrossRef]
- Hawkins, J.; Hires, C.; Dunne, E.; Baker, C. The Relationship between Lavender and Tea Tree Essential Oils and Pediatric Endocrine Disorders: A Systematic Review of the Literature. Complement. Ther. Med. 2020, 49, 102288. [Google Scholar] [CrossRef]
- Zhang, Y.; Long, Y.; Yu, S.; Li, D.; Yang, M.; Guan, Y.; Zhang, D.; Wan, J.; Liu, S.; Shi, A.; et al. Natural Volatile Oils Derived from Herbal Medicines: A Promising Therapy Way for Treating Depressive Disorder. Pharmacol. Res. 2021, 164, 105376. [Google Scholar] [CrossRef]
- Almanaa, T.N.; Alharbi, N.S.; Ramachandran, G.; Chelliah, C.K.; Rajivgandhi, G.; Manoharan, N.; Kadaikunnan, S.; Khaled, J.M.; Alanzi, K.F. Anti-biofilm Effect of Nerium oleander Essential Oils Against Biofilm Forming Pseudomonas aeruginosa on Urinary Tract Infections. J. King Saud Univ. Sci. 2021, 33, 101340. [Google Scholar] [CrossRef]
- Brandao, F.R.; Farias, C.F.S.; Souza, D.C.D.; de Oliveira, M.I.B.; de Matos, L.V.; Majolo, C.; de Oliveira, M.R.; Chaves, F.C.M.; O’Sullivan, F.L.D.; Chagas, E.C. Anesthetic Potential of the Essential Oils of Aloysia triphylla, Lippia sidoides and Mentha piperita for Colossoma macropomum. Aquaculture 2021, 534, 736275. [Google Scholar] [CrossRef]
- Valdivieso-Ugarte, M.; Plaza-Diaz, J.; Gomez-Llorente, C.; Gomez, E.L.; Sabes-Alsina, M.; Gil, A. In Vitro Examination of Antibacterial and Immunomodulatory Activities of Cinnamon, White Thyme, and Clove Essential Oils. J. Funct. Foods 2021, 81, 104436. [Google Scholar] [CrossRef]
- Chraibi, M.; Fadil, M.; Farah, A.; Lebrazi, S.; Fikri-Benbrahim, K. Antimicrobial Combined Action of Mentha pulegium, Ormenis mixta and Mentha piperita Essential Oils Against S. aureus, E. coli and C. tropicalis: Application of Mixture Design Methodology. LWT-Food Sci. Technol. 2021, 145, 111352. [Google Scholar] [CrossRef]
- Rocha, R.R.; Matos, M.N.C.; Guerrero, J.A.P.; Cavalcante, R.M.B.; Melo, R.S.; Azevedo, A.M.A.; Pereira, A.M.G.; Lopes, P.H.R.; Rodrigues, T.H.S.; Bandeira, P.N.; et al. Comparative Study of the Chemical Composition, Antibacterial Activity and Synergic Effects of the Essential Oils of Croton tetradenius Baill. and C. pulegiodorus Baill. Against Staphylococcus aureus Isolates. Microb. Pathog. 2021, 156, 104934. [Google Scholar] [CrossRef]
- Bogdan, M.A.; Bungau, S.; Tit, D.M.; Zaha, D.C.; Nechifor, A.C.; Behl, T.; Chambre, D.; Lupitu, A.I.; Copolovici, L.; Copolovici, D.M. Chemical Profile, Antioxidant Capacity, and Antimicrobial Activity of Essential Oils Extracted from Three Different Varieties (Moldoveanca 4, Vis Magic 10, and Alba 7) of Lavandula angustifolia. Molecules 2021, 26, 4381. [Google Scholar] [CrossRef] [PubMed]
- Delamare, A.P.L.; Moschen-Pistorello, I.T.; Artico, L.; Atti-Serafini, L.; Echeverrigaray, S. Antibacterial Activity of the Essential Oils of Salvia officinalis L. and Salvia triloba L. Cultivated in South Brazil. Food Chem. 2007, 100, 603–608. [Google Scholar] [CrossRef]
- Kulak, M.; Gul, F.; Sekeroglu, N. Changes in Growth Parameter and Essential Oil Composition of Sage (Salvia officinalis L.) Leaves in Response to Various Salt Stresses. Ind. Crops Prod. 2020, 145, 112078. [Google Scholar] [CrossRef]
- Rzepa, J.; Wojtal, L.; Staszek, D.; Grygierczyk, G.; Labe, K.; Hajnos, M.; Kowalska, T.; Waksmundzka-Hajnos, M. Fingerprint of Selected Salvia Species by HS-GC-MS Analysis of Their Volatile Fraction. J. Chromatogr. Sci. 2009, 47, 575–580. [Google Scholar] [CrossRef] [Green Version]
- Mohamed, A.; Mustafa, A. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of Essential Oil Salvia officinalis in Sudan. J. Multidis. Res. Rev. 2019, 1, 43–45. [Google Scholar]
- Ciko, L.; Andoni, A.; Ylli, F.; Plaku, E.; Taraj, K.; Armand, C. Extraction of Essential Oil from Albanian Salvia officinalis L. and its Characterization by FTIR Spectroscopy. Asian J. Chem. 2016, 28, 1401–1402. [Google Scholar] [CrossRef]
- Glevitzky, I.; Dumitrel, G.A.; Glevitzky, M.; Pasca, B.; Otrisal, P.; Bungau, S.; Cioca, G.; Pantis, C.; Popa, M. Statistical Analysis of the Relationship between Antioxidant Activity and the Structure of Flavonoid Compounds. Rev. Chim. 2019, 70, 3103–3107. [Google Scholar] [CrossRef]
- Rafi, N.; Khodadadizadeh, A.; Nematabad, M.S.; Sayadi, A.R. The Evaluation of the Effect of Aromatherapy with Lavender Essential Oil on the Quality of Sleep of Cardiac Patients Candidate for Angiography. Pak. J. Med. Health Sci. 2020, 14, 1143–1147. [Google Scholar]
- Russo, P.; Frustaci, A.; Del Bufalo, A.; Fini, M.; Cesario, A. From Traditional European Medicine to Discovery of New Drug Candidates for the Treatment of Dementia and Alzheimer’s Disease: Acetylcholinesterase Inhibitors. Curr. Med. Chem. 2013, 20, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, H.; Salmani, T.; Nourian, J.; Mirrezaie, S.M.; Abbasi, A.; Mardani, A.; Vlaisavljevic, Z. The Effects of Inhalation Aromatherapy Using Lavender Essential Oil on Postoperative Pain of Inguinal Hernia: A Randomized Controlled Trial. J. Perianesthesia Nurs. 2020, 35, 642–648. [Google Scholar] [CrossRef]
- Mohr, C.; Jensen, C.; Padden, N.; Besel, J.M.; Brant, J.M. Peppermint Essential Oil for Nausea and Vomiting in Hospitalized Patients: Incorporating Holistic Patient Decision Making Into the Research Design. J. Holist. Nurs. 2021, 39, 126–134. [Google Scholar] [CrossRef]
- Bertone, A.C.; Dekker, R.L. Aromatherapy in Obstetrics: A Critical Review of the Literature. Clin. Obstet. Gynecol. 2021, 64, 572–588. [Google Scholar] [CrossRef]
- Karan, N.B. Influence of lavender oil inhalation on vital signs and anxiety: A randomized clinical trial. Physiol. Behav. 2019, 211, 112676. [Google Scholar] [CrossRef]
- Lordani, T.V.A.; de Lara, C.E.; Ferreira, F.B.P.; Monich, M.D.T.; da Silva, C.M.; Lordani, C.R.F.; Bueno, F.G.; Teixeira, J.J.V.; Lonardoni, M.V.C. Therapeutic Effects of Medicinal Plants on Cutaneous Wound Healing in Humans: A Systematic Review. Mediat. Inflamm. 2018, 2018, 7354250. [Google Scholar] [CrossRef] [Green Version]
- Deng, C.; Xie, Y.; Liu, Y.; Li, Y.; Xiao, Y. Aromatherapy Plus Music Therapy Improve Pain Intensity and Anxiety Scores in Patients with Breast Cancer during Perioperative Periods: A Randomized Controlled Trial. Clin. Breast Cancer 2021, 22, 115–120. [Google Scholar] [CrossRef]
- Huang, H.; Wang, Q.; Guan, X.; Zhang, X.; Kang, J.; Zhang, Y.; Zhang, Y.; Zhang, Q.; Li, X. Effect of Aromatherapy on Preoperative Anxiety in Adult Patients: A Meta-Analysis of Randomized Controlled Trials. Complement. Ther. Clin. Pract. 2021, 42, 101302. [Google Scholar] [CrossRef]
- Tang, Y.; Gong, M.; Qin, X.; Su, H.; Wang, Z.; Dong, H. The Therapeutic Effect of Aromatherapy on Insomnia: A Meta-Analysis. J. Affect. Disord. 2021, 288, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpourhodki, R.; Sadeghnezhad, H.; Ebrahimi, H.; Basirinezhad, M.H.; Maleki, M.; Bossola, M. The Effect of Aromatherapy Massage with Lavender and Citrus Aurantium Essential Oil on Quality of Life of Patients on Chronic Hemodialysis: A Parallel Randomized Clinical Trial Study. J. Pain Symptom Manag. 2021, 61, 456–463.e1. [Google Scholar] [CrossRef] [PubMed]
- Varney, E.; Buckle, J. Effect of Inhaled Essential Oils on Mental Exhaustion and Moderate Burnout: A Small Pilot Study. J. Altern. Complement. Med. 2013, 19, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.-Y.; Lee, H.-F.; Chang, C.W.; Chiang, H.-C.; Tsai, Y.-H.; Liu, H.-E. The Immediate Effects of Lavender Aromatherapy Massage versus Massage in Work Stress, Burnout, and HRV Parameters: A Randomized Controlled Trial. Evid.-Based Complement. Altern. Med. 2020, 2020, 8830083. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, K.D.; Feitosa, B.D.; Cruz, J.N.; Ferreira, O.O.; Franco, C.D.; Cascaes, M.M.; Oliveira, M.S.; Andrade, E.H. Chemical composition and preliminary toxicity evaluation of the essential oil from Peperomia circinnata Link var. circinnata. (Piperaceae) in Artemia salina Leach. Molecules 2021, 26, 7359. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.F.; Carvalho, F.; Fernandes, E.; Bastos, M.L.; Santos-Gomes, P.C.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Evaluation of toxic/protective effects of the essential oil of Salvia officinalis on freshly isolated rat hepatocytes. Toxicol. In Vitro Int. J. Publ. Assoc. BIBRA 2004, 18, 457–465. [Google Scholar] [CrossRef]
- El Hadri, A.; del Rio, M.G.; Sanz, J.; Coloma, A.G.; Idaomar, M.; Ozonas, B.R.; González, J.B.; Reus, M.I.S. Cytotoxic Activity of alpha-Humulene and Transcaryophyllene from Salvia officinalis in Animal and Human Tumor Cells. An. R. Acad. Nac. Farm. 2010, 76, 343–356. [Google Scholar]
- Halicioglu, O.; Astarcioglu, G.; Yaprak, I.; Aydinlioglu, H. Toxicity of Salvia officinalis in a newborn and a child: An alarming report. Pediatr. Neurol. 2011, 45, 259–260. [Google Scholar] [CrossRef]
- Ahangari, F.; Farshbaf-Khalili, A.; Javadzadeh, Y.; Adibpour, M.; Oskouei, B.S. Comparing the Effectiveness of Salvia officinalis, Clotrimazole and their Combination on Vulvovaginal Candidiasis: A Randomized, Controlled Clinical Trial. J. Obstet. Gynaecol. Res. 2019, 45, 897–907. [Google Scholar] [CrossRef]
- Luca, T.; Napoli, E.; Privitera, G.; Musso, N.; Ruberto, G.; Castorina, S. Antiproliferative Effect and Cell Cycle Alterations Induced by Salvia officinalis Essential Oil and Its Three Main Components in Human Colon Cancer Cell Lines. Chem. Biodivers. 2020, 17, e2000309. [Google Scholar] [CrossRef]
- Russo, A.; Formisano, C.; Rigano, D.; Senatore, F.; Delfine, S.; Cardile, V.; Rosselli, S.; Bruno, M. Chemical Composition and Anticancer Activity of Essential Oils of Mediterranean sage (Salvia officinalis L.) Grown in Different Environmental Conditions. Food Chem. Toxicol. 2013, 55, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Sertel, S.; Eichhorn, T.; Plinkert, P.K.; Efferth, T. Anticancer Activity of Salvia officinalis Essential Oil against HNSCC Cell Line (UMSCC1). Hno 2011, 59, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Ovidi, E.; Masci, V.L.; Zambelli, M.; Tiezzi, A.; Vitalini, S.; Garzoli, S. Laurus nobilis, Salvia sclarea and Salvia officinalis Essential Oils and Hydrolates: Evaluation of Liquid and Vapor Phase Chemical Composition and Biological Activities. Plants 2021, 10, 707. [Google Scholar] [CrossRef]
- Ghorbani, A.; Esmaeilizadeh, M. Pharmacological Properties of Salvia officinalis and its Components. J. Tradit. Complement. Med. 2017, 7, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Ghaedi, M.; Naghiha, R.; Jannesar, R.; Dehghanian, N.; Mirtamizdoust, B.; Pezeshkpour, V. Antibacterial and Antifungal Activity of Flower Extracts of Urtica dioica, Chamaemelum nobile and Salvia officinalis: Effects of Zn[OH]2 Nanoparticles and Hp-2-minh on their Property. J. Ind. Eng. Chem. 2015, 32, 353–359. [Google Scholar] [CrossRef]
- Pearson, A.C.S.; Cutshall, S.M.; Hooten, W.M.; Rodgers, N.J.; Bauer, B.A.; Bhagra, A. Perspectives on the Use of Aromatherapy from Clinicians Attending an Integrative Medicine Continuing Education Event. BMC Complement. Altern. Med. 2019, 19, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, E.H.; Lee, M.-Y.; Hur, M.-H. The Effects of Aromatherapy on Intensive Care Unit Patients′ Stress and Sleep Quality: A Nonrandomised Controlled Trial. Evid.-Based Complement. Altern. Med. 2017, 2017, 2856592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasab, F.R.S.; Shahrbabaki, P.M.; Dehghan, M.; Tajadini, H.; Baniasadi, H.; Sabzevari, S. Effect of Abdominal Massage with and without Salvia officinalis on Nausea and Vomiting in Patients with Cancer Undergoing Chemotherapy: A Randomized Clinical Trial. J. Oncol. 2021, 2021, 9989228. [Google Scholar] [CrossRef]
- Jodaki, K.; Abdi, K.; Mousavi, M.S.; Mokhtari, R.; Asayesh, H.; Vandali, V.; Golitaleb, M. Effect of Rosa damascene Aromatherapy on Anxiety and Sleep Quality in Cardiac Patients: A Randomized Controlled Trial. Complement. Ther. Clin. Pract. 2021, 42, 101299. [Google Scholar] [CrossRef]
- Moslemi, F.; Alijaniha, F.; Naseri, M.; Kazemnejad, A.; Charkhkar, M.; Heidari, M.R. Citrus aurantium Aroma for Anxiety in Patients with Acute Coronary Syndrome: A Double-Blind Placebo-Controlled Trial. J. Altern. Complement. Med. 2019, 25, 833–839. [Google Scholar] [CrossRef]
- Gudmundsson, G.; Gislason, T.; Janson, C.; Lindberg, E.; Ulrik, C.S.; Brondum, E.; Nieminen, M.M.; Aine, T.; Hallin, R.; Bakke, P. Depression, Anxiety and Health Htatus after Hospitalisation for COPD: A Multicentre Study in the Nordic Countries. Respir. Med. 2006, 100, 87–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beicu, R.; Alexa, E.; Obistioiu, D.; Cocan, I.; Imbrea, F.; Pop, G.; Circioban, D.; Moisa, C.; Lupitu, A.; Copolovici, L.; et al. Antimicrobial Potential and Phytochemical Profile of Wild and Cultivated Populations of Thyme (Thymus sp.) Growing in Western Romania. Plants 2021, 10, 1833. [Google Scholar] [CrossRef] [PubMed]
- WMA Declaration of Helsinki-Ethical Principles for Medical Research Involving Human Subjects. Available online: https://www.wma.net/policies-post/wma-declaration-of-helsinki-ethical-principles-for-medical-research-involving-human-subjects/ (accessed on 23 June 2019).
- Moisa, C.; Lupitu, A.; Pop, G.; Chambre, D.R.; Copolovici, L.; Cioca, G.; Bungau, S.; Copolovici, D.M. Variation of the Chemical Composition of Thymus Vulgaris Essential Oils by Phenological Stages. Rev. De Chim. 2019, 70, 633–637. [Google Scholar] [CrossRef]
- Demirci, B.; Tabanca, N.; Baser, K.H.C. Enantiomeric Distribution of Some Monoterpenes in the Essential Oils of some Salvia Species. Flavour Fragr. J. 2002, 17, 54–58. [Google Scholar] [CrossRef]
- Granger, R.E.; Campbell, E.L.; Johnston, G.A.R. (+)- And (−)-Borneol: Efficacious Positive Modulators of GABA Action at Human Recombinant α1β2γ2L GABAA Receptors. Biochem. Pharmacol. 2005, 69, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Coates, J. Interpretation of Infrared Spectra, A Practical Approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., McKelvy, M.L., Eds.; John Wiley & Sons, Ltd.: New York, NY, USA, 2006. [Google Scholar] [CrossRef]
- Schulz, H.; Baranska, M. Identification and quantification of valuable plant substances by IR and Raman spectroscopy. Vib. Spectrosc. 2007, 43, 13–25. [Google Scholar] [CrossRef]
- Schulz, H.; Özkan, G.; Baranska, M.; Krüger, H.; Özcan, M. Characterisation of essential oil plants from Turkey by IR and Raman spectroscopy. Vib. Spectrosc. 2005, 39, 249–256. [Google Scholar] [CrossRef]
- Chambre, D.R.; Moisa, C.; Lupitu, A.; Copolovici, L.; Pop, G.; Copolovici, D.M. Chemical composition, antioxidant capacity, and thermal behavior of Satureja hortensis essential oil. Sci. Rep. 2020, 10, 21322. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Ristivojevic, P.; Gegechkori, V.; Litvinova, T.M.; Morton, D.W. Essential Oil Quality and Purity Evaluation via FT-IR Spectroscopy and Pattern Recognition Techniques. Appl. Sci. 2020, 10, 7294. [Google Scholar] [CrossRef]
- Samani, M.R.; Pirbalouti, A.G.; Moattar, F.; Golparvar, A.R. L-Phenylalanine and Bio-Fertilizers Interaction Effects on Growth, Yield and Chemical Compositions and Content of Essential Oil From the Sage (Salvia officinalis L.) Leaves. Ind. Crops Prod. 2019, 137, 1–8. [Google Scholar] [CrossRef]
- Ed-Dra, A.; Filali, F.R.; Lo Presti, V.; Zekkori, B.; Nalbone, L.; Bouymajane, A.; Trabelsi, N.; Lamberta, F.; Bentayeb, A.; Giuffrida, A.; et al. Chemical Composition, Antioxidant Capacity and Antibacterial Action of Five Moroccan Essential Oils Against Listeria Monocytogenes and Different Serotypes of Salmonella enterica. Microb. Pathog. 2020, 149, 104510. [Google Scholar] [CrossRef] [PubMed]
- Hayouni, E.A.; Chraief, I.; Abedrabba, M.; Bouix, M.; Leveau, J.Y.; Mohammed, H.; Hamdi, M. Tunisian Salvia officinalis L. and Schinus molle L. Essential Oils: Their Chemical Compositions an Their Preservative Effects Against Salmonella Inoculated in Minced Beef Meat. Int. J. Food Microbiol. 2008, 125, 242–251. [Google Scholar] [CrossRef]
- Craft, J.D.; Satyal, P.; Setzer, W.N. The Chemotaxonomy of Common Sage (Salvia officinalis) Based on the Volatile Constituents. Medicines 2017, 4, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jug-Dujaković, M.; Ristić, M.; Pljevljakušić, D.; Dajić-Stevanović, Z.; Liber, Z.; Hančević, K.; Radić, T.; Šatović, Z. High Diversity of Indigenous Populations of Dalmatian Sage (Salvia officinalis L.) in Essential-Oil Composition. Chem. Biodivers. 2012, 9, 2309–2323. [Google Scholar] [CrossRef] [PubMed]
- Kuštrak, D.; Kuftinec, J.; Blažević, N. Yields and Composition of Sage Oils from Different Regions of the Yugoslavian Adriatic Coast. J. Nat. Prod. 1984, 47, 520–524. [Google Scholar] [CrossRef]
- Valderrama, A.C.S.; Rojas De, G.C. Traceability of Active Compounds of Essential Oils in Antimicrobial Food Packaging Using a Chemometric Method by ATR-FTIR. Am. J. Anal. Chem. 2017, 8, 726–741. [Google Scholar] [CrossRef] [Green Version]
- Miguel, G.; Cruz, C.; Faleiro, M.L.; Simoes, M.T.F.; Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G. Salvia officinalis L. Essential Oils: Effect of Hydrodistillation Time on the Chemical Composition, Antioxidant and Antimicrobial Activities. Nat. Prod. Res. 2011, 25, 526–541. [Google Scholar] [CrossRef]
- Abou Baker, D.H.; Amarowicz, R.; Kandeil, A.; Ali, M.A.; Ibrahim, E.A. Antiviral Activity of Lavandula angustifolia L. and Salvia officinalis L. Essential Oils Against Avian Influenza H5N1 Virus. J. Agric. Food Res. 2021, 4, 100135. [Google Scholar] [CrossRef]
- Ben Farhat, M.; Jordan, M.J.; Chaouech-Hamada, R.; Landoulsi, A.; Sotomayor, J.A. Variations in Essential Oil, Phenolic Compounds, and Antioxidant Activity of Tunisian Cultivated Salvia officinalis L. J. Agric. Food Chem. 2009, 57, 10349–10356. [Google Scholar] [CrossRef]
- Bozin, B.; Mimica-Dukic, N.; Samojlik, I.; Jovin, E. Antimicrobial and antioxidant properties of rosemary and sage (Rosmarinus officinalis L. and Salvia officinalis L., Lamiaceae) essential oils. J. Agric. Food Chem. 2007, 55, 7879–7885. [Google Scholar] [CrossRef]
- Fu, Z.; Wang, H.; Hu, X.; Sun, Z.; Han, C. The Pharmacological Properties of Salvia Essential Oils. J. App. Pharm. Sci. 2013, 3, 122–127. [Google Scholar] [CrossRef]



| No. | KI | Compound/Class | Present Study | Turkey [17] | Poland [18] | Morocco [37] | Sudan [19] | Brazil [16] | ||
|---|---|---|---|---|---|---|---|---|---|---|
| B-SEO | EG-SEO | L-SEO | ||||||||
| 1 | 925 | Unidentified | 0.21 ± 0.01 | 0.24 ± 0.01 | ||||||
| 2 | 926 | Tricyclene/MH | 0.42 ± 0.01 | |||||||
| 3 | 930 | α-Thujene/MH | 0.17 ± 0.01 | 0.15 ± 0.01 | 0.45 ± 0.01 | 0.31 | ||||
| 4 | 939 | α-Pinene/MH | 5.96 ± 0.03 | 4.23 ± 0.02 | 7.8 ± 0.05 | 7.17 | 0.02 | 3.18 | 8.96 | 3.07 |
| 5 | 954 | Camphene/MH | 5.59 ± 0.07 | 6.92 ± 0.10 | 8.73 ± 0.12 | 8.40 | 3.67 | 5.09 | 4.40 | |
| 6 | 975 | Sabinene/MH | 1.70 ± 0.01 | 1.55 ± 0.03 | 0.14 ± 0.01 | 0.41 | 0.15 | |||
| 7 | 979 | β-Pinene/MH | 0.78 ± 0.03 | 0.55 ± 0.02 | 10.52 ± 0.15 | 2.92 | 0.40 | 2.57 | ||
| 8 | 990 | β-Myrcene/MH | 0.72 ± 0.06 | 1.16 | 1.94 | 3.65 | ||||
| 9 | 1012 | 4-Carene/MH | 0.42 ± 0.02 | 0.07 ± 0.01 | ||||||
| 10 | 1024 | p-Cymene/MH | 0.85 ± 0.02 | 1.01 ± 0.03 | 1.33 | |||||
| 11 | α-Terpinene/MH | 0.34 ± 0.02 | 0.18 | 0.2 | 0.22 | |||||
| 12 | 1029 | 1,8-Cineole/MH | 13.39 ± 0.21 | 14.22 ± 0.19 | 17.98 ± 0.23 | 18.54 | 23.72 | 17.52 | 14.8 | |
| 13 | 1050 | trans-β-Ocimene/MH | 0.52 ± 0.06 | |||||||
| 14 | 1051 | D-Limonene/MH | 2.46 | 1.7 | 0.37 | |||||
| 15 | 1056 | Linalool/MH | 0.79 | |||||||
| 16 | 1059 | γ-Terpinene/MH | 0.60 ± 0.05 | 0.15 ± 0.06 | 0.62 ± 0.08 | 0.18 | 0.42 | |||
| 17 | 1088 | α-Terpinolene/MH | 0.60 ± 0.10 | 0.20 ± 0.05 | 0.12 | |||||
| 18 | 1089 | 2-Carene/MH | 0.19 ± 0.01 | |||||||
| 19 | 1102 | α-Thujone/MO | 26.03 ± 0.25 | 26.73 ± 0.26 | 8.74 ± 0.12 | 22.30 | 21.22 | 0.91 | 24.8 | |
| 20 | 1109 | β-Thujone/MO | 4.65 ± 0.11 | 4.19 ± 0.12 | 1.34 ± 0.11 | 14.28 | 13.45 | 3.97 | ||
| 21 | 1146 | Camphor/MO | 20.09 ± 0.24 | 22.64 ± 0.27 | 2.56 ± 0.12 | 14.40 | 18.22 | 21.23 | 11.57 | 10.9 |
| 22 | 1169 | Borneol/MO | 3.1 ± 0.20 | 3.31 ± 0.19 | 15.86 ± 0.23 | 0.37 | 2.42 | 1.67 | 0.81 | 11.1 |
| 23 | 1188 | α-Terpineol/MO | 0.50 ± 0.07 | 0.46 ± 0.08 | ||||||
| 24 | 1287 | Bornyl acetate/MO | 1.93 ± 0.11 | 2.27 ± 0.13 | 0.88 ± 0.06 | 0.32 | 0.22 | |||
| 25 | 1290 | Thymol/MO | 0.33 ± 0.01 | |||||||
| 26 | 1351 | α-Cubebene/SH | 0.2 ± 0.06 | |||||||
| 27 | 1380 | α-Copaene/SH | 0.08 ± 0.01 | 0.13 | ||||||
| 28 | 1381 | α-Ylangene/SH | 0.26 ± 0.11 | |||||||
| 29 | 1389 | β-Bourbonene/SH | 0.15 ± 0.03 | |||||||
| 30 | 1393 | Unidentified | 0.21 ± 0.03 | |||||||
| 31 | 1402 | Unidentified | 0.23 ± 0.07 | |||||||
| 32 | 1419 | β-Caryophyllene/SH | 4.54 ± 0.23 | 3.28 ± 0.21 | 5.66 ± 0.22 | 0.58 | 3.76 | 2.89 | ||
| 33 | 1429 | Unidentified | 0.39 ± 0.01 | 0.29 ± 0.3 | ||||||
| 34 | 1432 | γ-Cadinen/SH | 0.24 ± 0.09 | |||||||
| 35 | 1439 | α-Guaiene/SH | 0.14 ± 0.01 | |||||||
| 36 | 1456 | α-Humulene/SH | 4.89 ± 021 | 4.29 ± 0.21 | 8.64 ± 0.25 | 0.94 | 1.45 | 1.47 | ||
| 37 | 1479 | γ-Muurolene/SH | 0.40 ± 0.08 | 0.26 ± 0.02 | 0.63 ± 0.06 | |||||
| 38 | 1500 | α-Muurolene/SH | 0.43 ± 0.07 | |||||||
| 39 | 1512 | Unidentified | 0.06 ± 0.01 | |||||||
| 40 | 1523 | δ-Cadinene/SH | 0.10 ± 0.01 | 0.03 ± 0.01 | 0.17 ± 0.01 | |||||
| 41 | 1576 | Isoledene/SH | 0.61 ± 0.05 | |||||||
| 42 | 1583 | Caryophyllene oxide/SO | 0.19 ± 0.01 | 0.29 ± 0.03 | 0.22 ± 0.07 | |||||
| 43 | 1592 | Viridiflorol/SO | 3.09 ± 0.19 | 0.6 | ||||||
| 44 | 1593 | Unidentified | 2.34 ± 0.19 | 1.88 ± 0.18 | ||||||
| 45 | 1594 | Unidentified | 0.35 ± 0.03 | 0.61 ± 0.08 | 0.6 ± 0.09 | |||||
| 46 | 1603 | Unidentified | 0.08 ± 0.01 | |||||||
| 47 | 1607 | Unidentified | 0.6 ± 0.08 | |||||||
| Wavenumber (cm−1) of ATR-FTIR Recorded Bands | Vibrational Assignment | ||
|---|---|---|---|
| L-SEO | B-SEO | EG-SEO | |
| 3459 | 3474 | 3474 | (O-H) stretching vibration [57] |
| 3066 | 3058 | 3058 | (CH) stretching vibration of CH3 and CH2 (Csp3 and Csp2) [57] |
| 2951 | 2958 | 2958 | |
| 2926 | 2929 | 2930 | |
| 2875 | 2875 | 2875 | |
| 1741 | 1741 | 1741 | (C=O) stretching vibration in carbonyl group; (C=C) stretching vibration in (>C=CH2), (-CH=CH-), and (-CH=C<) alkyl groups [20,56,57,58,59] |
| 1642 | 1637 | 1638 | |
| 1456 | 1455 | 1455 | (C-H) symmetric and asymmetric bending vibration of (CH3) and (CH2) groups, (C-H) in-plane bending, (C-O) symmetric and asymmetric stretching vibration, (O-H) in-plane bending, (CH3(CO)) symmetric bending, (C-O-C) symmetric and asymmetric stretching [20,57,59,60] |
| - | 1415 | 1415 | |
| 1371 | 1371 | 1371 | |
| 1303 | 1303 | 1303 | |
| 1266 | 1274 | 1274 | |
| 1236 | 1241 | 1241 | |
| 1214 | 1215 | 1215 | |
| 1166 | 1165 | 1165 | |
| 1106 | 1104 | 1104 | |
| 1080 | 1077 | 1078 | |
| 1054 | - | - | |
| - | 1045 | 1045 | |
| 1022 | 1022 | 1022 | |
| 982 | 983 | 983 | (CH2) and (CH) out-of-plane wagging, (O-H) out-of-plane banding vibration [57,59,60,61] |
| 877 | 880 | 879 | |
| 844 | 847 | 848 | |
| 817 | 810 | 810 | |
| 759 | 750 | 750 | |
| 642 | 642 | 642 | |
| Variable | Control Group | Salvia EO Group | Total | p-Value |
|---|---|---|---|---|
| n (%) | ||||
| Sex (Female/Male) | 14/36 | 46/78 | 174 (100) | |
| Weight (Kg) | 76.80 ± 13.74 | 74.98 ± 14.74 | 0.2173 * | |
| Educational level | 0.5877 ** | |||
| Middle school | 7 (14) | 11 (9) | 18 (10) | |
| High school | 25 (50) | 68 (55) | 93 (53) | |
| University | 18 (36) | 45 (36) | 63 (36) | |
| Social status | 0.3032 ** | |||
| Social aid | 3 (6) | 7 (6) | 10 (17) | |
| Active | 24 (48) | 75 (60) | 99 (57) | |
| Retired | 23 (46) | 42 (34) | 65 (37) | |
| Health status in the last year | 0.2839 ** | |||
| Excellent | 13 (26) | 34 (27) | 47 (27) | |
| Very good | 5 (10) | 24 (19) | 29 (17) | |
| Good | 17 (34) | 36 (29) | 53 (30) | |
| Bad | 15 (30) | 26 (21) | 41 (24) | |
| Very bad | 0 (0) | 4 (3) | 4 (2) | |
| Do you use fragrances/aroma in rooms/cars? | 0.1135 ** | |||
| Daily | 22 (44) | 63 (51) | 85 (49) | |
| 1–3 times/week | 5 (10) | 23 (19) | 28 (16) | |
| No | 23 (46) | 38 (31) | 61 (35) | |
| Do you use perfumes? | 0.4354 ** | |||
| Daily | 18 (36) | 57 (46) | 75 (43) | |
| 1–3 times/week | 22 (44) | 49 (40) | 71 (41) | |
| No | 10 (20) | 18 (15) | 28 (16) | |
| Do you smoke? | 0.0046 ** | |||
| Daily | 7 (14) | 46 (37) | 53 (30) | |
| Occasionally | 18 (36) | 42 (34) | 60 (34) | |
| No | 25 (50) | 36 (29) | 61 (35) | |
| Do you drink alcoholic drinks? | 0.1561 *** | |||
| Daily | 0 (0) | 1 (1) | 1 (1) | |
| Occasionally | 10 (20) | 51 (41) | 61 (35) | |
| No | 40 (80) | 72 (58) | 112 (64) | |
| Do you use sedatives/anxiolytics? | 0.0951 *** | |||
| Daily | 0 (0) | 0 (0) | 0 (0) | |
| Occasionally | 15 (30) | 31 (25) | 46 (26) | |
| No | 35 (70) | 93 (75) | 128 (74) | |
| Do you suffer from a chronic disease? | 0.6036 **** | |||
| Yes | 19 (38) | 42 (34) | 61 (35) | |
| No | 31 (62) | 82 (66) | 113 (65) | |
| Do you suffer from any drug/food allergies? | 0.0186 **** | |||
| Yes | 9 (18) | 46 (37) | 55 (32) | |
| No | 41 (82) | 78 (63) | 119 (68) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mot, M.-D.; Gavrilaș, S.; Lupitu, A.I.; Moisa, C.; Chambre, D.; Tit, D.M.; Bogdan, M.A.; Bodescu, A.-M.; Copolovici, L.; Copolovici, D.M.; et al. Salvia officinalis L. Essential Oil: Characterization, Antioxidant Properties, and the Effects of Aromatherapy in Adult Patients. Antioxidants 2022, 11, 808. https://doi.org/10.3390/antiox11050808
Mot M-D, Gavrilaș S, Lupitu AI, Moisa C, Chambre D, Tit DM, Bogdan MA, Bodescu A-M, Copolovici L, Copolovici DM, et al. Salvia officinalis L. Essential Oil: Characterization, Antioxidant Properties, and the Effects of Aromatherapy in Adult Patients. Antioxidants. 2022; 11(5):808. https://doi.org/10.3390/antiox11050808
Chicago/Turabian StyleMot, Maria-Daniela, Simona Gavrilaș, Andreea I. Lupitu, Cristian Moisa, Dorina Chambre, Delia Mirela Tit, Mihaela Alexandra Bogdan, Adina-Maria Bodescu, Lucian Copolovici, Dana Maria Copolovici, and et al. 2022. "Salvia officinalis L. Essential Oil: Characterization, Antioxidant Properties, and the Effects of Aromatherapy in Adult Patients" Antioxidants 11, no. 5: 808. https://doi.org/10.3390/antiox11050808
APA StyleMot, M. -D., Gavrilaș, S., Lupitu, A. I., Moisa, C., Chambre, D., Tit, D. M., Bogdan, M. A., Bodescu, A. -M., Copolovici, L., Copolovici, D. M., & Bungau, S. G. (2022). Salvia officinalis L. Essential Oil: Characterization, Antioxidant Properties, and the Effects of Aromatherapy in Adult Patients. Antioxidants, 11(5), 808. https://doi.org/10.3390/antiox11050808