Identification and Quantification of Urinary Microbial Phenolic Metabolites by HPLC-ESI-LTQ-Orbitrap-HRMS and Their Relationship with Dietary Polyphenols in Adolescents
Abstract
:1. Introduction
2. Materials and Methods
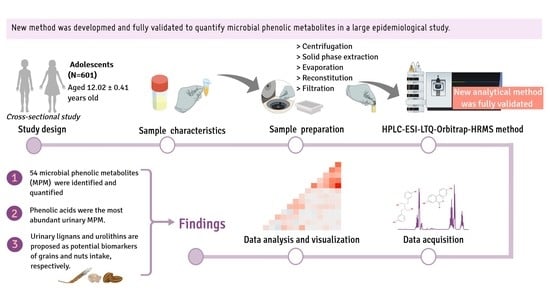
2.1. Study Design and Sample Selection
2.2. Chemicals and Urine Samples
2.3. Sample Preparation and Extraction of (Poly)Phenols
2.4. HPLC/ESI-LTQ-Orbitrap-HRMS Instrumentation
2.4.1. Chromatographic Conditions
2.4.2. Mass Spectrometry Parameters
2.5. Validation of the HPLC/ESI-LTQ-Orbitrap-HRMS Method
2.5.1. Linearity and Sensitivity
2.5.2. Accuracy and Precision
2.5.3. Recovery and Matrix Effect
2.5.4. Stability
2.5.5. Selectivity
2.6. Analysis of Urinary MPM by HPLC/ESI-LTQ-Orbitrap-HRMS in Adolescent Samples
2.6.1. Targeted Identification of MPM
2.6.2. Quantification of MPM
2.7. Dietary (Poly)Phenols
2.8. Data Analysis
3. Results and Discussion
3.1. Optimization of the HPLC/ESI-LTQ-Orbitrap-HRMS Method
3.2. Method Validation
3.2.1. Linearity, LOD, and LOQ
3.2.2. Precision and Accuracy
3.2.3. Matrix Effect and Recovery
3.2.4. Stability
3.2.5. Selectivity
3.3. Microbial Phenolic Metabolites Measured in Urine Samples
3.3.1. General Characteristics of the Study Population
3.3.2. Identification and Quantification of Urinary MPM
3.3.3. Urinary MPM and Dietary (Poly)Phenols
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AOAC | Association of Official Agricultural Chemists |
| BMI | body mass index |
| ESI | electrospray ionization |
| FDR | false discovery rate |
| FTMS | Fourier transform mass spectrometry |
| HPLC | high performance liquid chromatography |
| HRMS | high-resolution mass spectrometry |
| IQR | interquartile range |
| LOD | limit of detection |
| LOQ | limit of quantification |
| LTQ | linear ion trap quadrupole |
| MeOH | methanol |
| ME | matrix effect |
| MPM | microbial phenolic metabolites |
| MS/MS | two-stage mass analysis |
| MSn | multi-stage mass analysis |
| PCA | principal component analysis |
| R2 | coefficient of determination |
| RSD | relative standard deviation |
| SD | standard deviation |
| SEM | standard error of mean |
| S/N | signal-to-noise |
| SPE | solid-phase extraction |
| TPI | total (poly)phenol intake |
| UHPLC | ultra-high performance liquid chromatography |
References
- Tresserra-Rimbau, A.; Guasch-Ferré, M.; Salas-Salvadó, J.; Toledo, E.; Corella, D.; Castañer, O.; Guo, X.; Gómez-Gracia, E.; Lapetra, J.; Arós, F.; et al. Intake of Total Polyphenols and Some Classes of Polyphenols Is Inversely Associated with Diabetes in Elderly People at High Cardiovascular Disease Risk. J. Nutr. 2015, 146, 767–777. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Wedick, N.M.; Tworoger, S.S.; Pan, A.; Townsend, M.K.; Cassidy, A.; Franke, A.A.; Rimm, E.B.; Hu, F.B.; van Dam, R.M. Urinary Excretion of Select Dietary Polyphenol Metabolites Is Associated with a Lower Risk of Type 2 Diabetes in Proximate but Not Remote Follow-Up in a Prospective Investigation in 2 Cohorts of US Women. J. Nutr. 2015, 145, 1280–1288. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvadó, J.; Covas, M.-I.; Corella, D.; Arós, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Marhuenda-Muñoz, M.; Laveriano-Santos, E.P.; Tresserra-Rimbau, A.; Lamuela-Raventós, R.M.; Martínez-Huélamo, M.; Vallverdú-Queralt, A. Microbial Phenolic Metabolites: Which Molecules Actually Have an Effect on Human Health? Nutrients 2019, 11, 2725. [Google Scholar] [CrossRef] [Green Version]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food Sources and Bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef] [Green Version]
- Duarte, L.; Gasaly, N.; Poblete-Aro, C.; Uribe, D.; Echeverria, F.; Gotteland, M.; Garcia-Diaz, D.F. Polyphenols and Their Anti-Obesity Role Mediated by the Gut Microbiota: A Comprehensive Review. Rev. Endocr. Metab. Disord. 2021, 22, 367–388. [Google Scholar] [CrossRef]
- Bohn, T. Dietary Factors Affecting Polyphenol Bioavailability. Nutr. Rev. 2014, 72, 429–452. [Google Scholar] [CrossRef]
- Fan, Y.; Qian, H.; Wu, Z.; Li, Z.; Li, X.; Zhang, Y.; Xu, Q.; Lu, C.; Wang, X. Exploratory Analysis of the Associations between Urinary Phytoestrogens and Thyroid Hormones among Adolescents and Adults in the United States: National Health and Nutrition Examination Survey 2007–2010. Environ. Sci. Pollut. Res. 2022, 29, 2974–2984. [Google Scholar] [CrossRef]
- Gaya, P.; Medina, M.; Sánchez-Jiménez, A.; Landete, J. Phytoestrogen Metabolism by Adult Human Gut Microbiota. Molecules 2016, 21, 1034. [Google Scholar] [CrossRef] [Green Version]
- Perez-Cornago, A.; Appleby, P.N.; Boeing, H.; Gil, L.; Kyrø, C.; Ricceri, F.; Murphy, N.; Trichopoulou, A.; Tsilidis, K.K.; Khaw, K.-T.; et al. Circulating Isoflavone and Lignan Concentrations and Prostate Cancer Risk: A Meta-Analysis of Individual Participant Data from Seven Prospective Studies Including 2,828 Cases and 5,593 Controls. Int. J. Cancer 2018, 143, 2677–2686. [Google Scholar] [CrossRef]
- Selma, M.V.; González-Sarrías, A.; Salas-Salvadó, J.; Andrés-Lacueva, C.; Alasalvar, C.; Örem, A.; Tomás-Barberán, F.A.; Espín, J.C. The Gut Microbiota Metabolism of Pomegranate or Walnut Ellagitannins Yields Two Urolithin-Metabotypes That Correlate with Cardiometabolic Risk Biomarkers: Comparison between Normoweight, Overweight-Obesity and Metabolic Syndrome. Clin. Nutr. 2018, 37, 897–905. [Google Scholar] [CrossRef]
- López-Yerena, A.; Domínguez-López, I.; Vallverdú-Queralt, A.; Pérez, M.; Jáuregui, O.; Escribano-Ferrer, E.; Lamuela-Raventós, R.M. Metabolomics Technologies for the Identification and Quantification of Dietary Phenolic Compound Metabolites: An Overview. Antioxidants 2021, 10, 846. [Google Scholar] [CrossRef]
- Eliuk, S.; Makarov, A. Evolution of Orbitrap Mass Spectrometry Instrumentation. Annu. Rev. Anal. Chem. 2015, 8, 61–80. [Google Scholar] [CrossRef]
- Vallverdú-Queralt, A.; Jáuregui, O.; Medina-Remón, A.; Andrés-Lacueva, C.; Lamuela-Raventós, R.M. Improved Characterization of Tomato Polyphenols Using Liquid Chromatography/Electrospray Ionization Linear Ion Trap Quadrupole Orbitrap Mass Spectrometry and Liquid Chromatography/Electrospray Ionization Tandem Mass Spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 2986–2992. [Google Scholar] [CrossRef]
- Sasot, G.; Martínez-Huélamo, M.; Vallverdú-Queralt, A.; Mercader-Martí, M.; Estruch, R.; Lamuela-Raventós, R.M. Identification of Phenolic Metabolites in Human Urine after the Intake of a Functional Food Made from Grape Extract by a High Resolution LTQ-Orbitrap-MS Approach. Food Res. Int. 2017, 100, 435–444. [Google Scholar] [CrossRef]
- Grund, B.; Marvin, L.; Rochat, B. Quantitative Performance of a Quadrupole-Orbitrap-MS in Targeted LC–MS Determinations of Small Molecules. J. Pharm. Biomed. Anal. 2016, 124, 48–56. [Google Scholar] [CrossRef]
- Henry, H.; Sobhi, H.R.; Scheibner, O.; Bromirski, M.; Nimkar, S.B.; Rochat, B. Comparison between a High-Resolution Single-Stage Orbitrap and a Triple Quadrupole Mass Spectrometer for Quantitative Analyses of Drugs. Rapid Commun. Mass Spectrom. 2012, 26, 499–509. [Google Scholar] [CrossRef]
- Ordóñez, J.L.; Pereira-Caro, G.; Ludwig, I.; Muñoz-Redondo, J.M.; Ruiz-Moreno, M.J.; Crozier, A.; Moreno-Rojas, J.M. A Critical Evaluation of the Use of Gas Chromatography- and High Performance Liquid Chromatography-Mass Spectrometry Techniques for the Analysis of Microbial Metabolites in Human Urine after Consumption of Orange Juice. J. Chromatogr. A 2018, 1575, 100–112. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Ordóñez, J.L.; Ludwig, I.; Gaillet, S.; Mena, P.; del Rio, D.; Rouanet, J.-M.; Bindon, K.A.; Moreno-Rojas, J.M.; Crozier, A. Development and Validation of an UHPLC-HRMS Protocol for the Analysis of Flavan-3-Ol Metabolites and Catabolites in Urine, Plasma and Feces of Rats Fed a Red Wine Proanthocyanidin Extract. Food Chem. 2018, 252, 49–60. [Google Scholar] [CrossRef]
- Fernandez-Jimenez, R.; Santos-Beneit, G.; Tresserra-Rimbau, A.; Bodega, P.; de Miguel, M.; de Cos-Gandoy, A.; Rodríguez, C.; Carral, V.; Orrit, X.; Haro, D.; et al. Rationale and Design of the School-Based SI! Program to Face Obesity and Promote Health among Spanish Adolescents: A Cluster-Randomized Controlled Trial. Am. Heart J. 2019, 215, 27–40. [Google Scholar] [CrossRef]
- Martínez-Huélamo, M.; Tulipani, S.; Jáuregui, O.; Valderas-Martinez, P.; Vallverdú-Queralt, A.; Estruch, R.; Torrado, X.; Lamuela-Raventós, R.M. Sensitive and Rapid UHPLC-MS/MS for the Analysis of Tomato Phenolics in Human Biological Samples. Molecules 2015, 20, 20409–20425. [Google Scholar] [CrossRef] [Green Version]
- Roura, E.; Andrés-Lacueva, C.; Estruch, R.; Lamuela-Raventós, R.M. Total Polyphenol Intake Estimated by a Modified Folin–Ciocalteu Assay of Urine. Clin. Chem. 2006, 52, 749–752. [Google Scholar] [CrossRef] [Green Version]
- Lynch, J.; Horwitz, W.; Latimer, G.W. Appendix E: Laboratory Quality Assurance; Official Methods of Analysis of AOAC International: Washington, DC, USA, 2005; Available online: http://www.eoma.aoac.org/app_e.pdf (accessed on 1 January 2018).
- Matuszewski, B.K.; Constanzer, M.L.; Chavez-Eng, C.M. Strategies for the Assessment of Matrix Effect in Quantitative Bioanalytical Methods Based on HPLC−MS/MS. Anal. Chem. 2003, 75, 3019–3030. [Google Scholar] [CrossRef]
- Wishart, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N.; et al. HMDB 4.0: The Human Metabolome Database for 2018. Nucleic Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef]
- Medina-Remón, A.; Barrionuevo-González, A.; Zamora-Ros, R.; Andres-Lacueva, C.; Estruch, R.; Martínez-González, M.-Á.; Diez-Espino, J.; Lamuela-Raventos, R.M. Rapid Folin–Ciocalteu Method Using Microtiter 96-Well Plate Cartridges for Solid Phase Extraction to Assess Urinary Total Phenolic Compounds, as a Biomarker of Total Polyphenols Intake. Anal. Chim. Acta 2009, 634, 54–60. [Google Scholar] [CrossRef]
- Juton, C.; Castro-barquero, S.; Casas, R.; Freitas, T.; Ruiz-león, A.M.; Crovetto, F.; Domenech, M.; Crispi, F.; Vieta, E.; Gratacós, E.; et al. Reliability and Concurrent and Construct Validity of a Food Frequency Questionnaire for Pregnant Women at High Risk to Develop Fetal Growth Restriction. Nutrients 2021, 13, 1629. [Google Scholar] [CrossRef]
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An Online Comprehensive Database on Polyphenol Contents in Foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef]
- Willett, W.C.; Howe, G.R.; Kushi, L.H. Adjustment for Total Energy Intake in Epidemiologic Studies. Am. J. Clin. Nutr. 1997, 65, 1220S–1228S; discussion 1229S–1231S. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Zamora-Ros, R.; Achaintre, D.; Rothwell, J.A.; Rinaldi, S.; Assi, N.; Ferrari, P.; Leitzmann, M.; Boutron-Ruault, M.-C.; Fagherazzi, G.; Auffret, A.; et al. Urinary Excretions of 34 Dietary Polyphenols and Their Associations with Lifestyle Factors in the EPIC Cohort Study. Sci. Rep. 2016, 6, 26905. [Google Scholar] [CrossRef] [Green Version]
- Hurtado-Barroso, S.; Quifer-Rada, P.; Marhuenda-Muñoz, M.; Rinaldi de Alvarenga, J.F.; Tresserra-Rimbau, A.; Lamuela-Raventós, R.M. Increase of 4-Hydroxybenzoic, a Bioactive Phenolic Compound, after an Organic Intervention Diet. Antioxidants 2019, 8, 340. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Vervoort, J.; Beekmann, K.; Baccaro, M.; Kamelia, L.; Wesseling, S.; Rietjens, I.M.C.M. Interindividual Differences in Human Intestinal Microbial Conversion of (-)-Epicatechin to Bioactive Phenolic Compounds. J. Agric. Food Chem. 2020, 68, 14168–14181. [Google Scholar] [CrossRef]
- Mumford, S.L.; Sundaram, R.; Schisterman, E.F.; Sweeney, A.M.; Barr, D.B.; Rybak, M.E.; Maisog, J.M.; Parker, D.L.; Pfeiffer, C.M.; Buck Louis, G.M. Higher Urinary Lignan Concentrations in Women but Not Men Are Positively Associated with Shorter Time to Pregnancy. J. Nutr. 2014, 144, 352–358. [Google Scholar] [CrossRef] [Green Version]
- Campesi, I.; Marino, M.; Cipolletti, M.; Romani, A.; Franconi, F. Put “Gender Glasses” on the Effects of Phenolic Compounds on Cardiovascular Function and Diseases. Eur. J. Nutr. 2018, 57, 2677–2691. [Google Scholar] [CrossRef]
- Espín, J.C.; Larrosa, M.; García-Conesa, M.T.; Tomás-Barberán, F. Biological Significance of Urolithins, the Gut Microbial Ellagic Acid-Derived Metabolites: The Evidence So Far. Evid.-Based Complement. Altern. Med. 2013, 2013, 270418. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Huélamo, M.; Tulipani, S.; Torrado, X.; Estruch, R.; Lamuela-Raventós, R.M. Validation of a New LC-MS/MS Method for the Detection and Quantification of Phenolic Metabolites from Tomato Sauce in Biological Samples. J. Agric. Food Chem. 2012, 60, 4542–4549. [Google Scholar] [CrossRef]
- Quifer-Rada, P.; Martínez-Huélamo, M.; Jáuregui, O.; Chiva-Blanch, G.; Estruch, R.; Lamuela-Raventós, R.M. Analytical Condition Setting a Crucial Step in the Quantification of Unstable Polyphenols in Acidic Conditions: Analyzing Prenylflavanoids in Biological Samples by Liquid Chromatography-Electrospray Ionization Triple Quadruple Mass Spectrometry. Anal. Chem. 2013, 85, 5547–5554. [Google Scholar] [CrossRef]


| Phenolic Standards | Concentration Level (µg/L) | ||
|---|---|---|---|
| Low | Medium | High | |
| Enterodiol | 5 | 200 | 766 |
| 3′-Hydroxytyrosol-3′-glucuronide | 5 | 200 | 766 |
| 3-Hydroxybenzoic acid | 5 | 200 | 766 |
| 3-Hydroxytyrosol | 5 | 200 | 766 |
| 4-Hydroxybenzoic acid | 5 | 200 | 766 |
| Enterolactone | 5 | 200 | 766 |
| m-coumaric acid | 5 | 200 | 766 |
| p-coumaric acid | 5 | 200 | 766 |
| Protocatechuic acid | 5 | 200 | 766 |
| o-coumaric acid | 5 | 200 | 766 |
| Syringic acid | 5 | 200 | 766 |
| Urolithin-B | 5 | 200 | 766 |
| Vanillic acid | 5 | 200 | 766 |
| Dihydroresveratrol | 12.5 | 500 | 1915 |
| Gallic acid | 12.5 | 500 | 1915 |
| Urolithin-A | 12.5 | 500 | 1915 |
| 3,4-Dihydroxyphenylpropionic acid | 25 | 100 | 3830 |
| 3′-Hydroxyphenylacetic acid | 50 | 2000 | 7660 |
| N | Mean (SD) | Median (IQR) | |
|---|---|---|---|
| Age, years | 601 | 12.0 (0.4) | 12.0 (0.0) |
| Body mass, kg | 601 | 50.8 (12.2) | 48.5 (14.8) |
| Height, cm | 601 | 155.2 (6.9) | 155.2 (9.2) |
| BMI, kg/m2 | 601 | 20.9 (4.2) | 20.1 (5.0) |
| BMI z-score | 601 | 0.6 (1.0) | 0.6 (1.4) |
| Energy and nutrients intake | |||
| Energy, kcal/day | 546 | 2498.8 (579.6) | 2474.9 (828.6) |
| Carbohydrates, g/day | 546 | 132.1 (47.3) | 124.3 (63.0) |
| Fiber, g/day | 546 | 29.6 (10.6) | 28.1 (13.5) |
| Fat, g/da | 546 | 112.0 (32.8) | 109.4 (41.6) |
| Protein, g/day | 546 | 119.5 (32.3) | 117.8 (42.1) |
| Energy-adjusted (poly)phenol intake | |||
| Total (poly)phenol intake, mg/day | 546 | 683.5 (335.3) | 639.8 (354.9) |
| Flavonoids, mg/day | 546 | 533.9 (310.3) | 480.8 (298.8) |
| Stilbenes, mg/day | 546 | 0.2 (0.3) | 0.1 (0.2) |
| Tyrosols, mg/day | 546 | 21.3 (13.7) | 17.8 (12.6) |
| Lignans, mg/day | 546 | 3.7 (4.1) | 2.5 (2.5) |
| Phenolic acids, mg/day | 546 | 94.9 (50.4) | 89.2 (51.7) |
| Urinary MPM, µg/g Creatinine | <LOQ (n) | Mean * | SEM * | CV * |
|---|---|---|---|---|
| Lignans | ||||
| Enterodiol a | 136 | 4.5 | 0.9 | 1.0 |
| Enterodiol glucuronide I (ED) | 4 | 740.7 | 151.1 | 4.8 |
| Enterodiol glucuronide II (ED) | 3 | 209.2 | 67.8 | 5.1 |
| Enterodiol sulfate (ED) | 18 | 158.0 | 34.1 | 4.9 |
| Enterolactone a | 179 | 30.6 | 3.2 | 1.7 |
| Enterolactone glucuronide (EL) | 3 | 6984.5 | 419.2 | 1.5 |
| Enterolactone sulfate (EL) | 19 | 639.3 | 168.8 | 6.3 |
| Phenolic acids—Hydroxybenzoic acids | ||||
| Gallic acid a | 223 | 9.1 | 1.1 | 1.4 |
| Gallic acid glucuronide (GA) | 80 | 4.6 | 1.2 | 1.4 |
| Gallic acid sulfate (GA) | 87 | 22.8 | 1.5 | 1.3 |
| 3-Hydroxybenzoic acid a | 206 | 113.4 | 41.1 | 5.6 |
| 4-Hydroxybenzoic acid a | 1 | 824.5 | 157.8 | 4.6 |
| Hydroxybenzoic acid glucuronide I (HBA) | 229 | 33.4 | 4.2 | 1.3 |
| Hydroxybenzoic acid glucuronide II (HBA) | 13 | 69.5 | 6.0 | 1.8 |
| Hydroxybenzoic acid sulfate (HBA) | 0 | 25,034.4 | 1607.6 | 1.6 |
| Protocatechuic acid a | 1 | 173.8 | 31.7 | 4.2 |
| Protocatechuic acid glucuronide (PCA) | 57 | 30.2 | 2.1 | 1.5 |
| Protocatechuic acid sulfate I (PCA) | 3 | 33,703.3 | 5368.2 | 3.8 |
| Protocatechuic acid sulfate II (PCA) | 0 | 228.0 | 41.8 | 3.6 |
| Syringic acid a | 4 | 99.6 | 6.7 | 1.3 |
| Syringic acid glucuronide I (SA) | 0 | 297.6 | 26.8 | 2.0 |
| Syringic acid glucuronide II (SA) | 2 | 181.0 | 53.5 | 3.4 |
| Syringic acid sulfate (SA) | 32 | 249.9 | 26.9 | 1.8 |
| Vanillic acid a | 0 | 1027.5 | 198.9 | 3.5 |
| Vanillic acid glucuronide I (VA) | 16 | 6847.5 | 857.4 | 2.5 |
| Vanillic acid glucuronide II (VA) | 2 | 3795.8 | 1038.3 | 4.7 |
| Vanillic acid sulfate (VA) | 1 | 17,227.2 | 1610.6 | 2.2 |
| Phenolic acids—Hydroxycinnamic acids | ||||
| m-Coumaric acid a | 38 | 69.9 | 11.8 | 2.8 |
| o-Coumaric acid a | 42 | 15.8 | 2.4 | 1.6 |
| p-Coumaric acid a | 16 | 23.4 | 2.3 | 1.6 |
| Coumaric acid glucuronide I | 18 | 36.5 | 2.8 | 1.6 |
| Coumaric acid glucuronide II | 162 | 20.4 | 1.7 | 1.2 |
| Coumaric acid glucuronide III | 11 | 72.4 | 8.8 | 2.7 |
| Coumaric acid sulfate I | 39 | 46.8 | 9.2 | 2.6 |
| Coumaric acid sulfate II | 13 | 240.4 | 75.8 | 6.1 |
| Coumaric acid sulfate III | 5 | 788.7 | 208.6 | 5.3 |
| Phenolic acids—Hydroxyphenylacetic acids | ||||
| 3-Hydroxyphenylacetic acid a | 13 | 40,797.6 | 3248.4 | 1.8 |
| Hydroxyphenylacetic acid glucuronide (3-HPAA) | 122 | 13,860.5 | 4363.6 | 5.3 |
| Hydroxyphenylacetic acid sulfate (3-HPAA) | 22 | 45,815.5 | 6160.0 | 2.4 |
| Phenolic acids—Hydroxyphenylpropanoic acids | ||||
| 3,4-dihydroxyphenylpropionic acid a | 25 | 132.8 | 17.1 | 2.0 |
| Dihydroxyphenylpropionic acid sulfate (3,4-DHPPA) | 1 | 30,942.7 | 2700.1 | 2.0 |
| Stilbenes | ||||
| Dihydroresveratrol a | 78 | 3.3 | 0.5 | 0.5 |
| Dihydroresveratrol sulfate I (DHR) | 4 | 753.5 | 57.8 | 1.8 |
| Dihydroresveratrol sulfate II (DHR) | 47 | 991.6 | 379.0 | 5.2 |
| Other polyphenols—Hydroxycoumarins | ||||
| Urolithin A a | 57 | 1338.1 | 270.3 | 2.4 |
| Urolithin A glucuronide (Uro A) | 41 | 3030.2 | 482.1 | 2.7 |
| Urolithin A sulfate (Uro A) | 26 | 801.0 | 399.9 | 3.5 |
| Urolithin B a | 86 | 1334.4 | 1067.1 | 4.4 |
| Urolithin B glucuronide (Uro B) | 63 | 3062.8 | 1565.1 | 6.1 |
| Other polyphenols-Tyrosols | ||||
| 3-Hydroxytyrosol a | 143 | 9.1 | 0.9 | 0.6 |
| 3′hydroxytyrosol-3′-glucuronide a | 71 | 62.4 | 28.9 | 7.5 |
| Hydroxytyrosol sulfate (3-HT) | 5 | 398.0 | 88.8 | 5.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laveriano-Santos, E.P.; Marhuenda-Muñoz, M.; Vallverdú-Queralt, A.; Martínez-Huélamo, M.; Tresserra-Rimbau, A.; Miliarakis, E.; Arancibia-Riveros, C.; Jáuregui, O.; Ruiz-León, A.M.; Castro-Baquero, S.; et al. Identification and Quantification of Urinary Microbial Phenolic Metabolites by HPLC-ESI-LTQ-Orbitrap-HRMS and Their Relationship with Dietary Polyphenols in Adolescents. Antioxidants 2022, 11, 1167. https://doi.org/10.3390/antiox11061167
Laveriano-Santos EP, Marhuenda-Muñoz M, Vallverdú-Queralt A, Martínez-Huélamo M, Tresserra-Rimbau A, Miliarakis E, Arancibia-Riveros C, Jáuregui O, Ruiz-León AM, Castro-Baquero S, et al. Identification and Quantification of Urinary Microbial Phenolic Metabolites by HPLC-ESI-LTQ-Orbitrap-HRMS and Their Relationship with Dietary Polyphenols in Adolescents. Antioxidants. 2022; 11(6):1167. https://doi.org/10.3390/antiox11061167
Chicago/Turabian StyleLaveriano-Santos, Emily P., María Marhuenda-Muñoz, Anna Vallverdú-Queralt, Miriam Martínez-Huélamo, Anna Tresserra-Rimbau, Elefterios Miliarakis, Camila Arancibia-Riveros, Olga Jáuregui, Ana María Ruiz-León, Sara Castro-Baquero, and et al. 2022. "Identification and Quantification of Urinary Microbial Phenolic Metabolites by HPLC-ESI-LTQ-Orbitrap-HRMS and Their Relationship with Dietary Polyphenols in Adolescents" Antioxidants 11, no. 6: 1167. https://doi.org/10.3390/antiox11061167
APA StyleLaveriano-Santos, E. P., Marhuenda-Muñoz, M., Vallverdú-Queralt, A., Martínez-Huélamo, M., Tresserra-Rimbau, A., Miliarakis, E., Arancibia-Riveros, C., Jáuregui, O., Ruiz-León, A. M., Castro-Baquero, S., Estruch, R., Bodega, P., Miguel, M. d., Cos-Gandoy, A. d., Martínez-Gómez, J., Santos-Beneit, G., Fernández-Alvira, J. M., Fernández-Jiménez, R., & Lamuela-Raventós, R. M. (2022). Identification and Quantification of Urinary Microbial Phenolic Metabolites by HPLC-ESI-LTQ-Orbitrap-HRMS and Their Relationship with Dietary Polyphenols in Adolescents. Antioxidants, 11(6), 1167. https://doi.org/10.3390/antiox11061167