Melatonin Prevents NaAsO2-Induced Developmental Cardiotoxicity in Zebrafish through Regulating Oxidative Stress and Apoptosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fish Husbandry and Embryo Collection
2.2. Chemical Exposure
2.3. ROS Measurement
2.4. Antioxidant Activity Assays
2.5. Acridine Orange (AO) Staining
2.6. Oxidative Damage Assays
2.7. Quantitative Real-Time PCR (qPCR)
2.8. Statistical Analysis
3. Results
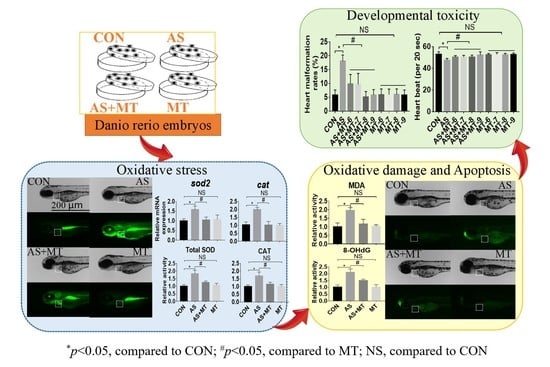
3.1. Melatonin Attenuated NaAsO2-Induced Heart Malformations
3.2. Melatonin Inhibited NaAsO2-Induced Oxidative Stress
3.3. Melatonin Prevented NaAsO2-Induced Oxidative Damage and Apoptosis
3.4. Melatonin Prevented NaAsO2-Induced Effects in Heart Development-Related Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rodriguez-Lado, L.; Sun, G.; Berg, M.; Zhang, Q.; Xue, H.; Zheng, Q.; Johnson, C.A. Groundwater arsenic contamination throughout China. Science 2013, 341, 866–868. [Google Scholar] [CrossRef] [PubMed]
- Hossain, K.; Suzuki, T.; Hasibuzzaman, M.M.; Islam, S.; Rahman, A.; Paul, S.K.; Tanu, T.; Hossain, S.; Alam Saud, Z.; Rahman, M.; et al. Chronic exposure to arsenic, LINE-1 hypomethylation, and blood pressure: A cross-sectional study in Bangladesh. Environ. Health 2017, 16, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.; Shi, Y.; Mo, X.; Xu, J.; Zhao, B.; Lin, Y.; Yang, S.; Xu, Z.; Dai, J.; Pan, S.; et al. A genome-wide association study identifies two risk loci for congenital heart malformations in Han Chinese populations. Nat. Genet. 2013, 45, 818–821. [Google Scholar] [CrossRef] [PubMed]
- Kalisch-Smith, J.I.; Ved, N.; Sparrow, D.B. Environmental Risk Factors for Congenital Heart Disease. Cold Spring Harb. Perspect. Biol. 2019, 12, a037234. [Google Scholar] [CrossRef] [Green Version]
- Rollins, C.K.; Ortinau, C.M.; Ms, C.S.; Friedman, K.G.; Tworetzky, W.; Gagoski, B.; Bs, C.V.; Afacan, O.; Vasung, L.; Bs, J.I.B.; et al. Regional Brain Growth Trajectories in Fetuses with Congenital Heart Disease. Ann. Neurol. 2020, 89, 143–157. [Google Scholar] [CrossRef]
- Rudnai, T.; Sándor, J.; Kádár, M.; Borsányi, M.; Béres, J.; Métneki, J.; Maráczi, G.; Rudnai, P. Arsenic in drinking water and congenital heart anomalies in Hungary. Int. J. Hyg. Environ. Health 2014, 217, 813–818. [Google Scholar] [CrossRef]
- Jin, X.; Tian, X.; Liu, Z.; Hu, H.; Li, X.; Deng, Y.; Li, N.; Zhu, J. Maternal exposure to arsenic and cadmium and the risk of congenital heart defects in offspring. Reprod. Toxicol. 2016, 59, 109–116. [Google Scholar] [CrossRef]
- Gao, X.; Zhang, C.; Zheng, P.; Dan, Q.; Luo, H.; Ma, X.; Lu, C. Arsenic suppresses GDF1 expression via ROS-dependent downregulation of specificity protein 1. Environ. Pollut. 2020, 271, 116302. [Google Scholar] [CrossRef]
- Gholami, A.; Ataei, S.; Ahmadimoghaddam, D.; Omidifar, N.; Nili-Ahmadabadi, A. Pentoxifylline Attenuates Arsenic Trioxide-Induced Cardiac Oxidative Damage in Mice. Oxidative Med. Cell. Longev. 2021, 2021, 6406318. [Google Scholar] [CrossRef]
- Reiter, R.J.; Mayo, J.C.; Tan, D.-X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Zhai, M.; Li, B.; Duan, W.; Jing, L.; Zhang, B.; Zhang, M.; Yu, L.; Liu, Z.; Yu, B.; Ren, K.; et al. Melatonin ameliorates myocardial ischemia reperfusion injury through SIRT3-dependent regulation of oxidative stress and apoptosis. J. Pineal Res. 2017, 63, e12419. [Google Scholar] [CrossRef] [PubMed]
- Sakotnik, A.; Liebmann, P.; Stoschitzky, K.; Lercher, P.; Schauenstein, K.; Klein, W.; Eber, B. Decreased melatonin synthesis in patients with coronary artery disease. Eur. Heart J. 1999, 20, 1314–1317. [Google Scholar] [CrossRef] [PubMed]
- Girotti, L.; Lago, M.; Ianovsky, O.; Carbajales, J.; Elizari, M.V.; Brusco, L.I.; Cardinali, D.P. Low urinary 6-sulphatoxymelatonin levels in patients with coronary artery disease. J. Pineal Res. 2000, 29, 138–142. [Google Scholar] [CrossRef]
- Seron-Ferre, M.; Torres-Farfan, C.; Valenzuela, F.J.; Castillo-Galan, S.; Rojas, A.; Mendez, N.; Reynolds, H.; Valenzuela, G.J.; Llanos, A.J. Deciphering the Function of the Blunt Circadian Rhythm of Melatonin in the Newborn Lamb: Impact on Adrenal and Heart. Endocrinology 2017, 158, 2895–2905. [Google Scholar] [CrossRef] [PubMed]
- Benaldo, F.A.; Llanos, A.J.; Araya-Quijada, C.; Rojas, A.; Gonzalez-Candia, A.; Herrera, E.A.; Ebensperger, G.; Cabello, G.; Valenzuela, G.J.; Serón-Ferré, M. Effects of Melatonin on the Defense to Acute Hypoxia in Newborn Lambs. Front. Endocrinol. 2019, 10, 433. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Du, J.; Xu, R.; Shen, Y.; Yang, D.; Li, D.; Hu, H.; Pei, H.; Yang, Y. Melatonin alleviates angiotensin-II-induced cardiac hypertrophy via activating MICU1 pathway. Aging 2020, 13, 493–515. [Google Scholar] [CrossRef]
- Lowe, V.; Wisniewski, L.; Pellet-Many, C. The Zebrafish Cardiac Endothelial Cell-Roles in Development and Regeneration. J. Cardiovasc. Dev. Dis. 2021, 8, 49. [Google Scholar] [CrossRef]
- Bakkers, J. Zebrafish as a model to study cardiac development and human cardiac disease. Cardiovasc. Res. 2011, 91, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Ji, C.; Guo, Y.; Yan, R.; Hong, T.; Dou, Y.; An, Y.; Tao, S.; Qin, F.; Nie, J.; et al. Mechanisms underlying melatonin-mediated prevention of fenvalerate-induced behavioral and oxidative toxicity in zebrafish. J. Toxicol. Environ. Health Part A 2017, 80, 1331–1341. [Google Scholar] [CrossRef]
- Liu, X.; Gao, Q.; Feng, Z.; Tang, Y.; Zhao, X.; Chen, D.; Feng, X. Protective Effects of Spermidine and Melatonin on Deltamethrin-Induced Cardiotoxicity and Neurotoxicity in Zebrafish. Cardiovasc. Toxicol. 2020, 21, 29–41. [Google Scholar] [CrossRef]
- Yue, C.; Ji, C.; Zhang, H.; Zhang, L.W.; Tong, J.; Jiang, Y.; Chen, T. Protective effects of folic acid on PM2.5-induced cardiac developmental toxicity in zebrafish embryos by targeting AhR and Wnt/beta-catenin signal pathways. Environ. Toxicol. 2017, 32, 2316–2322. [Google Scholar] [CrossRef] [PubMed]
- Na, L.; Xiumei, Z.; Lingzi, Z.; Deqin, H.; Xuanxuan, Z.; Huanhuan, G.; Yuan, L.; Xiujuan, C. Research into the intervention effect of folic acid on arsenic-induced heart abnormalities in fetal rats during the periconception period. BMC Cardiovasc. Disord. 2020, 20, 139. [Google Scholar] [CrossRef]
- Wang, L.; Yan, R.; Yang, Q.; Li, H.; Zhang, J.; Shimoda, Y.; Kato, K.; Yamanaka, K.; An, Y. Role of GH/IGF axis in arsenite-induced developmental toxicity in zebrafish embryos. Ecotoxicol. Environ. Saf. 2020, 201, 110820. [Google Scholar] [CrossRef] [PubMed]
- Sonmez, M.F.; Narin, F.; Akkuş, D.; Özdamar, S. Effect of melatonin and vitamin C on expression of endothelial NOS in heart of chronic alcoholic rats. Toxicol. Ind. Health 2009, 25, 385–393. [Google Scholar] [CrossRef] [PubMed]
- Hendawy, A.K.; El-Toukhey, N.E.S.; AbdEl-Rahman, S.S.; Ahmed, H.H. Ameliorating effect of melatonin against nicotine induced lung and heart toxicity in rats. Environ. Sci. Pollut. Res. 2021, 28, 35628–35641. [Google Scholar] [CrossRef]
- Lan, H.; Su, Y.; Liu, Y.; Deng, C.; Wang, J.; Chen, T.; Jules, K.E.D.; Masau, J.F.; Li, H.; Wei, X. Melatonin protects circulatory death heart from ischemia/reperfusion injury via the JAK2/STAT3 signalling pathway. Life Sci. 2019, 228, 35–46. [Google Scholar] [CrossRef]
- Niehoff, J.; Matzkies, M.; Nguemo, F.; Hescheler, J.; Reppel, M. The influence of melatonin on the heart rhythm—An in vitro simulation with murine embryonic stem cell derived cardiomyocytes. Biomed. Pharmacother. 2021, 136, 111245. [Google Scholar] [CrossRef]
- Jiang, J.; Liang, S.; Zhang, J.; Du, Z.; Xu, Q.; Duan, J.; Sun, Z. Melatonin ameliorates PM2.5-induced cardiac perivascular fibrosis through regulating mitochondrial redox homeostasis. J. Pineal Res. 2021, 70, e12686. [Google Scholar] [CrossRef]
- Ma, H.; Wang, X.; Zhang, W.; Li, H.; Zhao, W.; Sun, J.; Yang, M. Melatonin Suppresses Ferroptosis Induced by High Glucose via Activation of the Nrf2/HO-1 Signaling Pathway in Type 2 Diabetic Osteoporosis. Oxidative Med. Cell. Longev. 2020, 2020, 9067610. [Google Scholar] [CrossRef]
- Jiang, L.L.; Zhang, F.; He, Y.-F.; Fan, W.-G.; Zheng, M.-M.; Kang, J.; Huang, F.; He, H.-W. Melatonin regulates mitochondrial function and biogenesis during rat dental papilla cell differentiation. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 5967–5979. [Google Scholar] [CrossRef]
- Yang, X.; Wang, D.; Ma, Y.; Xu, X.; Zhu, Z.; Wang, X.; Deng, H.; Li, C.; Chen, M.; Tong, J.; et al. Continuous activation of Nrf2 and its target antioxidant enzymes leads to arsenite-induced malignant transformation of human bronchial epithelial cells. Toxicol. Appl. Pharmacol. 2015, 289, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.J.; Zhang, J.-Y.; Wang, Q.; Zhu, E.; Chen, W.; Lin, H.; Chen, J.; Hong, H. Environmentally relevant concentrations of arsenite induces developmental toxicity and oxidative responses in the early life stage of zebrafish. Environ. Pollut. 2019, 254 Pt A, 113022. [Google Scholar] [CrossRef]
- NaveenKumar, S.K.; Hemshekhar, M.; Jagadish, S.; Manikanta, K.; Ks, G.; Kemparaju, K.; Girish, K.S. Melatonin restores neutrophil functions and prevents apoptosis amid dysfunctional glutathione redox system. J. Pineal Res. 2020, 69, e12676. [Google Scholar] [CrossRef]
- Zhi, S.M.; Fang, G.-X.; Xie, X.-M.; Liu, L.-H.; Yan, J.; Liu, D.-B.; Yu, H.-Y. Melatonin reduces OGD/R-induced neuron injury by regulating redox/inflammation/apoptosis signaling. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 1524–1536. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Huang, Y.; Tao, Y.; Ji, C.; Aniagu, S.; Jiang, Y.; Chen, T. Resveratrol protects against PM2.5-induced heart defects in zebrafish embryos as an antioxidant rather than as an AHR antagonist. Toxicol. Appl. Pharmacol. 2020, 398, 115029. [Google Scholar] [CrossRef] [PubMed]
- Elbatreek, M.H.; Mucke, H.; Schmidt, H. NOX Inhibitors: From Bench to Naxibs to Bedside. Handb. Exp. Pharmacol. 2021, 264, 145–168. [Google Scholar] [CrossRef]
- Totten, S.P.; Im, Y.K.; Cañedo, E.C.; Najyb, O.; Nguyen, A.; Hébert, S.; Ahn, R.; Lewis, K.; Lebeau, B.; La Selva, R.; et al. STAT1 potentiates oxidative stress revealing a targetable vulnerability that increases phenformin efficacy in breast cancer. Nat. Commun. 2021, 12, 3299. [Google Scholar] [CrossRef]
- Dikalova, A.E.; Pandey, A.; Xiao, L.; Arslanbaeva, L.; Sidorova, T.; Lopez, M.G.; Iv, F.T.B.; Verdin, E.; Auwerx, J.; Harrison, D.G.; et al. Mitochondrial Deacetylase Sirt3 Reduces Vascular Dysfunction and Hypertension While Sirt3 Depletion in Essential Hypertension Is Linked to Vascular Inflammation and Oxidative Stress. Circ. Res. 2020, 126, 439–452. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Sun, L.; Chen, W.; Zhou, Y.; Liu, K.; Chen, J.; Zhang, Z.; Zhang, C.; Tian, H. Sirtuin 3 Therapy Attenuates Aging Expression, Oxidative Stress Parameters, and Neointimal Hyperplasia Formation in Vein Grafts. Ann. Vasc. Surg. 2019, 64, 303–317. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, W.; Xiong, Z.; Kong, J.; Wang, L.; Qiu, Y.; Shen, F.; Huang, Z. Resveratrol protects against triptolide-induced cardiotoxicity through SIRT3 signaling pathway in vivo and in vitro. Pharmazie 2016, 71, 514–523. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, H.; Niu, J.; Luo, M.; Gou, Y.; Miao, L.; Zou, Z.; Cheng, Y. Induction of ROS Overload by Alantolactone Prompts Oxidative DNA Damage and Apoptosis in Colorectal Cancer Cells. Int. J. Mol. Sci. 2016, 17, 558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Li, S.; Guo, Y.; Yu, H.; Bao, Y.; Xin, X.; Yang, H.; Ni, X.; Wu, N.; Jia, D. Astaxanthin Attenuates Hypertensive Vascular Remodeling by Protecting Vascular Smooth Muscle Cells from Oxidative Stress-Induced Mitochondrial Dysfunction. Oxidative Med. Cell. Longev. 2020, 2020, 4629189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorda-Diez, C.I.; Solis-Mancilla, M.E.; Fernández, C.S.; Garcia-Porrero, J.A.; Hurle, J.M.; Montero, J.A. Cell senescence, apoptosis and DNA damage cooperate in the remodeling processes accounting for heart morphogenesis. J. Anat. 2019, 234, 815–829. [Google Scholar] [CrossRef] [PubMed]
- Gawdzik, J.C.; Yue, M.S.; Martin, N.R.; Elemans, L.M.H.; Lanham, K.A.; Heideman, W.; Rezendes, R.; Baker, T.R.; Taylor, M.R.; Plavicki, J.S. sox9b is required in cardiomyocytes for cardiac morphogenesis and function. Sci. Rep. 2018, 8, 13906. [Google Scholar] [CrossRef] [PubMed]
- Dixit, R.; Narasimhan, C.; Balekundri, V.I.; Agrawal, D.; Kumar, A.; Mohapatra, B. Functional analysis of novel genetic variants of NKX2-5 associated with nonsyndromic congenital heart disease. Am. J. Med. Genet. Part A 2021, 185, 3644–3663. [Google Scholar] [CrossRef]





| Gene Name | Sequence of the Primer (5′–3′) | |
|---|---|---|
| β-actin | NM_131031.2 | Forward: CGAGCAGGAGATGGGAACC |
| Reverse: CAACGGAAACGCTCATTGC | ||
| bax | NM_131562.2 | Forward: GGCTATTTCAACCAGGGTTCC |
| Reverse: TGCGAATCACCAATGCTGT | ||
| bcl2 | NM_001030253.2 | Forward: AGGAAAATGGAGGTTGGGATG |
| Reverse: TGTTAGGTATGAAAACGGGTGGA | ||
| caspase3 | NM_131877.3 | Forward: CCGCTGCCCATCACTA |
| Reverse: ATCCTTTCACGACCATCT | ||
| cat | NM_130912.2 | Forward: AGGGCAACTGGGATCTTACA |
| Reverse: TTTATGGGACCAGACCTTGG | ||
| gpx | NM_001007281.2 | Forward: AGATGTCATTCCTGCACACG |
| Reverse: AAGGAGAAGCTTCCTCAGCC | ||
| ho-1 | NM_001127516.1 | Forward: GGAAGAGCTGGACAGAAACG |
| Reverse: CGAAGAAGTGCTCCAAGTCC | ||
| nkx2.5 | NM_131421.2 | Forward: GCATCAGAGCTTGGTGAACA |
| Reverse: ATGCGCACGCATAAACATTA | ||
| nrf2 | NM_182889.1 | Forward: GACAAAATCGGCGACAAAAT |
| Reverse: TTAGGCCATGTCCACACGTA | ||
| sod2 | NM_199976.1 | Forward: GCTTGGGATAGATGTCTGGG |
| Reverse: CTTGGAAACGCTCGCTGA | ||
| sox9b | NM_131644.1 | Forward: CGAGAAGCGTCCGTTTGTG |
| Reverse: CCGTCTGGGCTGGTATTTGTA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, R.; Ding, J.; Wei, Y.; Yang, Q.; Zhang, X.; Huang, H.; Shi, Z.; Feng, Y.; Li, H.; Zhang, H.; et al. Melatonin Prevents NaAsO2-Induced Developmental Cardiotoxicity in Zebrafish through Regulating Oxidative Stress and Apoptosis. Antioxidants 2022, 11, 1301. https://doi.org/10.3390/antiox11071301
Yan R, Ding J, Wei Y, Yang Q, Zhang X, Huang H, Shi Z, Feng Y, Li H, Zhang H, et al. Melatonin Prevents NaAsO2-Induced Developmental Cardiotoxicity in Zebrafish through Regulating Oxidative Stress and Apoptosis. Antioxidants. 2022; 11(7):1301. https://doi.org/10.3390/antiox11071301
Chicago/Turabian StyleYan, Rui, Jie Ding, Yuanjie Wei, Qianlei Yang, Xiaoyun Zhang, Hairu Huang, Zhuoyue Shi, Yue Feng, Heran Li, Hengdong Zhang, and et al. 2022. "Melatonin Prevents NaAsO2-Induced Developmental Cardiotoxicity in Zebrafish through Regulating Oxidative Stress and Apoptosis" Antioxidants 11, no. 7: 1301. https://doi.org/10.3390/antiox11071301
APA StyleYan, R., Ding, J., Wei, Y., Yang, Q., Zhang, X., Huang, H., Shi, Z., Feng, Y., Li, H., Zhang, H., Ding, W., & An, Y. (2022). Melatonin Prevents NaAsO2-Induced Developmental Cardiotoxicity in Zebrafish through Regulating Oxidative Stress and Apoptosis. Antioxidants, 11(7), 1301. https://doi.org/10.3390/antiox11071301