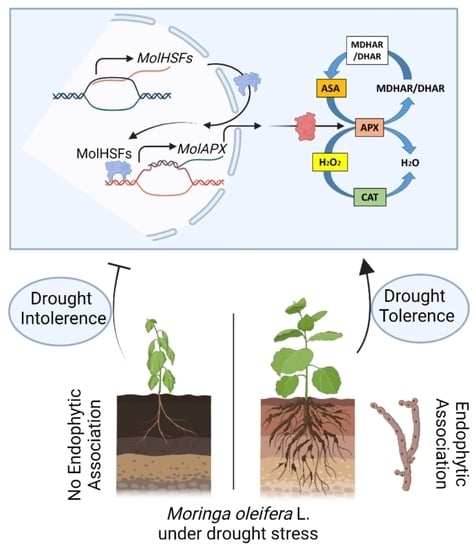
Endophytic Fungal Consortia Enhance Basal Drought-Tolerance in Moringa oleifera by Upregulating the Antioxidant Enzyme (APX) through Heat Shock Factors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Endophytic Fungal Isolation, Characterization, and Identification
2.1.1. Isolation and Purification of Endophytic Fungi
2.1.2. Fungal Strains Microscopy
2.1.3. Strain Identification
2.1.4. Screening of Fungal Endophytes in PEG (Polyethylene Glycol) Containing Czapek Medium
2.1.5. Analysis of the Endophytic Fungal Culture Filtrate
2.2. Application of Endophytic Fungi on M. oleifera
2.2.1. In Vitro Drought Stress Tolerance Bioassay and Field Experiment of M. oleifera
2.2.2. Analysis of Growth Attribute and Stomatal Aperture
2.2.3. 3,3-Diaminobenzidine (DAB) Activity
2.2.4. Biochemical Analysis
2.2.5. Determination of Phytohormones in M. oleifera
2.2.6. Quantification of Catalase Activity
2.2.7. Multivariate Analysis
2.2.8. Stem Anatomical Evaluation
2.3. RT-qPCR Analysis for Gene Expression
2.4. Statistical Analysis
3. Results
3.1. Endophytic Fungal Isolation
3.2. Assessment of Isolated Strains for Drought Stress Tolerance
3.3. Molecular Identification Based on ITS Sequences and Phylogenetic Analysis
3.4. Quantification of Essential Metabolites in Culture Filtrate under Drought Stress
3.5. Quantification of Hormonal Content in Culture Filtrate under Drought Stress
3.6. Quantification of H2O2 and Antioxidants in Culture Filtrate under Drought Stress
3.7. PEG-Induced Drought Stress Tolerance Response of M. oleifera Seedlings under In Vitro Conditions
3.8. Effect of Fungal Endophytes on the Growth Attributes of M. oleifera Plants under PEG-Induced Drought Stress under Field Conditions
3.9. Effect of Fungal Endophytes on Stem Anatomical Features in M. oleifera Plants under PEG-Induced Drought Stress
3.10. Effect of Fungal Endophytes on Photosynthetic Pigments and Growth-Related Metabolites Production in M. oleifera Plants under PEG-Induced Drought Stress
3.11. Effect of Fungal Endophytes on Hormonal Content in M. oleifera Plants under PEG-Induced Drought Stress
3.12. Effect of Fungal Isolates on Stomatal Aperture and Water Potential (Ψw) in M. oleifera Plants under PEG-Induced Drought Stress
3.13. Effect of Fungal Endophytes on Antioxidant System in M. oleifera Plants under PEG-Induced Drought Stress
3.14. Multivariant Assessment by Principal Component Analysis (PCA)
3.15. Quantitative Gene Expression Analysis of MolHSFs and MolAPX under Drought Stress
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Ethics Approval and Consent to Participate
References
- Costa, M.-C.D.; Farrant, J.M. Plant Resistance to Abiotic Stresses. Plants 2019, 8, 553. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Lu, C.; Guo, J.; Qiao, Z.; Sui, N.; Qiu, N.; Wang, B. C2H2 zinc finger proteins: Master regulators of abiotic stress responses in plants. Front. Plant Sci. 2020, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, N. Plants and salt: Plant response and adaptations to salinity. In Model Ecosystems in Extreme Environments; Elsevier: Amsterdam, The Netherlands, 2019; pp. 101–112. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.J. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, J.; Sharma, A.; Tao, S.; Zheng, B.; Landi, M.; Yuan, H.; Yan, D. Melatonin stimulates activities and expression level of antioxidant enzymes and preserves functionality of photosynthetic apparatus in hickory plants (Carya cathayensis Sarg.) under PEG-Promoted Drought. Agronomy 2019, 9, 702. [Google Scholar] [CrossRef]
- UN. 2010. Available online: http://www.un.org/en/events/desertification_decade/whynow.shtml (accessed on 14 February 2022).
- Nadeem, M.; Li, J.; Wang, M.; Shah, L.; Lu, S.; Wang, X.; Ma, C. Unraveling field crops sensitivity to heat stress: Mechanisms, approaches, and future prospects. Agronomy 2018, 8, 128. [Google Scholar] [CrossRef]
- Kogan, F.; Guo, W.; Yang, W. Drought and food security prediction from NOAA new generation of operational satellites. Geomat. Nat. Hazards Risk 2019, 10, 651–666. [Google Scholar] [CrossRef]
- Boyer, J.S. Plant productivity and environment. Science 1982, 218, 443–448. [Google Scholar] [CrossRef]
- Kohli, S.K.; Khanna, K.; Bhardwaj, R.; Abd Allah, E.F.; Ahmad, P.; Corpas, F.J. Assessment of Subcellular ROS and NO Metabolism in Higher Plants: Multifunctional Signaling Molecules. Antioxidants 2019, 8, 641. [Google Scholar] [CrossRef]
- Ahmad, P.; Tripathi, D.K.; Deshmukh, R.; Singh, V.P.; Corpas, F.J. Revisiting the role of ROS and RNS in plants under changing environment. Environ. Exp. Bot. 2019, 161, 1–3. [Google Scholar] [CrossRef]
- Mansoor, S.; Wani, O.A.; Lone, J.K.; Manhas, S.; Kour, N.; Alam, P.; Ahmad, A.; Ahmad, P. Reactive Oxygen Species in Plants: From Source to Sink. Antioxidants 2022, 11, 225. [Google Scholar] [CrossRef]
- Khan, S.; Basit, A.; Hafeez, M.B.; Irshad, S.; Bashir, S.; Bashir, S.; Maqbool, M.M.; Saddiq, M.S.; Hasnain, Z.; Aljuaid, B.S.; et al. Moringa leaf extract improves biochemical attributes, yield and grain quality of rice (Oryza sativa L.) under drought stress. PLoS ONE 2021, 16, e0254452. [Google Scholar] [CrossRef]
- Ezzo, M.; Ebtihal, M.; Elhamid, A.; Sadak, M.S.; Abdalla, A.M. Improving drought tolerance of moringa plants by using trehalose foliar treatments. Biosci. Res. 2018, 15, 4203–4214. [Google Scholar]
- Sadak, M.S.; Abdalla, A.M.; Abd Elhamid, E.M.; Ezzo, M.I. Role of melatonin in improving growth, yield quantity and quality of M. oleifera L. plant under drought stress. Bull. Natl. Res. Cent. 2020, 44, 1–13. [Google Scholar] [CrossRef]
- Mabapa, P.M.; Ayisi, K.K.; Mariga, I.K. Comparison of gas exchange in Moringa oleifera and other drought tolerant tree species for climate change mitigation under semi-arid condition of Northern South Africa. Int. J. Agric. Biol. 2018, 20, 2669–2676. [Google Scholar]
- Padayachee, B.; Baijnath, H. An updated comprehensive review of the medicinal, phytochemical and pharmacological properties of Moringa oleifera. S. Afr. J. Bot. 2020, 129, 304–316. [Google Scholar] [CrossRef]
- Boumenjel, A.; Papadopoulos, A.; Ammari, Y. Growth response of M. oleifera (Lam) to water stress and to arid bioclimatic conditions. Agrofor. Syst. 2021, 95, 823–833. [Google Scholar] [CrossRef]
- Tian, Y.; Zeng, Y.; Zhang, J.; Yang, C.; Yan, L.; Wang, X.; Shi, C.; Xie, J.; Dai, T.; Peng, L.; et al. High quality reference genome of drumstick tree (Moringa oleifera Lam.), a potential perennial crop. Sci. China Life Sci. 2015, 58, 627–638. [Google Scholar] [CrossRef]
- Pasha, S.N.; Shafi, K.M.; Joshi, A.G.; Meenakshi, I.; Harini, K.; Mahita, J.; Sajeevan, R.S.; Karpe, S.D.; Ghosh, P.; Nitish, S.; et al. The transcriptome enables the identification of candidate genes behind medicinal value of Drumstick tree (Moringa oleifera). Genomics 2020, 112, 621–628. [Google Scholar] [CrossRef]
- Shyamli, P.S.; Pradhan, S.; Panda, M.; Parida, A. De novo Whole-Genome Assembly of M. oleifera Helps Identify Genes Regulating Drought Stress Tolerance. Front. Plant Sci. 2021, 12, 766999. [Google Scholar]
- Li, M.; Xie, F.; Li, Y.; Gong, L.; Luo, Y.; Zhang, Y.; Chen, Q.; Wang, Y.; Lin, Y.; Zhang, Y.; et al. Genome-Wide Analysis of the heat shock transcription factor gene family in Brassica juncea: Structure, evolution, and expression profiles. DNA Cell Biol. 2020, 39, 1990–2004. [Google Scholar] [CrossRef]
- Rauf, M.; Ur-Rahman, A.; Arif, M.; Gul, H.; Ud-Din, A.; Hamayun, M.; Lee, I.-J. Immunomodulatory Molecular Mechanisms of Luffa cylindrica for Downy Mildews Resistance Induced by Growth-Promoting Endophytic Fungi. J. Fungi 2022, 8, 689. [Google Scholar] [CrossRef]
- Poudel, M.; Mendes, R.; Costa, L.A.S.; Bueno, C.G.; Meng, Y.; Folimonova, S.Y.; Garrett, K.A.; Martins, S.J. The Role of Plant-Associated Bacteria, Fungi, and Viruses in Drought Stress Mitigation. Front. Microbiol. 2021, 12, 743512. [Google Scholar] [CrossRef]
- Shamly, V.; Kali, A.; Srirangaraj, S.; Umadevi, S. Comparison of microscopic morphology of fungi using lactophenol cotton blue (LPCB), iodine glycerol and congo red formaldehyde staining. J. Clin. Diagn.Res. JCDR 2014, 8, DL01. [Google Scholar] [CrossRef]
- Rauf, M.; Awais, M.; Ud-Din, A.; Ali, K.; Gul, H.; Rahman, M.M.; Hamayun, M.; Arif, M. Molecular mechanisms of the 1-aminocyclopropane-1-carboxylic acid (ACC) deaminase producing Trichoderma asperellum MAP1 in enhancing wheat tolerance to waterlogging stress. Front. Plant Sci. 2021, 11, 2213. [Google Scholar] [CrossRef]
- Khatiwora, E.; Adsul, V.B.; Kulkarni, M.; Deshpande, N.R.; Kashalkar, R.V. Antibacterial activity of Dibutyl Phthalate: A secondary metabolite isolated from Ipomoea carnea stem. J. Pharm. Res. 2012, 5, 150–152. [Google Scholar]
- Mohammad khani, N.; Heidari, R. Drought-induced accumulation of soluble sugars and proline in two maize varieties. World Appl. Sci. J. 2008, 3, 448–453. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Vogel, H.J. A convenient growth medium for Neurospora (Medium N). Microb. Genet. Bull. 1956, 13, 42–43. [Google Scholar]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Benizri, E.; Courtade, A.; Picard, C.; Guckert, A. Role of maize root exudates in the production of auxins by Pseudomonas fluorescens M. 3.1. Soil Biol. Biochem. 1998, 30, 1481–1484. [Google Scholar] [CrossRef]
- Ergün, N.; Topcuoğlu, Ş.F. Auxin (Indole-3-acetic acid), gibberellic acid (GA3), abscisic acid (ABA) and cytokinin (zeatin) production by some species of mosses and lichens. J. Bot. 2002, 26, 13–18. [Google Scholar]
- Warrier, R.R.; Paul, M.; Vineetha, M.V. Estimation of salicylic acid in Eucalyptus leaves using spectrophotometric methods. Genet. Plant Physiol. 2013, 3, 90–97. [Google Scholar]
- Asada, K.; Takahashi, M. Production and scavenging of active oxygen in photosynthesis. In Photoinhibition; Kyle, D.J., Osmond, C.B., Arntzen, C.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1987; pp. 227–287. [Google Scholar]
- Chandlee, J.M.; Scandalios, J.G. Regulation of Cat1 gene expression in the scutellum of maize during early sporophytic development. Proc. Natl. Acad. Sci. USA 1984, 81, 4903–4907. [Google Scholar] [CrossRef] [PubMed]
- Yen, G.C.; Chen, H.Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Malik, C.P.; Singh, M.B. Plant Enzymology and Histo-Enzymology; Kalyani Publishers: New Delhi, India, 1980. [Google Scholar]
- Moloi, M.J.; van der Westhuizen, A.J. The reactive oxygen species are involved in resistance responses of wheat to the Russian wheat aphid. J. Plant Physiol. 2006, 163, 1118–1125. [Google Scholar] [CrossRef]
- Fozia, A.; Muhammad, A.Z.; Muhammad, A.; Zafar, M.K. Effect of chromium on growth attributes in sunflower (Helianthus annuus L.). J. Environ. Sci. 2008, 20, 1475–1480. [Google Scholar] [CrossRef]
- Thordal-Christensen, H.; Zhang, Z.; Wei, Y.; Collinge, D.B. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley—powdery mildew interaction. Plant J. 1997, 11, 1187–1194. [Google Scholar] [CrossRef]
- Fryer, M.J.; Oxborough, K.; Mullineaux, P.M.; Baker, N.R. Imaging of photo-oxidative stress responses in leaves. J. Exp. Bot. 2002, 53, 1249–1254. [Google Scholar]
- Maclachlan, S.; Zalik, S. Plastid structure, chlorophyll concentration, and free amino acid composition of a chlorophyll mutant of barley. Can. J. Bot. 1963, 41, 1053–1062. [Google Scholar] [CrossRef]
- Khatiwora, E.; Adsul, V.B.; Torane, R.C.; Gaikwad, S.; Deshpande, N.R.; Kashalkar, R.V. Spectroscopic determination of total phenol and flavonoid contents of citrus limon peel from north eastern region of India. J. Drug Deliv. Ther. 2017, 7, 21–24. [Google Scholar] [CrossRef]
- Rauf, M.; Arif, M.; Fisahn, J.; Xue, G.P.; Balazadeh, S.; Mueller-Roeber, B. NAC transcription factor speedy hyponastic growth regulates flooding-induced leaf movement in Arabidopsis. Plant Cell. 2013, 25, 4941–4955. [Google Scholar] [CrossRef]
- Pareek, A.; Dhankher, O.P.; Foyer, C.H. Mitigating the impact of climate change on plant productivity and ecosystem sustainability. J. Exp. Bot. 2020, 71, 451–456. [Google Scholar] [CrossRef]
- Drost, S.M.; Rutgers, M.; Wouterse, M.; De Boer, W.; Bodelier, P.L. Decomposition of mixtures of cover crop residues increases microbial functional diversity. Geoderma 2020, 361, 114060. [Google Scholar] [CrossRef]
- Aziz, L.; Hamayun, M.; Rauf, M.; Iqbal, A.; Husssin, A.; Khan, S.A.; Irshad, M.; Lee, I.J. Aspergillus Flavus reprogrammed morphological and chemical attributes of Solanum lycopersicum through SlGSH1 and SlPCS1 genes modulation under heavy metal stress. J. Plant Interact. 2021, 16, 104–115. [Google Scholar] [CrossRef]
- Khan, Z.; Gul, H.; Rauf, M.; Arif, M.; Hamayun, M.; Ud-Din, A.; Sajid, Z.A.; Khilji, S.; Rehman, A.; Tabassum, A.; et al. Sargassum wightii Aqueous Extract Improved Salt Stress Tolerance in Abelmoschus esculentus by Mediating Metabolic and Ionic Rebalance. Front. Mar. Sci. 2022, 9, 853272. [Google Scholar] [CrossRef]
- Ghazghazi, H.; Riahi, L.; Yangui, I.; Messaoud, C.; Rzigui, T.; Nasr, Z. Effect of Drought Stress on Physio-biochemical Traits and Secondary Metabolites Production in the Woody Species Pinus Halepensis Mill. At a Juvenile Development Stage. J. Sustain. For. 2022, 1–17. [Google Scholar] [CrossRef]
- Ali, R.; Gul, H.; Hamayun, M.; Rauf, M.; Iqbal, A.; Shah, M.; Hussain, A.; Bibi, H.; Lee, I. Aspergillus awamori ameliorates the physicochemical characteristics and mineral profile of mung bean under salt stress. Chem. Biol. Technol. Agric. 2021, 8, 1–13. [Google Scholar] [CrossRef]
- Ali, R.; Gul, H.; Rauf, M.; Arif, M.; Hamayun, M.; Husna; Khilji, S.A.; Ud-Din, A.; Sajid, Z.A.; Lee, I.-J. Growth-Promoting Endophytic Fungus (Stemphylium lycopersici) Ameliorates Salt Stress Tolerance in Maize by Balancing Ionic and Metabolic Status. Front. Plant Sci. 2022, 13, 890565. [Google Scholar] [CrossRef]
- Aziz, L.; Hamayun, M.; Rauf, M.; Iqbal, A.; Arif, M.; Husssin, A.; Khan, S.A. Endophytic Aspergillus niger reprograms the physicochemical traits of tomato under cadmium and chromium stress. Environ. Exp. Bot. 2021, 186, 104456. [Google Scholar] [CrossRef]
- Zhou, X.R.; Dai, L.; Xu, G.F.; Wang, H.S. A strain of Phoma species improves drought tolerance of Pinus tabulaeformis. Sci. Rep. 2021, 11, 1–11. [Google Scholar]
- Decunta, F.A.; Pérez, L.I.; Malinowski, D.P.; Molina-Montenegro, M.A.; Gundel, P.E. A systematic review on the effects of Epichloë fungal endophytes on drought tolerance in cool-season grasses. Front. Plant Sci. 2021, 12, 644731. [Google Scholar] [CrossRef]
- Hereme, R.; Morales-Navarro, S.; Ballesteros, G.; Barrera, A.; Ramos, P.; Gundel, P.E.; Molina-Montenegro, M.A. Fungal endophytes exert positive effects on Colobanthus quitensis under water stress but neutral under a projected climate change scenario in Antarctica. Front. Microbiol. 2020, 11, 264. [Google Scholar] [CrossRef]
- Hubbard, M.; Germida, J.; Vujanovic, V. Fungal endophytes improve wheat seed germination under heat and drought stress. Botany 2012, 90, 137–149. [Google Scholar] [CrossRef]
- Khan, Z.; Rho, H.; Firrincieli, A.; Hung, S.H.; Luna, V.; Masciarelli, O.; Kim, S.H.; Doty, S.L. Growth enhancement and drought tolerance of hybrid poplar upon inoculation with endophyte consortia. Curr. Plant Biol. 2016, 6, 38–47. [Google Scholar] [CrossRef]
- Langeroodi, A.R.S.; Osipitan, O.A.; Radicetti, E.; Mancinelli, R. To what extent arbuscular mycorrhiza can protect chicory (Cichorium intybus L.) against drought stress. Sci. Hortic. 2020, 263, 109109. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, J.; Liao, X.; Yan, Q.; Liang, G.; Liu, J.; Wang, D.; Guan, R. Different Arbuscular Mycorrhizal Fungi Established by Two Inoculation Methods Improve Growth and Drought Resistance of Cinnamomum Migao Seedlings Differently. Biology 2022, 11, 220. [Google Scholar] [CrossRef]
- Halo, B.A.; Al-Yahyai, R.A.; Al-Sadi, A.M. An endophytic Talaromyces omanensis enhances reproductive, physiological and anatomical characteristics of drought-stressed tomato. J. Plant Physiol. 2020, 249, 15. [Google Scholar] [CrossRef]
- Mahpara, S.; Zainab, A.; Ullah, R.; Kausar, S.; Bilal, M.; Latif, M.I.; Arif, M.; Akhtar, I.; Al-Hashimi, A.; Elshikh, M.S.; et al. The impact of PEG-induced drought stress on seed germination and seedling growth of different bread wheat (Triticum aestivum L.) genotypes. PLoS ONE 2022, 17, e0262937. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiao, C.; Adeyeye, O.; Yang, W.; Liang, X. Source and mobilization mechanism of iron, manganese and arsenic in groundwater of Shuangliao City, Northeast China. Water 2020, 12, 534. [Google Scholar] [CrossRef] [Green Version]
- Bali, A.S.; Sidhu, G.P.S. Abiotic stress-induced oxidative stress in wheat. In Wheat Production in Changing Environments; Springer: Berlin/Heidelberg, Germany, 2019; pp. 225–239. [Google Scholar]
- Ma, Y.; Dias, M.C.; Freitas, H. Drought and salinity stress responses and microbe-induced tolerance in plants. Front. Plant Sci. 2020, 11, 591911. [Google Scholar] [CrossRef]
- Semida, W.M.; Abdelkhalik, A.; Rady, M.O.; Marey, R.A.; Abd El-Mageed, T.A. Exogenously applied proline enhances growth and productivity of drought stressed onion by improving photosynthetic efficiency, water use efficiency and up-regulating osmoprotectants. Sci. Hortic. 2020, 272, 109580. [Google Scholar] [CrossRef]
- Raja, V.; Qadir, S.U.; Alyemeni, M.N.; Ahmad, P. Impact of drought and heat stress individually and in combination on physio-biochemical parameters, antioxidant responses, and gene expression in Solanum lycopersicum. 3 Biotech 2020, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Environ. 2010, 3, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Kar, R.K. Plant responses to water stress. Role of reactive oxygen species. Plant Signal. Behav. 2011, 6, 1741–1745. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Bhuyan, M.H.M.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive oxygen species and antioxidant defense in plants under abiotic stress: Revisiting the crucial role of a universal defense regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Kosar, F.; Akram, N.A.; Ashraf, M.; Ahmad, A.; Alyemeni, M.N.; Ahmad, P. Impact of exogenously applied trehalose on leaf biochemistry, achene yield and oil composition of sunflower under drought stress. Physiol. Plant 2021, 172, 317–333. [Google Scholar] [CrossRef]
- Redman, R.S.; Kim, Y.O.; Woodward, C.J.; Greer, C.; Espino, L.; Doty, S.L.; Rodriguez, R.J. Increased fitness of rice plants to abiotic stress via habitat adapted symbiosis: A strategy for mitigating impacts of climate change. PLoS ONE 2011, 6, e14823. [Google Scholar] [CrossRef]
- Fang, Y.; Liao, K.; Du, H.; Xu, Y.; Song, H.; Li, X.; Xiong, L. A stress-responsive NAC transcription factor SNAC3 confers heat and drought tolerance through modulation of reactive oxygen species in rice. J. Exp. Bot. 2015, 66, 6803–6817. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, Y.; Tao, X.; Wang, J.-Z.; Cheng, H.-Y.; Yang, H.; Ma, X.-R. Heat Shock Factor Genes of Tall Fescue and Perennial Ryegrass in Response to Temperature Stress by RNA-Seq Analysis. Front. Plant Sci. 2016, 6, 1226. [Google Scholar] [CrossRef] [Green Version]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F. The role of salicylic acid and gibberellin signaling in plant responses to abiotic stress with an emphasis on heavy metals. Plant Signal. Behav. 2020, 15, 1777372. [Google Scholar] [CrossRef]
- Sedaghat, M.; Tahmasebi-Sarvestani, Z.; Emam, Y.; Mokhtassi-Bidgoli, A. Physiological and antioxidant responses of winter wheat cultivars to strigolactone and salicylic acid in drought. Plant Physiol. Biochem. 2017, 119, 59–69. [Google Scholar] [CrossRef]
- Brunetti, C.; Loreto, F.; Ferrini, F.; Gori, A.; Guidi, L.; Remorini, D.; Centritto, M.; Fini, A.; Tattini, M. Metabolic plasticity in the hygrophyte M. oleifera exposed to water stress. Tree Physiol. 2018, 38, 1640–1654. [Google Scholar]
- Yoon, Y.; Seo, D.H.; Shin, H.; Kim, H.J.; Kim, C.M.; Jang, G. The role of stress-responsive transcription factors in modulating abiotic stress tolerance in plants. Agronomy 2020, 10, 788. [Google Scholar] [CrossRef]
- Zhu, M.D.; Zhang, M.; Gao, D.J.; Zhou, K.; Tang, S.J.; Zhou, B.; Lv, Y.M. Rice OsHSFA3 gene improves drought tolerance by modulating polyamine biosynthesis depending on abscisic acid and ROS levels. Int. J. Mol. Sci. 2020, 21, 1857. [Google Scholar] [CrossRef] [Green Version]
- Dang, J.; Jiang, M.; Lin, F. ABA up-regulate the expression of OsHsf gene in leaves of rice plants. J. Nanjing Agric. Univ. 2010, 33, 11–15. [Google Scholar]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Javed, J.; Rauf, M.; Arif, M.; Hamayun, M.; Gul, H.; Ud-Din, A.; Ud-Din, J.; Sohail, M.; Rahman, M.M.; Lee, I.-J. Endophytic Fungal Consortia Enhance Basal Drought-Tolerance in Moringa oleifera by Upregulating the Antioxidant Enzyme (APX) through Heat Shock Factors. Antioxidants 2022, 11, 1669. https://doi.org/10.3390/antiox11091669
Javed J, Rauf M, Arif M, Hamayun M, Gul H, Ud-Din A, Ud-Din J, Sohail M, Rahman MM, Lee I-J. Endophytic Fungal Consortia Enhance Basal Drought-Tolerance in Moringa oleifera by Upregulating the Antioxidant Enzyme (APX) through Heat Shock Factors. Antioxidants. 2022; 11(9):1669. https://doi.org/10.3390/antiox11091669
Chicago/Turabian StyleJaved, Javeria, Mamoona Rauf, Muhammad Arif, Muhammad Hamayun, Humaira Gul, Aziz Ud-Din, Jalal Ud-Din, Mohammad Sohail, Muhammad Mizanur Rahman, and In-Jung Lee. 2022. "Endophytic Fungal Consortia Enhance Basal Drought-Tolerance in Moringa oleifera by Upregulating the Antioxidant Enzyme (APX) through Heat Shock Factors" Antioxidants 11, no. 9: 1669. https://doi.org/10.3390/antiox11091669
APA StyleJaved, J., Rauf, M., Arif, M., Hamayun, M., Gul, H., Ud-Din, A., Ud-Din, J., Sohail, M., Rahman, M. M., & Lee, I. -J. (2022). Endophytic Fungal Consortia Enhance Basal Drought-Tolerance in Moringa oleifera by Upregulating the Antioxidant Enzyme (APX) through Heat Shock Factors. Antioxidants, 11(9), 1669. https://doi.org/10.3390/antiox11091669