Light Quality Potentiates the Antioxidant Properties of Brassica rapa Microgreen Extracts against Oxidative Stress and DNA Damage in Human Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
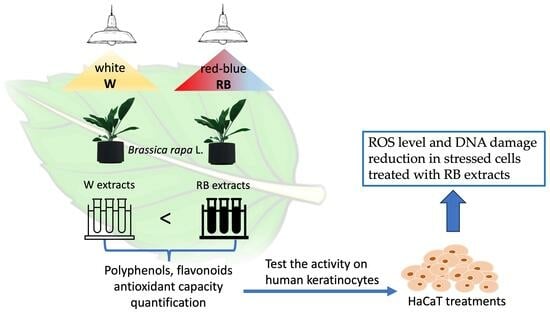
2.2. Experimental Design and Plant Material
2.3. Preparation of Methanolic Extracts
2.4. Polyphenols and Flavonoids Determination
2.5. Antioxidant Activity Assay
2.6. Cell Culture, Reagents, and Preparation Samples
2.7. Cell Viability (MTT) Assay
2.8. Dichlorofluorescein Diacetate (DCFDA) Assay
2.9. Immunofluorescence Microscopy on HaCaT Cells
2.10. Western Blot Analysis
3. Results
3.1. Evaluation of the Antioxidant Capacity of B. rapa Microgreens Exposed to Different Light Regimes
3.2. Cytotoxicity of B. rapa Microgreens MeOH Extract on Human Cells
3.3. Antioxidant Activity of B. rapa W and RB Extracts
3.4. Effect of W and RB Crude Extracts on Stress Granules Formation and DNA Damage
4. Discussion
- Inizio modulo
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dhalaria, R.; Verma, R.; Kumar, D.; Puri, S.; Tapwal, A.; Kumar, V.; Nepovimova, E.; Kuca, K. Bioactive Compounds of Edible Fruits with Their Anti-Aging Properties: A Comprehensive Review to Prolong Human Life. Antioxidants 2020, 9, 1123. [Google Scholar] [CrossRef] [PubMed]
- Teodoro, A.J. Bioactive Compounds of Food: Their Role in the Prevention and Treatment of Diseases. Oxid Med. Cell. Longev. 2019, 2019, 3765986. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Singh, S.; Dulta, K.; Singh, N.; Ali, B.; Hafeez, A.; Vodnar, D.C.; Marc, R.A. Nutritional evaluation, phytochemical makeup, antibacterial and antioxidant properties of wild plants utilized as food by the Gaddis-a tribal tribe in the Western Himalayas. Front. Agron. 2022, 4, 1010309. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T.; Moreno-Rojas, J.M. Potential Health Benefits of Plant Food-Derived Bioactive Components: An overview. Foods 2021, 10, 839. [Google Scholar] [CrossRef] [PubMed]
- Simunkova, M.; Alwasel, S.H.; Alhazza, I.M.; Jomova, K.; Kollar, V.; Rusko, M.; Valko, M. Management of oxidative stress and other pathologies in Alzheimer’s disease. Arch. Toxicol. 2019, 93, 2491–2513. [Google Scholar] [CrossRef]
- Lourenço, S.C.; Moldão-Martinsand, M.; Alves, V.D. Antioxidants of Natural Plant Origins: From Sourcesto Food Industry Applications. Molecules 2019, 24, 4132. [Google Scholar] [CrossRef]
- Ferreres, F.; Sousa, C.; Valentão, P.; Seabra, R.M.; Pereira, J.A.; Andrade, P.B. Tronchuda cabbage (Brassica oleracea L. var. costata DC) seeds: Phytochemical characterization and antioxidant potential. Food Chem. 2007, 101, 549–558. [Google Scholar] [CrossRef]
- Vallejo, F.; Tomás-Barberán, F.; García-Viguera, C. Health-Promoting Compounds in Broccoli as Influenced by Refrigerated Transport and Retail Sale Period. J. Agric. Food Chem. 2003, 51, 3029–3034. [Google Scholar] [CrossRef] [PubMed]
- Jahangir, M.; Kim, H.K.; Choi, Y.H.; Verpoorte, R. Health-Affecting Compounds in Brassicaceae. Compr. Rev. Food Sci. Food Saf. 2009, 8, 31–43. [Google Scholar] [CrossRef]
- Robbins, R.J.; Keck, A.S.; Banuelos, G.; Finley, J.W. Cultivation Conditions and Selenium Fertilization Alter the Phenolic Profile, Glucosinolate, and Sulforaphane Content of Broccoli. J. Med. Food 2005, 8, 204–214. [Google Scholar] [CrossRef] [PubMed]
- Birt, D.F.; Hendrich, S.; Wang, W. Dietary agents in cancer prevention: Flavonoids and isoflavonoids. Pharmacol Ther. 2001, 90, 157–177. [Google Scholar] [CrossRef]
- Satterfield, S.; Cutler, J.A.; Langford, H.G.; Applegate, W.B.; Borhani, N.O.; Brittain, E.; Cohen, J.D.; Kuller, L.H.; Lasser, N.L.; Oberman, A.; et al. Trials of hypertension prevention phase I design. Ann. Epidemiol. 1991, 1, 455–471. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Q.; Heying, E.; Tanumihardjo, S.A. History, Global Distribution, and Nutritional Importance of Citrus Fruits. Compr. Rev. Food Sci. Food Saf. 2012, 11, 530–545. [Google Scholar] [CrossRef]
- Pagare, S.; Bhatia, M.; Tripathi, N.; Pagare, S.; Bansal, Y.K. Secondary metabolites of plants and their role: Overview. Curr. Trends Biotechnol. Pharm. 2015, 9, 294–305. [Google Scholar]
- Izzo, L.G.; Arena, C.; De Micco, V.; Capozzi, F.; Aronne, G. Light quality shapes morpho-functional traits and pigment content of green and red leaf cultivars of Atriplex hortensis. Sci. Hortic. 2019, 246, 942–950. [Google Scholar] [CrossRef]
- Hasan, M.M.; Bashir, T.; Ghosh, R.; Lee, S.K.; Bae, H. An overview of LEDs’ effects on the production of bioactive compounds and crop quality. Molecules 2017, 22, 1420. [Google Scholar] [CrossRef]
- Costanzo, G.; Vitale, E.; Iesce, M.R.; Naviglio, D.; Amoresano, A.; Fontanarosa, C.; Spinelli, M.; Ciaravolo, M.; Arena, C. Antioxidant Properties of Pulp, Peel and Seeds of Phlegrean Mandarin (Citrus reticulata Blanco) at Different Stages of Fruit Ripening. Antioxidants 2022, 11, 187. [Google Scholar] [CrossRef]
- Lefsrud, M.G.; Kopsell, D.A.; Sams, C.E. Irradiance from distinct wavelength light-emitting diodes affect secondary metabolites in kale. Hortic. Sci. 2008, 43, 2243–2244. [Google Scholar] [CrossRef]
- Costanzo, G.; Vitale, E.; Iesce, M.R.; Spinelli, M.; Fontanarosa, C.; Paradiso, R.; Amoresano, A.; Arena, C. Modulation of Antioxidant Compounds in Fruits of Citrus reticulata Blanco Using Postharvest LED Irradiation. Biology 2023, 12, 1029. [Google Scholar] [CrossRef]
- Dutta Gupta, S. Light Emitting Diodes for Agriculture: Smart Lighting. Light Emit; Springer: Cham, Switzerland, 2017; Volume XIX, pp. 1–334. [Google Scholar]
- Lee, S.U.; Lee, J.H.; Choi, S.H.; Lee, J.S.; Kameyama, M.O.; Kozukue, N.; Friedman, M. Flavonoid content in fresh, home-processed, and light-exposed onions and in dehydrated commercial onion products. J. Agric. Food Chem. 2008, 56, 8541–8548. [Google Scholar] [CrossRef]
- Loi, M.; Villani, A.; Paciolla, F.; Mulè, G.; Paciolla, C. Challenges and Opportunities of Light-Emitting Diode (LED) as Key to Modulate Antioxidant Compounds in Plants. A Review. Antioxidants 2021, 10, 42. [Google Scholar] [CrossRef] [PubMed]
- Paradiso, R.; Proietti, S. Light-Quality Manipulation to Control Plant Growth and Photomorphogenesis in Greenhouse Horticulture: The State of the Art and the Opportunities of Modern LED Systems. J. Plant Growth Regul. 2021, 41, 742–780. [Google Scholar] [CrossRef]
- Darko, E.; Heydarizadeh, P.; Schoefs, B.; Sabzalian, M.R. Photosynthesis under artificial light: The shift in primary and secondary metabolism. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 1471–2970. [Google Scholar] [CrossRef] [PubMed]
- Dou, H.; Niu, G.; Gu, M.; Masabni, J. Effects of light quality on growth and phytonutrient accumulation of herbs under controlled environments. Horticulturae 2017, 3, 36. [Google Scholar] [CrossRef]
- Haghi, A.; Azimi, H.; Rahimi, R. A Comprehensive Review on Pharmacotherapeutics of Three Phytochemicals, Curcumin, Quercetin, and Allicin, in the Treatment of Gastric Cancer. J. Gastrointest. Cancer 2017, 48, 314–320. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y.; Di Gioia, F.; Kyratzis, A.; Serio, F.; Renna, M.; De Pascale, S.; Santamaria, P. Micro-scale vegetable production and the rise of microgreens. Trends Food Sci. Technol. 2016, 57, 103–115. [Google Scholar] [CrossRef]
- Świeca, M.; Dziki, D. Improvement in sprouted wheat flour functionality: Effect of time, temperature and elicitation. Int. J. Food Sci. Technol. 2015, 50, 2135–2142. [Google Scholar] [CrossRef]
- Sun, B.; Richardo-da-Silva, J.M.; Sprenger, I. Critical factors of vanillin assay for catechins and proanthocyanidins. J. Agric. Food Chem. 1998, 46, 4267–4274. [Google Scholar] [CrossRef]
- Moulehi, I.; Bourgou, S.; Ourghemmi, I.; Tounsi, M.S. Variety and ripening impact on phenolic composition and Antioxidant activity of mandarin (Citrus reticulate Blanco) and bitter orange (Citrus aurantium L.) seeds extracts. Ind. Crop. Prod. 2012, 39, 74–80. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- George, B.; Kaur, C.; Khurdiya, D.S.; Kapoor, H.C. Antioxidants in tomato (Lycopersium esculentum) as a function of genotype. Food Chem. 2004, 84, 45–51. [Google Scholar] [CrossRef]
- Sangermano, F.; Masi, M.; Vivo, M.; Ravindra, P.; Cimmino, A.; Pollice, A.; Evidente, A.; Calabrò, V. Higginsianins A and B, two fungal diterpenoid α-pyrones with cytotoxic activity against human cancer cells. Toxicol In Vitro 2019, 61, 104614. [Google Scholar] [CrossRef] [PubMed]
- Fontana, R.; Guidone, D.; Sangermano, F.; Calabrò, V.; Pollice, A.; La Mantia, G.; Vivo, M. PKC Dependent p14ARF Phosphorylation on Threonine 8 Drives Cell Proliferation. Sci. Rep. 2018, 8, 7056. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, O.; Troiano, A.; Guarino, A.M.; Pollice, A.; Vivo, M.; La Mantia, G.; Calabrò, V. ΔNp63α controls YB-1 protein stability: Evidence on YB-1 as a new player in keratinocyte differentiation. Genes Cells. 2016, 21, 648–660. [Google Scholar] [CrossRef]
- Vivo, M.; Matarese, M.; Sepe, M.; Di Martino, R.; Festa, L.; Calabrò, V.; La Mantia, G.; Pollice, A. MDM2-mediated degradation of p14ARF: A novel mechanism to control ARF levels in cancer cells. PLoS ONE 2015, 10, e0117252. [Google Scholar] [CrossRef]
- Xu, F.; Cao, S.; Shi, L.; Chen, W.; Su, X.; Yang, Z. Blue light irradiation affects anthocyanin content and enzyme activities involved in postharvest strawberry fruit. J. Agric. Food Chem. 2014, 62, 4778–4783. [Google Scholar] [CrossRef]
- Xu, F.; Shi, L.; Chen, W.; Cao, S.; Su, X.; Yang, Z. Scientia Horticulturae Effect of blue light treatment on fruit quality, antioxidant enzymes and radical-scavenging activity in strawberry fruit. Sci. Hortic. 2014, 175, 181–186. [Google Scholar] [CrossRef]
- Zhan, L.; Hu, J.; Li, Y.; Pang, L. Combination of light exposure and low temperature in preserving quality and extending shelf-life of fresh-cut broccoli (Brassica oleracea L.). Postharvest Biol. Technol. 2012, 72, 76–81. [Google Scholar] [CrossRef]
- Zhan, L.; Hu, J.; Li, Y.; Pang, L.; Fan, H. Browning inhibition and quality preservation of fresh-cut romaine lettuce exposed to high-intensity light. Innov. Food Sci. Emerg. Technol. 2012, 14, 70–76. [Google Scholar] [CrossRef]
- Nassarawa, S.S.; Abdelshafy, A.M.; Xu, Y.; Luo, Z. Effect of Light-Emitting Diodes (LEDs) on the Quality of Fruits and Vegetables During Postharvest Period: A Review. Food Bioprocess Technol. 2021, 14, 388–414. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, C.T.; Wang, C.Y. The influence of light and maturity on fruit quality and flavonoid content of red raspberries. Food Chem. 2009, 112, 676–684. [Google Scholar] [CrossRef]
- Silva, F.G.D.; O’Callagahan, Y.; O’Brien, N.M.; Netto, F.M. Antioxidant capacity of flaxseed products: The effect of in vitro digestion. Plant Food Hum. Nutr. 2013, 68, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Sarker, U.; Oba, S. Augmentation of leaf color parameters, pigments, vitamins, phenolic acids, flavonoids and antioxidant activity in selected Amaranthus tricolor under salinity stress. Sci. Rep. 2018, 8, 12349. [Google Scholar] [CrossRef] [PubMed]
- Mahboubi, H.; Stochaj, U. Cytoplasmic stress granules: Dynamic modulators of cell signaling and disease. Biochim. Biophys. Acta Mol. Basis. Dis 2017, 1863, 884–895. [Google Scholar] [CrossRef]
- Marcelo, A.; Koppenol, R.; de Almeida, L.P.; Matos, C.A.; Nóbrega, C. Stress granules, RNA-binding proteins, and polyglutamine diseases: Too much aggregation? Cell Death Dis. 2021, 12, 592. [Google Scholar] [CrossRef] [PubMed]
- Barzilai, A.; Yamamoto, K.I. DNA damage responses to oxidative stress. DNA Repair 2004, 3, 1109–1115. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, K.; Almasan, A. Histone H2AX phosphorylation: A marker for DNA damage. DNA Repair Protoc. 2012, 920, 613–626. [Google Scholar]
- Bellezza, I.; Giambanco, I.; Minelli, A.; Donato, R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 721–733. [Google Scholar] [CrossRef]
- O’Reilly, M.A. Redox activation of p21Cip1/WAF1/Sdi1: A multifunctional regulator of cell survival and death. Antioxid. Redox Signal. 2005, 7, 108–118. [Google Scholar] [CrossRef]
- Espinosa-Diez, C.; Miguel, V.; Mennerich, D.; Kietzmann, T.; Sánchez-Pérez, P.; Cadenas, S.; Lamas, S. Antioxidant responses and cellular adjustments to oxidative stress. Redox Biol. 2015, 6, 183–197. [Google Scholar] [CrossRef]






| W Light Regime | RB Light Regime | |
|---|---|---|
| Antioxidant capacity (µmol TE g−1 FW) | 9.46 ± 0.11 b | 13.56 ± 0.16 a |
| Total polyphenols (mg GAE g−1 FW) | 2.96 ± 0.07 b | 4.45 ± 0.07 a |
| Total flavonoids (mg CE g−1 FW) | 3.86 ± 0.06 b | 4.40 ± 0.09 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paolillo, I.; Costanzo, G.; Delicato, A.; Villano, F.; Arena, C.; Calabrò, V. Light Quality Potentiates the Antioxidant Properties of Brassica rapa Microgreen Extracts against Oxidative Stress and DNA Damage in Human Cells. Antioxidants 2023, 12, 1895. https://doi.org/10.3390/antiox12101895
Paolillo I, Costanzo G, Delicato A, Villano F, Arena C, Calabrò V. Light Quality Potentiates the Antioxidant Properties of Brassica rapa Microgreen Extracts against Oxidative Stress and DNA Damage in Human Cells. Antioxidants. 2023; 12(10):1895. https://doi.org/10.3390/antiox12101895
Chicago/Turabian StylePaolillo, Ida, Giulia Costanzo, Antonella Delicato, Filippo Villano, Carmen Arena, and Viola Calabrò. 2023. "Light Quality Potentiates the Antioxidant Properties of Brassica rapa Microgreen Extracts against Oxidative Stress and DNA Damage in Human Cells" Antioxidants 12, no. 10: 1895. https://doi.org/10.3390/antiox12101895
APA StylePaolillo, I., Costanzo, G., Delicato, A., Villano, F., Arena, C., & Calabrò, V. (2023). Light Quality Potentiates the Antioxidant Properties of Brassica rapa Microgreen Extracts against Oxidative Stress and DNA Damage in Human Cells. Antioxidants, 12(10), 1895. https://doi.org/10.3390/antiox12101895